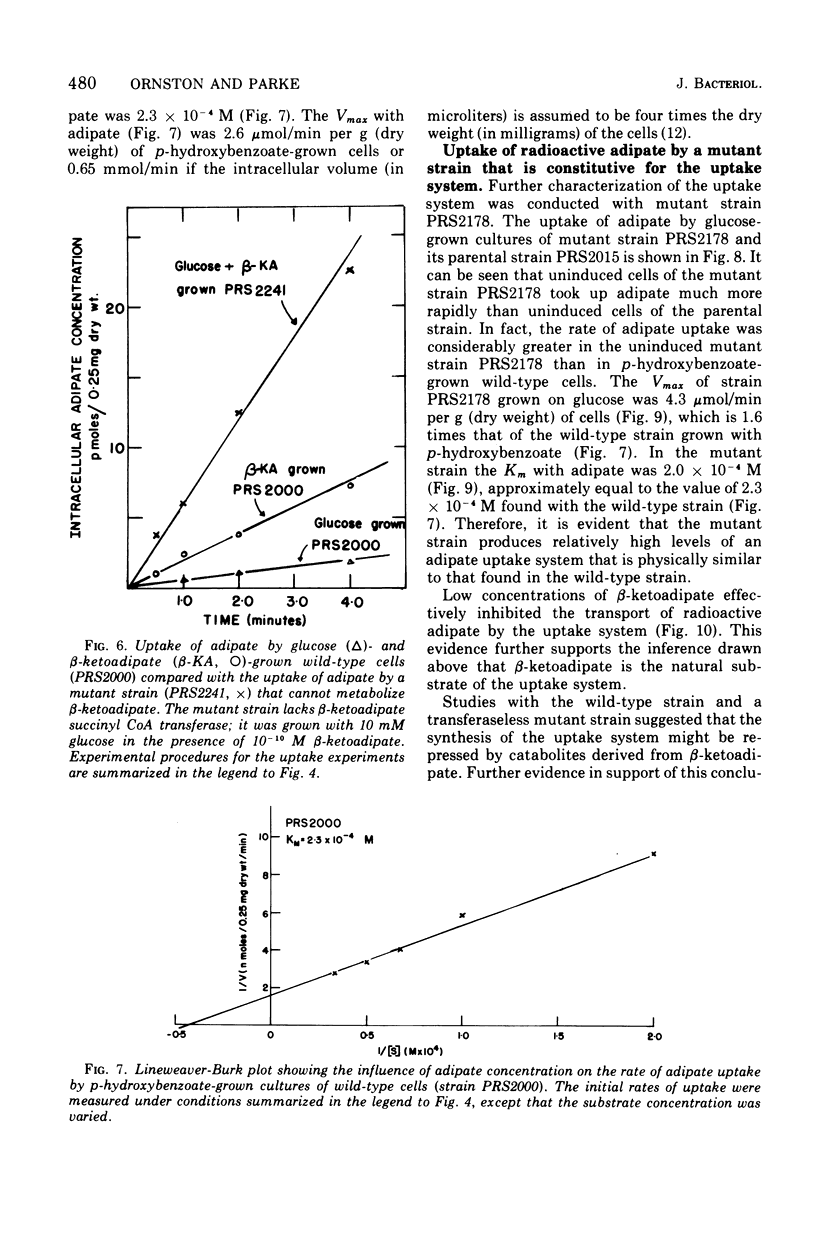
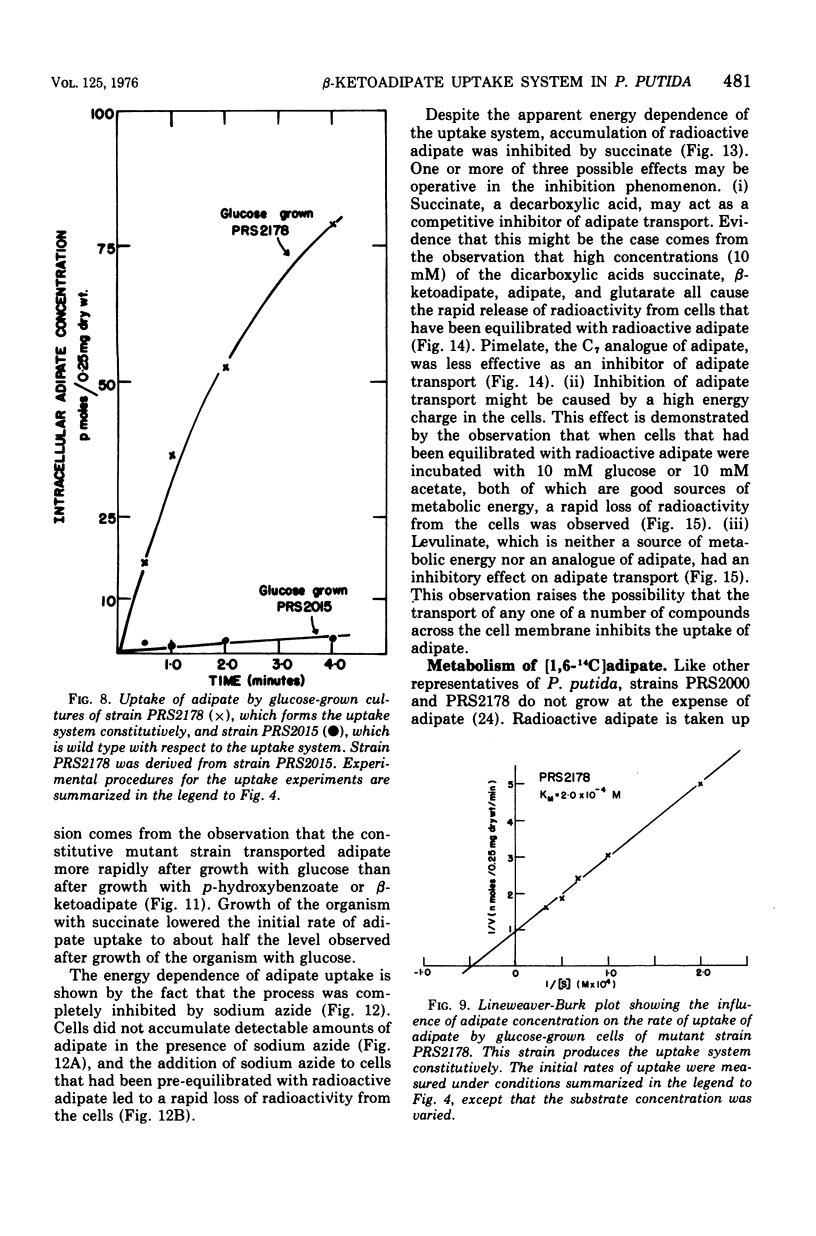
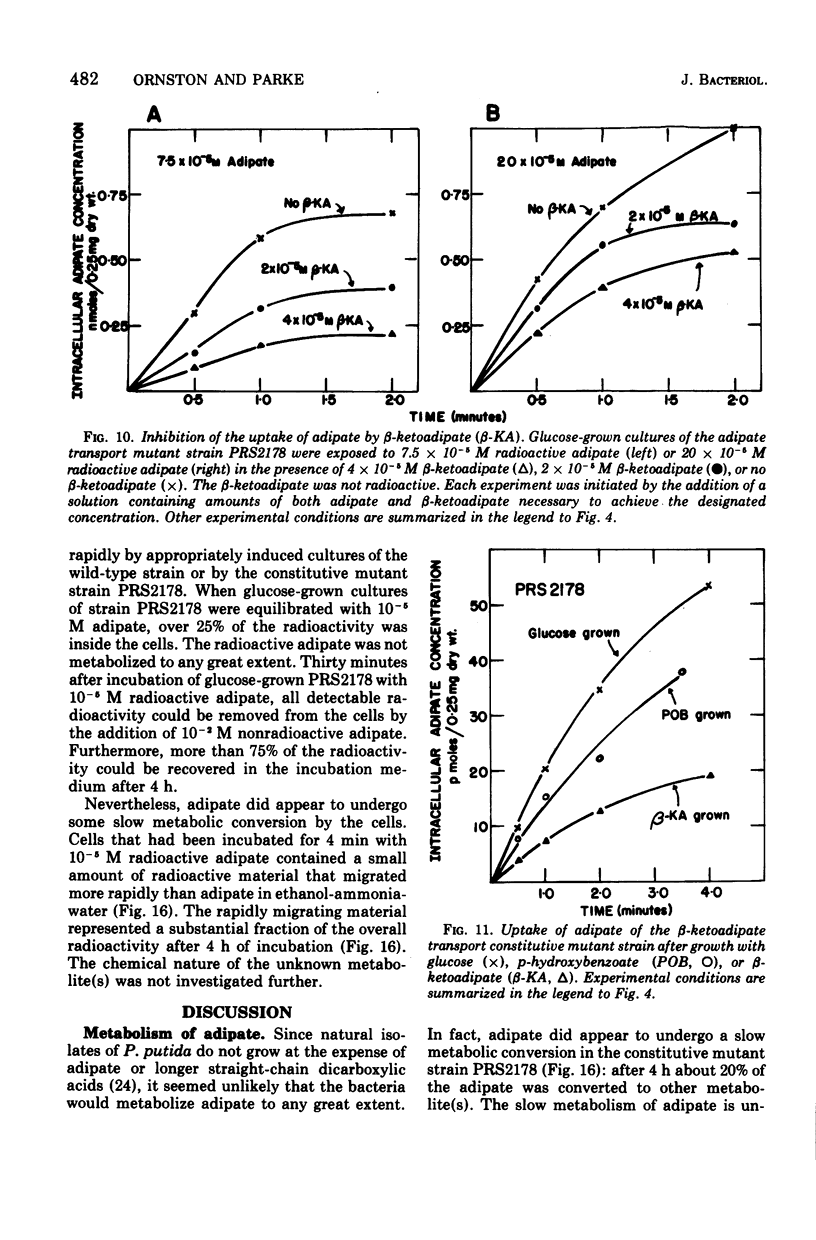
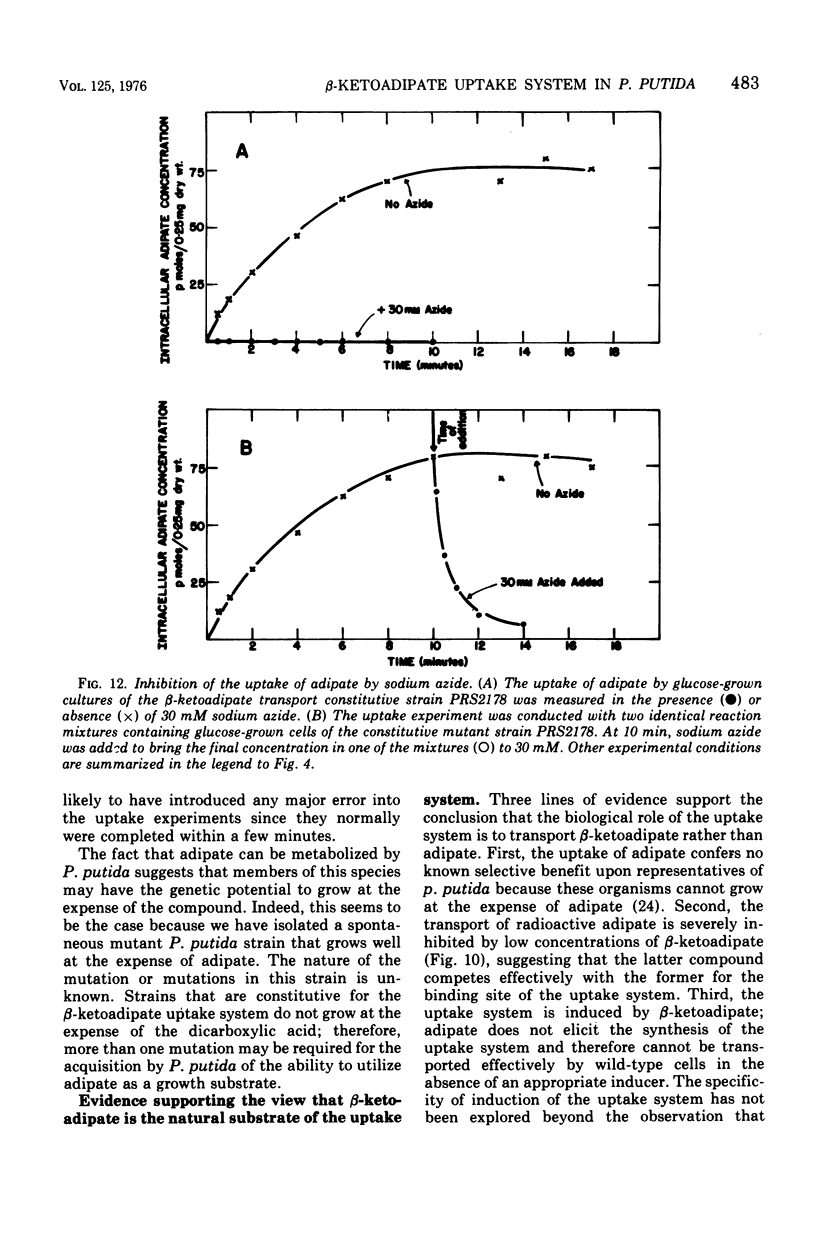
Abstract
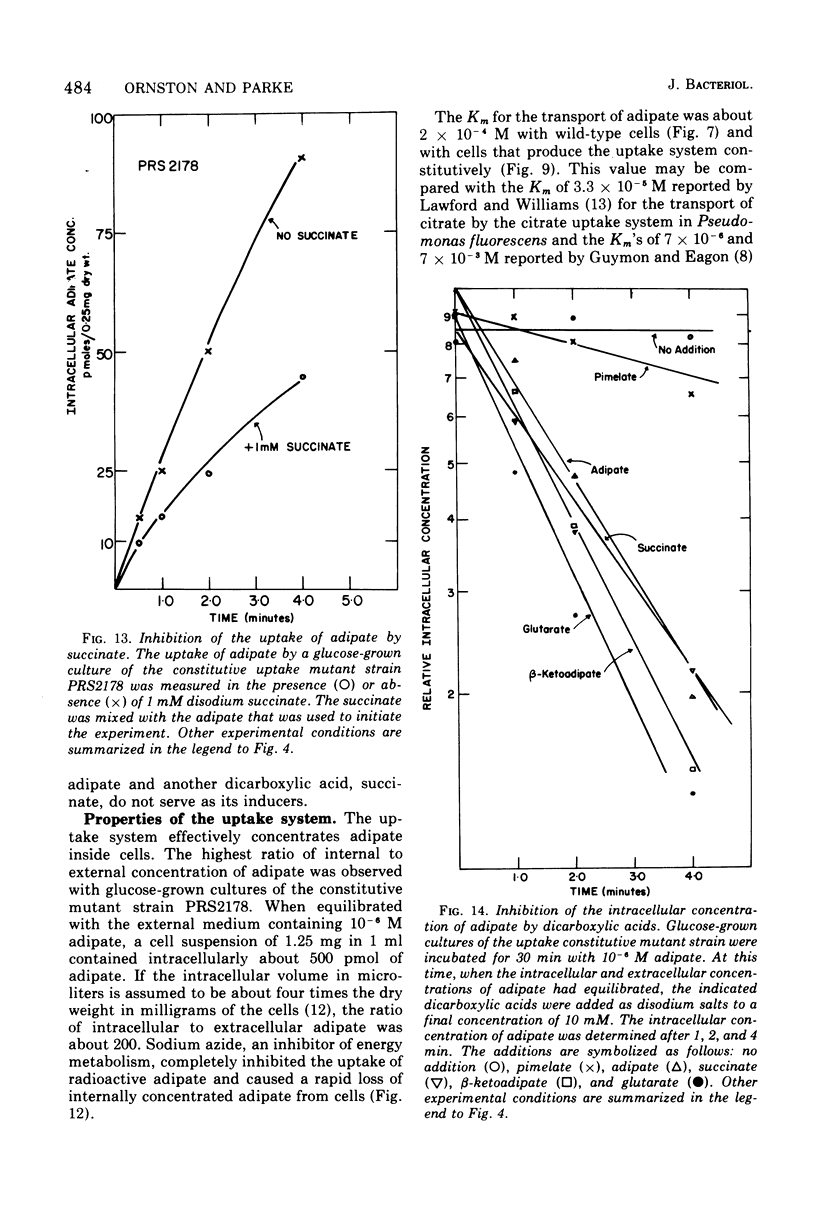
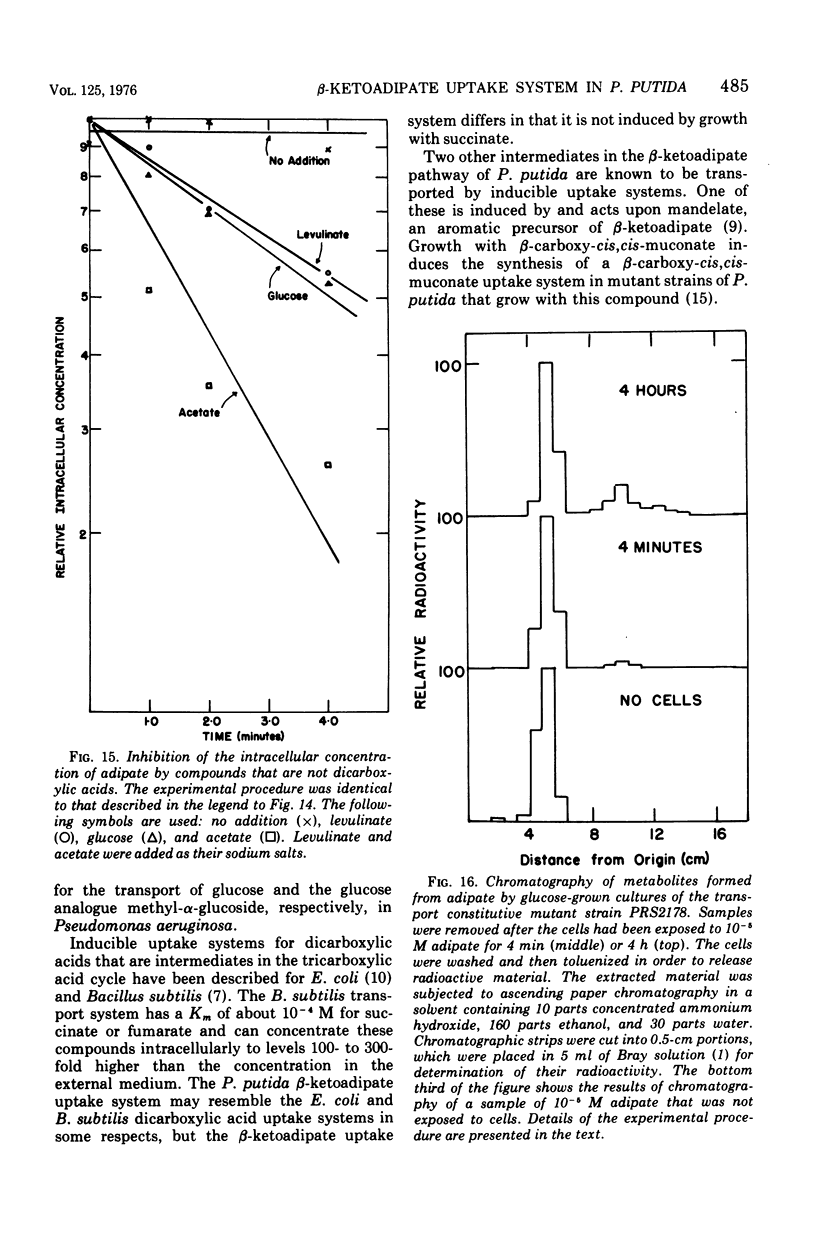
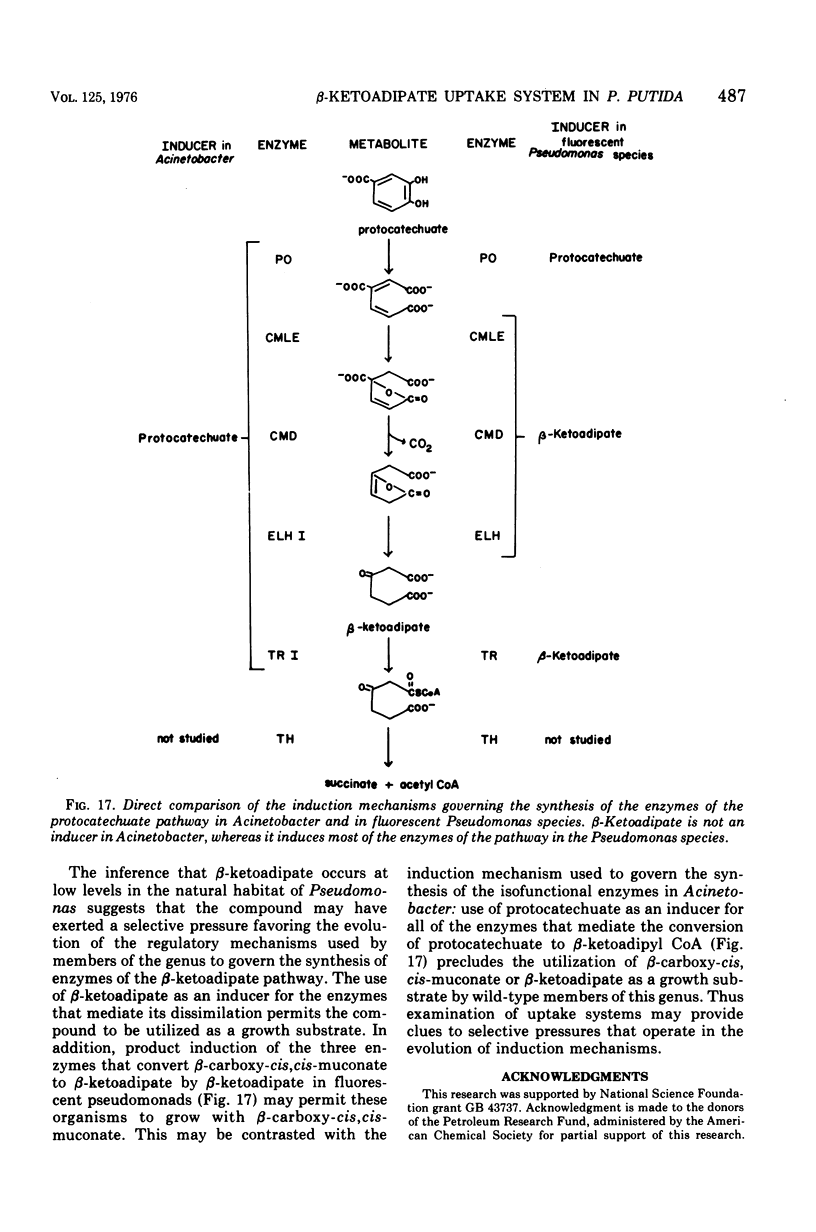
Wild-type strains of Pseudomonas putida form an inducible uptake system that appears to act on beta-ketoadipate under normal physiological conditions. The system is induced by beta-ketoadipate and is represented by catabolites derived from it. Adipate is metabolized very slowly by wild-type P. putida cultures; [14C]adipate was used as an analogue of beta-ketoadipate to measure the transport activity in wild-type cells and in cells that constitutively produced the uptake system. Constitutive cells that contained high levels of the uptake system concentrated adipate to a level up to 200-fold above the concentration in the external medium. The process was energy dependent. The activity of the system with radioactive adipate was inhibited by beta-ketoadipate, by beta-ketoadipate analogues, and by some compounds (e.g., acetate, glucose) that are structurally unrelated to beta-ketoadipate; it is not known if the inhibitory effects are exerted directly by the compounds themselves or indirectly by catabolites derived from the compounds. The discovery of the beta-ketoadipate uptake system is surprising in view of earlier studies that had indicated that beta-ketoadipate does not permeate the membrane of wild-type P. putida cells. Contradictions between the former investigations and the present analysis are due primarily to the relatively high concentrations of substrate used in the earlier experiments. The existence of the beta-ketoadipate uptake system indicates that beta-ketoadipate may exist as a selective nutrient in the natural niche of P. putida and may play a determinative role in the evolution of induction mechanisms that are characteristic of fluorescent pseudomonads.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- COHEN-BAZIRE G., JOLIT M. Isolement par sélection de mutants d'Escherichia coli synthétisant spontanément l'amylomaltase et la beta-galactosidase. Ann Inst Pasteur (Paris) 1953 May;84(5):937–945. [PubMed] [Google Scholar]
- Cánovas J. L., Johnson B. F. Regulation of the enzymes of the beta-ketoadipate pathway in Moraxella calcoacetica. 4. Constitutive synthesis of beta-ketoadipate succinyl-CoA transferases II and 3. Eur J Biochem. 1968 Jan;3(3):312–317. doi: 10.1111/j.1432-1033.1968.tb19531.x. [DOI] [PubMed] [Google Scholar]
- Cánovas J. L., Ornston L. N., Stanier R. Y. Evolutionary significance of metabolic control systems. The beta-ketoadipate pathway provides a case history in bacteria. Science. 1967 Jun 30;156(3783):1695–1699. doi: 10.1126/science.156.3783.1695. [DOI] [PubMed] [Google Scholar]
- Cánovas J. L., Stanier R. Y. Regulation of the enzymes of the beta-ketoadipate pathway in Moraxella calcoacetica. 1. General aspects. Eur J Biochem. 1967 May;1(3):289–300. doi: 10.1007/978-3-662-25813-2_40. [DOI] [PubMed] [Google Scholar]
- Cánovas J. L., Wheelis M. L., Stanier R. Y. Regulation of the enzymes of the beta-ketoadipate pathway in Moraxella calcoacetica. 2. The role of protocatechuate as inducer. Eur J Biochem. 1968 Jan;3(3):293–304. doi: 10.1111/j.1432-1033.1968.tb19529.x. [DOI] [PubMed] [Google Scholar]
- Ghei O. K., Kay W. W. Properties of an inducible C 4 -dicarboxylic acid transport system in Bacillus subtilis. J Bacteriol. 1973 Apr;114(1):65–79. doi: 10.1128/jb.114.1.65-79.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guymon L. F., Eagon R. G. Transport of glucose, gluconate, and methyl alpha-D-glucoside by Pseudomonas aeruginosa. J Bacteriol. 1974 Mar;117(3):1261–1269. doi: 10.1128/jb.117.3.1261-1269.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins S. J., Mandelstam J. Evidence for induced synthesis of an active transport factor for mandelate in Pseudomonas putida. Biochem J. 1972 Feb;126(4):917–922. doi: 10.1042/bj1260917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay W. W., Kornberg H. L. The uptake of C4-dicarboxylic acids by Escherichia coli. Eur J Biochem. 1971 Jan;18(2):274–281. doi: 10.1111/j.1432-1033.1971.tb01240.x. [DOI] [PubMed] [Google Scholar]
- Kemp M. B., Hegeman G. D. Genetic control of the beta-ketoadipate pathway in Pseudomonas aeruginosa. J Bacteriol. 1968 Nov;96(5):1488–1499. doi: 10.1128/jb.96.5.1488-1499.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawford H. G., Williams G. R. The transport of citrate and other tricarboxylic acids in two species of Pseudomonas. Biochem J. 1971 Jul;123(4):571–577. doi: 10.1042/bj1230571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- London J., Meyer E. Y. Malate utilization by a group D Streptococcus: regulation of malic enzyme synthesis by an inducible malate permease. J Bacteriol. 1970 Apr;102(1):130–137. doi: 10.1128/jb.102.1.130-137.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meagher R. B., McCorkle G. M., Ornston M. K., Ornston L. N. Inducible uptake system for -carboxy-cis, cis-muconate in a permeability mutant of Pseudomonas putida. J Bacteriol. 1972 Aug;111(2):465–473. doi: 10.1128/jb.111.2.465-473.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ornston L. N., Stanier R. Y. The conversion of catechol and protocatechuate to beta-ketoadipate by Pseudomonas putida. J Biol Chem. 1966 Aug 25;241(16):3776–3786. [PubMed] [Google Scholar]
- Ornston L. N. The conversion of catechol and protocatechuate to beta-ketoadipate by Pseudomonas putida. IV. Regulation. J Biol Chem. 1966 Aug 25;241(16):3800–3810. [PubMed] [Google Scholar]
- Patel R. N., Meagher R. B., Ornston L. N. Relationships among enzymes of the beta-ketoadipate pathway. II. Properties of crystalline beta-carboxy-cis,cis-muconate-lactonizing enzyme from Pseudomonas putida. Biochemistry. 1973 Aug 28;12(18):3531–3537. doi: 10.1021/bi00742a028. [DOI] [PubMed] [Google Scholar]
- Patel R. N., Meagher R. B., Ornston L. N. Relationships among enzymes of the beta-ketoadipate pathway. IV. Muconolactone isomerase from Acinetobacter calcoaceticus and Pseudomonas putida. J Biol Chem. 1974 Dec 10;249(23):7410–7419. [PubMed] [Google Scholar]
- Romano A. H., Van Vranken N. J., Preisand P., Brustolon M. Regulation of the Thiobacillus intermedius glucose uptake system by thiosulfate. J Bacteriol. 1975 Feb;121(2):577–582. doi: 10.1128/jb.121.2.577-582.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STANIER R. Y. Problems of bacterial oxidative metabolism. Bacteriol Rev. 1950 Sep;14(3):179–191. doi: 10.1128/br.14.3.179-191.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanier R. Y., Ornston L. N. The beta-ketoadipate pathway. Adv Microb Physiol. 1973;9(0):89–151. [PubMed] [Google Scholar]
- Stanier R. Y., Palleroni N. J., Doudoroff M. The aerobic pseudomonads: a taxonomic study. J Gen Microbiol. 1966 May;43(2):159–271. doi: 10.1099/00221287-43-2-159. [DOI] [PubMed] [Google Scholar]
- Wheelis M. L., Ornston L. N. Genetic control of enzyme induction in the -ketoadipate pathway of Pseudomonas putida: deletion mapping of cat mutations. J Bacteriol. 1972 Feb;109(2):790–795. doi: 10.1128/jb.109.2.790-795.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson D. B. The regulation and properties of the galactose transport system in Escherichia coli K12. J Biol Chem. 1974 Jan 25;249(2):553–558. [PubMed] [Google Scholar]
- Winkler H. H., Wilson T. H. Inhibition of beta-galactoside transport by substrates of the glucose transport system in Escherichia coli. Biochim Biophys Acta. 1967;135(5):1030–1051. doi: 10.1016/0005-2736(67)90073-9. [DOI] [PubMed] [Google Scholar]



