Abstract
A major obstacle to the expansion of abnormal cells with significant proliferative potential is the induction of programmed cell death. Consequently, oncogene-driven hyperproliferation must be associated with apoptosis inhibition to allow malignant outgrowth. The oncogenic cooperation of N-Myc and Twist-1 in the development of neuroblastoma, the most common and deadly solid tumour of childhood, perfectly illustrates such a process. N-Myc promotes cell proliferation, whereas Twist-1 counteracts its pro-apoptotic properties by knocking-down the ARF/p53 pathway. On the basis of numerous recent studies reporting its overexpression in a variety of human cancers, we discuss in this review the role of Twist-1 as a potent inhibitor of the cell safety programs engaged in response to an abnormal mitogenic activity.
Keywords: twist-1, neuroblastoma, breast cancer, apoptosis, metastasis
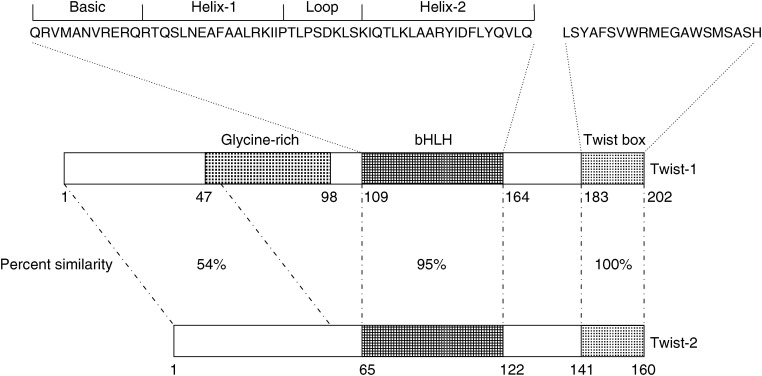
The Twist-1 protein (also called Twist) is a highly conserved transcription factor that belongs to the family of basic helix–loop–helix (bHLH) proteins (Thisse et al, 1987). bHLH proteins are structurally and functionally characterised by a conserved domain containing a stretch of basic amino acids adjacent to two amphipathic α-helices separated by an interhelical loop. The α-helices mediate dimerisation with a second bHLH factor, leading to the formation of a bipartite DNA-binding domain that specifically binds to hexanucleotide sequences (CANNTG), referred to as E-boxes. Functional E-boxes are found in the regulatory elements of many lineage-specific genes. Consistently, bHLH are transcription factors acting in various differentiation processes, either as positive or negative regulators, and play key roles in different developmental events like neurogenesis and myogenesis (for review see Jan and Jan, 1993). Drosophila twist is a zygotic developmental gene involved in early mesoderm patterning (Thisse et al, 1987). It is regulated by a nuclear gradient of the protein Dorsal, a Rel-containing sequence-specific transcription factor (Ip et al, 1992), leading to twist expression in the ventral mesoderm. The encoded Twist protein forms a steep gradient across the presumptive mesoderm–neuroectoderm border in the early embryo. Drosophila twist mutants fail to gastrulate indicating that it is not only necessary for mesoderm differentiation but also for the morphogenetic movement during gastrulation (Leptin, 1991). Two Twist genes exist in vertebrates, Twist-1 (Twist) and Twist-2 (formerly known as Dermo-1) (Li et al, 1995). The two Twist proteins display more than 90% identity in the bHLH and carboxy-terminal domains (Figure 1). The C-terminal part of the proteins comprises a ‘Twist box’ which is involved in the antiosteogenic function of Twist-1 and Twist-2 (Bialek et al, 2004). The N-termini of Twist-1 and Twist-2 are more divergent, and Twist-2 lacks a glycine-rich region that is present in Twist-1 (Li et al 1995). Twist-1 and Twist-2 have been implicated in the differentiation of multiple cell lineages including muscle, cartilage and osteogenic cells. In mice, Twist-1 was shown to be required for proper development of the head mesenchyme, somites and limb buds. Mice lacking Twist-1 die at E10.5, whereas mice homozygous for a Twist-2 null allele show elevated expression of pro-inflammatory cytokines causing perinatal death (Sosic et al, 2003). Interestingly, mice heterozygous for Twist-1 or Twist-2 mutant alleles are viable, and double heterozygous mutants phenocopy the severe cachexia seen in twist-2 null mice, suggesting that Twist-1 and Twist-2 may act redundantly to control an essential post-natal process (Sosic et al, 2003). Heterozygosity for Twist-1-null mutations results in a moderate phenotype including minor skull and limb anomalies. Consistently, germ-line mutations of the coding sequence of the human Twist-1 gene, leading to haploinsufficiency, have been identified in the Saethre–Chotzen syndrome (SCS) (acrocephalosyndactyly type III, ACS III; MIM No.101400). SCS is a hereditary disorder transmitted as an autosomal dominant trait and characterised by abnormalities of the limbs, asymmetric head and face, and premature fusion of cranial sutures (Pantke et al, 1975).
Figure 1.
Functional domains of human Twist-1 and Twist-2 proteins. The glycine-rich region is specific to Twist-1. The Runx2-binding ‘Twist box’ has been implicated in the antiosteogenic function of Twist-1 and Twist-2 (Bialek et al, 2004).
Besides this important role in differentiation, various observations strongly support a regulatory effect of Twist proteins on programmed cell death. Notably, Twist-1 haploinsufficiency in SCS induces calvarial osteoblast apoptosis and Twist-1 homozygous inactivation in animal models induces massive apoptosis during mammalian development (Chen and Behringer, 1995). Twist-2−/− cells are also dramatically sensitised to cytokine-induced apoptosis (Sosic et al, 2003). In vitro experiments on mouse embryo fibroblasts further showed that Twist-1 interferes with the ARF-p53 regulation pathway, thus blocking c-myc-induced apoptotic response (Maestro et al, 1999). Moreover, DNazyme-mediated cleavage of mouse Twist-1 transcripts in mouse mesenchymal C3H10T1/2 cells increases apoptosis (Hjiantoniou et al, 2003).
TWIST, APOPTOTIC FAILSAFE PROGRAM AND CANCER: THE MODEL OF NEUROBLASTOMA
Neuroblastoma (NB) stands out among paediatric solid tumours because of its relative frequency, intriguing natural history, prognostic biologic features, and therapeutic challenges (Brodeur, 2003). Neuroblastoma is considered as an embryonal malignancy of the postganglionic sympathetic nervous system, generally arising in the adrenal gland. It is the most common extracranial paediatric solid tumour, accounting for 7–10% of all childhood cancers, and the most common neoplasm in infancy. NB is characterised by a highly heterogeneous clinical behaviour, ranging from spontaneous regression without treatment to aggressive therapy-resistant disease. At present, patients are classified into risk categories on the basis of a combination of clinical and biological markers. These include age at diagnosis, stage of disease, histopathology findings, and amplification of the proto-oncogene N-Myc. N-Myc amplification, observed in about 25% of primary tumours, is strongly associated with rapid tumour progression and a poor outcome, independently from the stage of the tumour or the age of the patient, and has thus become an important factor in clinical decision-making and therapy (Brodeur, 2003). Myc oncoproteins are known examples of cellular oncoproteins that act both as growth promoting factors and apoptosis promoters (Evan et al, 1992). Apoptosis is mainly triggered by the ARF-p53 pathway (Zindy et al, 1998). Interestingly, an in vivo analysis of p53-dependent functions in Myc-induced lymphomas arising in Eμ-myc transgenic mice has indicated that apoptosis is the only p53 effector program that is selected against during tumour formation (Schmitt et al, 2002), suggesting that cell cycle checkpoint defects and genomic instability are by-products of p53 loss. Apoptosis is therefore a crucial failsafe program to counteract oncogenic Myc activation, and tumour development requires both hyperproliferation and prosurvival signalling. Intriguingly, in contrast with most human cancer types exhibiting an oncogenic Myc activation, primary NBs rarely display p53 mutations, and deletions or loss of heterozygosity (LOH) at 17p (where p53 maps) are uncommon (Vogan et al, 1993). This suggests that other defects in p53-dependent pathways may substitute for intragenic p53 mutations. Although amplification of the p53-inhibitory MDM2 locus has been identified in rare cases of NBs and abnormal cytoplasmic p53 localisation has been observed by immunohistochemistry in undifferentiated NBs (for review see Borriello et al, 2002), the mechanism of inhibition of p53-dependent apoptosis remains largely unknown. Interestingly, a deregulation of important regulators of apoptosis has been reported in NBs, including overexpression of survivin, a member of the IAP family, and down-expression of caspase 8. However, no obvious correlation with N-Myc amplification has been observed (Borriello et al, 2002).
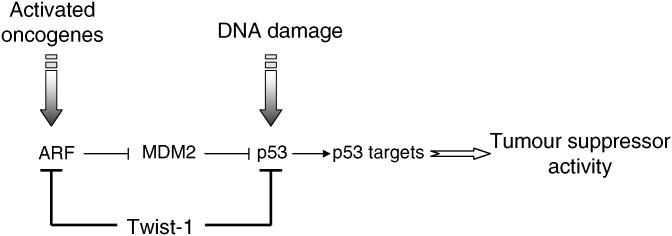
N-Myc-amplified NBs are thus remarkable examples of tumours displaying a high mitogenic activity, in the presence of a wild-type p53. With the aim to identify oncogenes or tumour suppressor genes whose alterations are able to cooperate with N-Myc in NB development, we performed a genome-wide microarray analysis of human NBs (Valsesia-Wittmann et al, 2004). This study showed that N-Myc amplification was constantly associated to the overexpression of Twist-1. On the basis of previous observations suggesting an anti-apoptotic activity of Twist-1, we hypothesised that Twist-1 was able to interfere with the cellular mechanism that triggers apoptosis in NB cells overexpressing N-Myc. This hypothesis was first verified by inhibiting endogenous Twist-1 expression in NB cell lines endogenously overexpressing N-Myc and Twist-1. Such inactivation by RNA interference was sufficient to promote massive apoptosis. In line with this observation, forced expression of Twist-1 in non N-Myc amplified NB cells was able to inhibit p53 response to genotoxic stress. We next demonstrated that N-Myc and Twist-1 co-expression transforms early-passage mouse embryo fibroblasts (MEFs), whereas each oncogene separately fails to do so. The oncogenic cooperation between N-Myc and Twist-1 was finally confirmed in vivo, the co-expression of the two oncogenes increasing tumourigenicity of human NB cells after injection in nude mice. Overall, this study demonstrated the oncogenic cooperation of two major regulators of embryogenesis, N-Myc and Twist-1, in the development of NB, supporting the current hypothesis of an embryonic origin of this tumour. N-Myc induces cell proliferation, whereas Twist-1 inhibits the ARF-p53 dependent pathway and prevents the apoptotic response normally triggered by Myc overexpression. By identifying Twist-1 overexpression as a cause of p53 inactivation, we not only provide a mechanistic explanation for the rarity of p53 mutations in NBs, but also show that the collaboration between N-Myc and Twist-1 is based on escape from natural safeguard response. Nevertheless, the mechanisms by which Twist-1 compromises p53-response remain elusive. Several observations suggest that Twist-1 may act at different steps of p53-regulation including transcriptional control and post-translational modifications (Figure 2). Recently, the group of Raman demonstrated that Twist-1 was able to physically interact with HOXA5, a potent transactivator of the p53 promoter, and to modulate its activity (Stasinopoulos et al, 2005). Twist-1 was also found to affect p53 stabilisation by reducing the expression of the ARF tumour suppressor, an upstream regulator of p53 (Maestro et al, 1999), and to prevent post-translational modifications, such as serine 20 phosphorylation, essential for p53 activation in response to DNA damage (Stasinopoulos et al, 2005). Finally, Twist-1 could also inhibit p53-mediated gene transcription through its demonstrated ability to inhibit p300-mediated acetylation, another post-translational process involved in the control of p53 activity (Hamamori et al, 1999).
Figure 2.
Twist-1 as a potent inhibitor of p53 tumour suppressor activity. Twist inhibits p53-mediated response to cellular stress (activated oncogenes or DNA damage) by modulating both ARF expression and p53 post-translational modifications such as phosphorylation and acetylation.
Recent data demonstrated that the anti-apoptotic role of Twist-1 is not restricted to NB cells. Indeed, Twist-1 overexpression was reported in a variety of solid cancers including breast, prostate and gastric carcinomas, melanomas, osteosarcomas, rhabdomyosarcomas, as well as in Sezary syndrome, a malignancy of CD4+ memory skin-homing T cells (Maestro et al, 1999; Rosivatz et al, 2002; Hoek et al, 2004; van Doorn et al, 2004; Watanabe et al, 2004; Entz-Werle et al, 2005; Kwok et al, 2005; Martin et al, 2005). Of note, Twist-2 has been shown recently to be deregulated in chronic lymphocytic leukaemia (Raval et al, 2005). Experimental downregulation of Twist-1 through small interfering RNA promotes apoptosis in human breast cancer and melanoma cell lines (unpublished data). Interestingly, the downregulation of Twist-1 increases cell death sensitivity of PC3 and DU145 prostate cancer cells, that both lack functional p53, suggesting that Twist-1 might regulate apoptosis through both p53-dependent and p53-independent pathways (Kwok et al, 2005). As mentioned previously, Twist-2−/− cells both upregulate TNFα expression and are sensitised to TNFα-induced apoptosis (Sosic et al, 2003). This phenotype is recapitulated in Twist-1 and Twist-2 compound heterozygotes reflecting redundancy and dosage dependence of these genes in inhibition of cytokine expression (Sosic et al, 2003). In addition to inactivating p53, Twist proteins may thus also act as NF-κB-controlled anti-apoptotic factors.
Considering that N-Myc amplified NBs are highly metastatic tumours and that Twist-1 overexpression in melanomas is associated to an aggressive phenotype (Hoek et al, 2004), we hypothesise that the anti-apoptotic activity of Twist-1 is likely to play a significant role in the metastatic process. Our hypothesis is strengthened by previous reports demonstrating that abrogation of p53-mediated apoptosis facilitates experimental metastasis by promoting the survival of tumour cells in circulation (Nikiforov et al, 1996). The overexpression of Twist-1 may thus be a selective advantage for a primary tumour cell to survive in settings of limited survival signals when contact with the original tumour is lost. Indeed, besides the necessity to avoid effective immune clearance and to survive in multiple contexts (local stroma, blood or lymph, secondary site of expansion), malignant cells have to override a significant number of barriers to metastasise. Hence, the metastatic process requires a complex set of cellular functions, including changes in adhesion, initiation of motility, extracellular matrix proteolysis, intravasation, extravasation into the surrounding tissue, molecular interactions of cancer cells with the environment of the secondary site, initiation of angiogenesis for ensuring independent nutrient supply and maintenance of growth leading to the metastatic colonisation.
TWIST, EMT AND METASTASIS: THE MODEL OF BREAST CARCINOMA
Although still a subject of controversy (Thompson and Newgreen, 2005; Tarin, 2005), the epithelial–mesenchymal transition (EMT) is proposed as an important step of carcinoma progression. EMT is a variant of transdifferentiation and a mechanism for dispersing cell lineages in vertebrate embryos. It is seen early in the course of development during gastrulation, and plays a major role during the formation of the heart, the musculoskeletal system, most craniofacial structures and the peripheral nervous system. Twist participates in EMTs during mesoderm differentiation in Drosophila and during neural crest migration in vertebrates. Hallmarks of developmental EMT include derangements of apicobasal polarity, lack of basal laminal integrity, and cell shape plasticity, allowing an increased migration capability. These processes are associated to a switch from E-cadherin (epithelial cadherin) to N-cadherin (neural cadherin) expression. E-cadherin is a homotypic cell–cell adhesion molecule which is required for the formation of epithelia in the embryo. It is then expressed on most epithelial cells, where it forms the adherens junction and facilitates the formation of the entire epithelial junctional complex. N-cadherin typically forms homotypic homophilic interactions, but also heterotytpic and heterophilic interactions have been described. In the early embryo, N-cadherin is found in the mesoderm, whereas in the late embryo it is present in neural tissue, lens and several other epithelial tissues (Derycke and Bracke, 2004). In the adult, N-cadherin expression is mainly restricted to neural tissue, retina, endothelial cells, fibroblasts and myocytes. Because strong migratory and invasive abilities are also characteristic of metastatic cells, it has been suggested that epithelial cell-derived tumours acquire a mesenchymal character during tumour progression. Indeed, several genes implicated in EMT during embryogenesis are deregulated during tumorigenesis, including the zinc-finger proteins Snail, SIP-1 and Slug that act as E-cadherin repressors (Huber et al, 2005, for review). Loss of E-cadherin-mediated cell–cell adhesion is a frequent event during tumour cell invasion and metastasis formation (Heimann et al, 2000). Consistent with this finding, noninvasive transformed cells in vitro become invasive upon abolishing E-cadherin function or expression (Vleminckx et al, 1991). Conversely, transfection of highly invasive epithelial tumor cell lines by E-cadherin cDNA totally abrogates their invasiveness potential. Several mechanisms are found to underlie the loss of E-cadherin function during tumorigenesis: these include mutational events, mostly in breast lobular carcinomas, or epigenetic mechanisms such as promoter hypermethylation or transcriptional repression of the gene by repressor factors. Conversely, gain of expression of N-cadherin is frequently observed in carcinomas and its forced expression promotes motility and metastasis of prostate and breast cancer cells. Interestingly, Twist has been shown to be essential for the initiation of Drosophila N-cadherin expression during gastrulation (Oda et al, 1998), and Twist-1 overexpression is correlated to abnormal expression of N-cadherin mRNA in human diffuse-type gastric cancer (Rosivatz et al, 2002). As previously mentioned, Twist-1 is upregulated in several types of epithelial cancers including breast, prostate and gastric carcinomas (Rosivatz et al, 2002; Watanabe et al, 2004; Kwok et al, 2005; Martin et al, 2005). Although the mechanisms underlying this overexpression remain unknown, Twist-1 expression has been shown to be induced in response to Wnt-1 in mouse mammary epithelial cells, suggesting that it might be regulated by Wnt signalling (Howe et al, 2003). Recently, by using a mouse mammary tumour model, in which a set of otherwise isogenic tumour cell populations is able to complete distinct steps of metastasis when implanted into the mammary glands of BALB/c mice, the group of R. Weinberg showed that the murine homologue of Twist-1 was required for intravasation and metastasis of breast cancer cells in the lung (Yang et al, 2004). Indeed, inhibition of Twist-1 expression by RNAi in metastatic cancer cells significantly decreases the efficiency of lung metastasis from the mammary gland as well as the number of tumour cells that could be recovered from the circulation. The same authors also demonstrated that ectopic expression of Twist-1 leads to molecular and morphological features associated with an EMT, including induction of cell motility, loss of E-cadherin-mediated cell–cell adhesion and activation of mesenchymal markers. Similar observations were recently reported in androgen-independent prostate cancer cells (Kwok et al, 2005). The link between Twist-1 and E-Cadherin was strengthened by the demonstration that Twist-1, directly or indirectly, causes the transcriptional repression of E-cadherin through the E-box elements present in its promoter region (Yang et al, 2004). Nevertheless, the reported observation that Twist-1-overexpression is more frequent in breast lobular carcinomas than in breast ductal carcinomas (Yang et al, 2004) is intriguing as more than 50% of lobular carcinomas have E-cadherin mutations. The fact that forced expression of E-cadherin is not sufficient to reverse the process in Twist-1-expressing cells (Yang et al, 2004) further supports the hypothesis that Twist-1 might modulate several important processes during cancer development, including inhibition of cell safeguard programs, alteration of cell–cell adhesion (through control of E- and N-cadherin expression) and deregulation of differentiation, leading to both cell survival and invasion capability.
CONCLUDING REMARKS
Over the last year, numerous studies have reported the upregulation of Twist-1 in a variety of human cancers. Functional studies have indicated that Twist-1 may play a major role in tumour promotion and progression, by inhibiting differentiation, interfering with the p53 tumour suppressor pathway and favouring cell survival, and/or inducing an epithelial–mesenchymal-like transition. Although further studies required to determine the exact nature of Twist-1 involvement in a defined cancer type, this novel identification of a crucial regulator of embryogenesis as an oncogene supports the view of tumours as corrupt forms of normal development.
At last, the observation of Twist-1 activation in human cancers might have important clinical implications, both as a prognostic and/or predictive factor and as a novel therapeutic target. In support of the first hypothesis, Twist-1 overexpression was shown, recently, to be a factor of poor prognosis in melanomas (Hoek et al, 2004), and Twist-1 forced expression triggers the resistance of human cancer cells to microtubule agents such as taxol and vincristine (Wang et al, 2004; Kwok et al, 2005). Furthermore, as discussed previously, Twist-1 probably inhibits both p53-dependent and p53-independent apoptosis. Consequently, the experimental inactivation of Twist-1 by RNA interference leads to cell death in a variety of cancer cell lines, including NB, melanoma and breast cancer lines. Downregulation of Twist-1 by antisense technology in mouse fibroblasts also increases the sensitivity to etoposide, a DNA-damaging anticancer drug (Dupont et al, 2001). Overall, these observations suggest that Twist-1 might be an interesting therapeutic target for increasing cell sensitivity to drug-induced apoptosis.
References
- Bialek P, Kern B, Yang X, Schrock M, Sosic D, Hong N, Wu H, Yu K, Ornitz DM, Olson EN, Justice MJ, Karsenty G (2004) A twist code determines the onset of osteoblast differentiation. Dev Cell 6: 423–435 [DOI] [PubMed] [Google Scholar]
- Borriello A, Roberto R, Della Ragione F, Iolascon A (2002) Proliferate and survive: cell division cycle and apoptosis in human neuroblastoma. Haematologica 87: 196–214 [PubMed] [Google Scholar]
- Brodeur GM (2003) Neuroblastoma: biological insights into a clinical enigma. Nat Rev Cancer 3: 203–216 [DOI] [PubMed] [Google Scholar]
- Chen ZF, Behringer RR (1995) Twist is required in head mesenchyme for cranial neural tube morphogenesis. Genes Dev 9: 686–699 [DOI] [PubMed] [Google Scholar]
- Derycke LDM, Bracke ME (2004) N-cadherin in the spotlight of cell–cell adhesion, differentiation, embryogenesis, invasion and signalling. Int J Dev Biol 48: 463–476 [DOI] [PubMed] [Google Scholar]
- Dupont J, Fernandez AM, Glackin CA, Helman L, LeRoith D (2001) Insulin-like growth factor 1 (IGF-1)-induced twist expression is involved in the anti-apoptotic effects of the IGF-1 receptor. J Biol Chem 276: 26699–26707 [DOI] [PubMed] [Google Scholar]
- Entz-Werle N, Stoetzel C, Berard-Marec P, Kalifa C, Brugiere L, Pacquement H, Schmitt C, Tabone MD, Gentet JC, Quillet R, Oudet P, Lutz P, Babin-Boilletot A, Gaub MP, Perrin-Schmitt F (2005) Frequent genomic abnormalities at TWIST in human pediatric osteosarcomas. Int J Cancer 117: 349–355 [DOI] [PubMed] [Google Scholar]
- Evan GI, Wyllie AH, Gilbert CS, Littlewood TD, Land H, Brooks M, Waters CM, Penn LZ, Hancock DC (1992) Induction of apoptosis in fibroblasts by c-myc protein. Cell 69: 119–128 [DOI] [PubMed] [Google Scholar]
- Hamamori Y, Sartorelli V, Ogrysko V, Puri PL, Wu H-Y, Wang JYJ, Nakatani Y, Kedes L (1999) Regulation of HAT p300 and pCAF by the bHLH Twist and adenoviral oncoprotein E1A. Cell 96: 405–413 [DOI] [PubMed] [Google Scholar]
- Heimann R, Lan F, McBride R, Hellman S (2000) Separating favourable from unfavourable prognostic markers in breast cancer: the role of E-cadherin. Cancer Res 60: 298–304 [PubMed] [Google Scholar]
- Hjiantoniou E, Iseki S, Uney JB, Phylactou LA (2003) DNazyme-mediated cleavage of Twist transcripts and increase in cellular apoptosis. Biochem Biophys Res Commun 300: 178–181 [DOI] [PubMed] [Google Scholar]
- Hoek K, Rimm DL, Williams KR, Zhao H, Ariyan S, Lin A, Kluger HM, Berger AJ, Cheng E, Trombetta ES, Wu T, Niinobe M, Yoshikawa K, Hannigan GE, Halaban R (2004) Expression profiling reveals novel pathways in the transformation of melanocytes to melanomas. Cancer Res 64: 5270–5282 [DOI] [PubMed] [Google Scholar]
- Howe LR, Watanabe O, Leonard J, Brown AMC (2003) Twist is up-regulated in response to Wnt1 and inhibits mouse mammary cell differentiation. Cancer Res 63: 1906–1913 [PubMed] [Google Scholar]
- Huber MA, Kraut N, Beug H (2005) Molecular requirements for epithelial–mesenchymal transition during tumor progression. Curr Opin Cell Biol 17: 548–558 [DOI] [PubMed] [Google Scholar]
- Ip YT, Park RE, Kosman D, Bier E, Levine M (1992) The dorsal gradient morphogen regulates stripes of rhomboid expression in the presumptive neuroectoderm of the Drosophila embryo. Genes Dev 6: 1728–1739 [DOI] [PubMed] [Google Scholar]
- Jan YN, Jan LY (1993) Functional gene cassettes in development. Proc Natl Acad Sci USA 90: 8305–8307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwok WK, Ling MT, Lee TW, Lau TC, Zhou C, Zhang X, Chua CW, Chan KW, Chan FL, Glackin C, Wong YC, Wang X (2005) Up-regulation of TWIST in prostate cancer and its implication as a therapeutic target. Cancer Res 65: 5153–5162 [DOI] [PubMed] [Google Scholar]
- Leptin M (1991) twist and snail as positive and negative regulators during Drosophila mesoderm development. Genes Dev 5: 1568–1576 [DOI] [PubMed] [Google Scholar]
- Li L, Cserjesi P, Olson EN (1995) Dermo-1: a novel twist-related bHLH protein expressed in the developing dermis. Dev Biol 172: 280–292 [DOI] [PubMed] [Google Scholar]
- Maestro R, Dei Tos AP, Hamamori Y, Krasnokutsky S, Sartorelli V, Kedes L, Doglioni C, Beach DH, Hannon GJ (1999) Twist is a potential oncogene that inhibits apoptosis. Genes Dev 13: 2207–2217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin TA, Goyal A, Watkins G, Jiang WG (2005) Expression of the transcription factors snail, slug, and twist and their clinical significance in human breast cancer. Ann Surg Oncol 12: 488–496 [DOI] [PubMed] [Google Scholar]
- Nikiforov MA, Hagen K, Ossovskaya VS, Connor TM, Lowe SW, Deichman GI, Gudkov AV (1996) p53 modulation of anchorage independent growth and experimental metastasis. Oncogene 13: 1709–1719 [PubMed] [Google Scholar]
- Oda H, Tsukita S, Takeichi M (1998) Dynamic behavior of the cadherin-based cell-cell adhesion system during Drosophila gastrulation. Dev Biol 203: 435–450 [DOI] [PubMed] [Google Scholar]
- Pantke OA, Cohen Jr MM, Witkop Jr CJ, Feingold M, Schaumann B, Pantke HC, Gorlin RJ (1975) The Saethre–Chotzen syndrome. Birth Defects Orig Arti Ser 11: 190–225 [PubMed] [Google Scholar]
- Raval A, Lucas DM, Matkovic JJ, Bennett KL, Liyanarachchi S, Young DC, Rassenti L, Kipps TJ, Grever MR, Byrd JC, Plass C (2005) TWIST2 demonstrates differential methylation in immunoglobulin variable heavy chain mutated and unmutated chronic lymphocytic leukemia. J Clin Oncol 23: 3877–3885 [DOI] [PubMed] [Google Scholar]
- Rosivatz E, Becker I, Specht K, Fricke E, Luber B, Busch R, Hofler H, Becker KF (2002) Differential expression of the epithelial–mesenchymal transition regulators snail, SIP1, and twist in gastric cancer. Am J Pathol 161: 1881–1891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt CA, Fridman JS, Yang M, Baranov E, Hoffman RM, Lowe SW (2002) Dissecting p53 tumor suppressor functions in vivo. Cancer Cell 1: 289–298 [DOI] [PubMed] [Google Scholar]
- Sosic D, Richardson JA, Yu K, Ornitz DM, Olson EN (2003) Twist regulates cytokine gene expression through a negative feedback loop that represses NF-B activity. Cell 112: 169–180 [DOI] [PubMed] [Google Scholar]
- Stasinopoulos IA, Mironchik Y, Raman A, Wildes F, Winnard Jr P, Raman V (2005) HOXA5-twist interaction alters p53 homeostasis in breast cancer cells. J Biol Chem 280: 2294–2299 [DOI] [PubMed] [Google Scholar]
- Tarin D (2005) The fallacy of epithelial mesenchymal transition in neoplasia. Cancer Res 65: 5996–6000 [DOI] [PubMed] [Google Scholar]
- Thisse B, Stoetzel C, El Messal M, Perrin-Schmitt F (1987) The twist gene: isolation of a Drosophila zygotic gene necessary for the establishment of dorso-ventral pattern. Nucleic Acids Res 15: 3439–3453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson EW, Newgreen DF (2005) Carcinoma invasion and metastasis: a role for epithelial-mesenchymal transition? Cancer Res 65: 5991–5995 [DOI] [PubMed] [Google Scholar]
- Valsesia-Wittmann S, Magdeleine M, Dupasquier S, Garin E, Jallas AC, Combaret V, Krause A, Leissner P, Puisieux A (2004) Oncogenic cooperation between H-Twist and N-Myc overrides failsafe programs in cancer cells. Cancer Cell 6: 625–630 [DOI] [PubMed] [Google Scholar]
- van Doorn R, Dijkman R, Vermeer MH, Out-Luiting JJ, van der Raaij-Helmer EM, Willemze R, Tensen CP (2004) Aberrant expression of the tyrosine kinase receptor EphA4 and the transcription factor twist in Sezary syndrome identified by gene expression analysis. Cancer Res 64: 5578–5586 [DOI] [PubMed] [Google Scholar]
- Vleminckx K, Vakaet L, Mareel M, Fiers W, Van Roy F (1991) Genetic manipulation of E-cadherin expression by epithelial tumor cells reveals an invasion suppressor role. Cell 66: 107–119 [DOI] [PubMed] [Google Scholar]
- Vogan K, Bernstein M, Leclerc JM, Brisson L, Brossard J, Brodeur GM, Pelletier J, Gros P (1993) Absence of p53 gene mutations in primary neuroblastomas. Cancer Res 53: 5269–5273 [PubMed] [Google Scholar]
- Wang X, Ling MT, Guan XY, Tsao SW, Cheung HW, Lee DT, Wong YC (2004) Identification of a novel function of TWIST, a bHLH protein, in the development of acquired taxol resistance in human cancer cells. Oncogene 23: 474–482 [DOI] [PubMed] [Google Scholar]
- Watanabe O, Imamura H, Shimizu T, Kinoshita J, Okabe T, Hirano A, Yoshimatsu K, Konno S, Aiba M, Ogawa K (2004) Expression of twist and wnt in human breast cancer. Anticancer Res 24: 3851–3856 [PubMed] [Google Scholar]
- Yang J, Mani SA, Donaher JL, Ramaswamy S, Itzykson RA, Come C, Savagner P, Gitelman I, Richardson A, Weinberg RA (2004) Twist, a master regulator of morphogenesis, plays an essential role in tumor metastasis. Cell 117: 927–939 [DOI] [PubMed] [Google Scholar]
- Zindy F, Eischen CM, Randle DH, Kamijo T, Cleveland JL, Sherr CJ, Roussel MF (1998) Myc signalling via the ARF tumor suppressor regulates p53-dependent apoptosis and immortalization. Genes Dev 12: 2424–2433 [DOI] [PMC free article] [PubMed] [Google Scholar]