Abstract
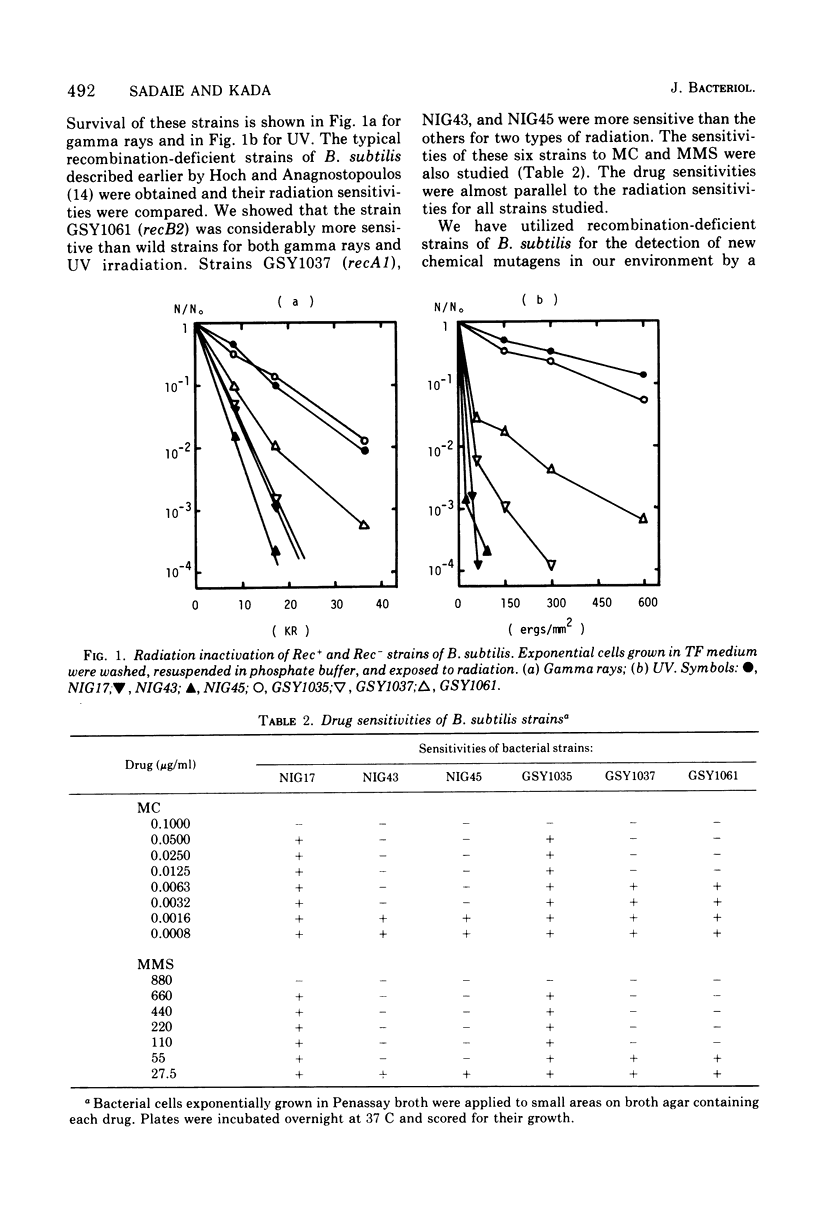
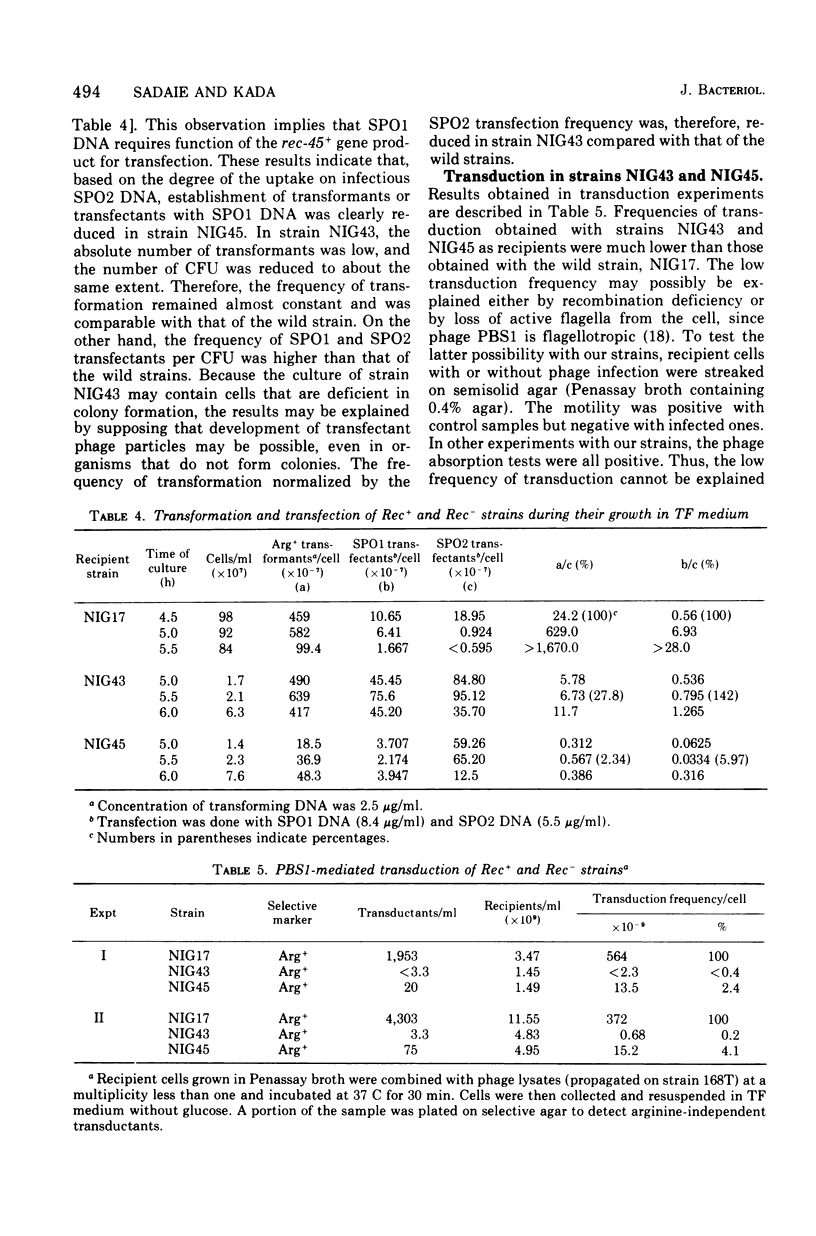
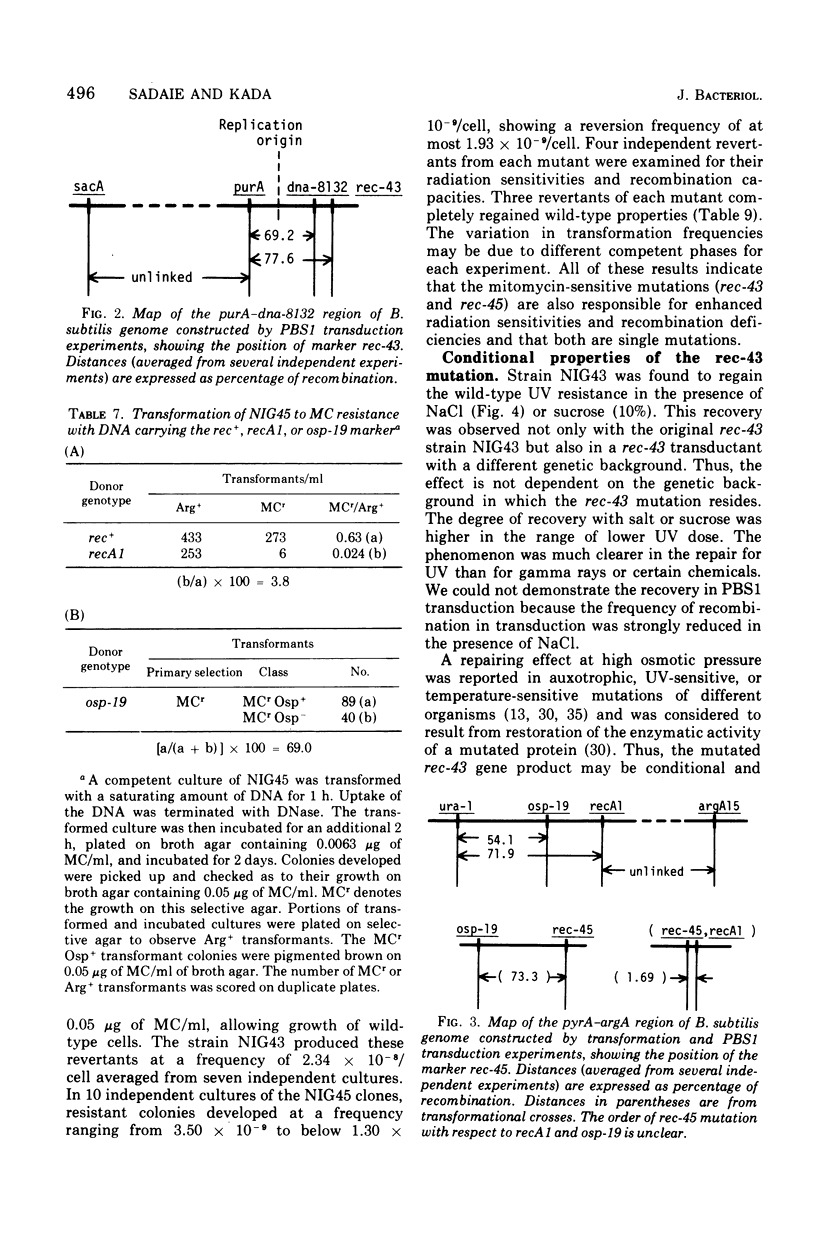
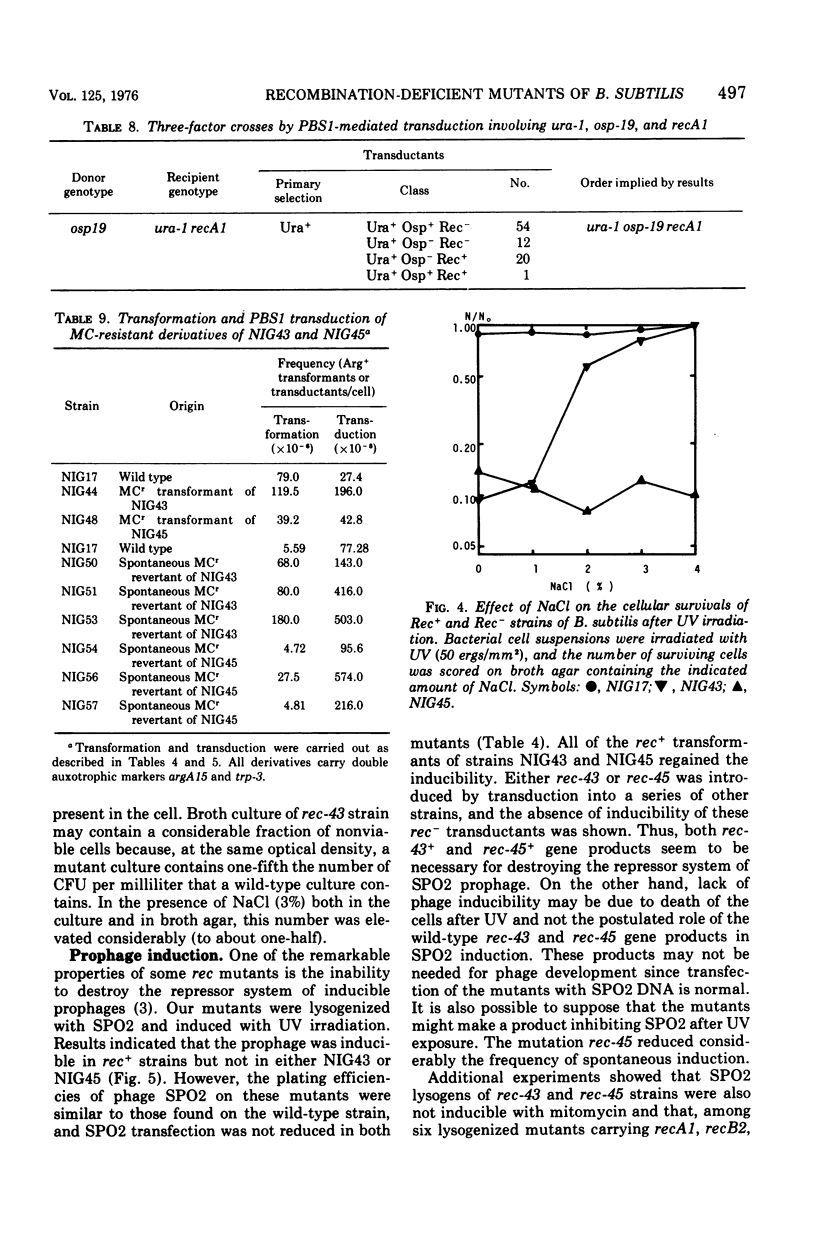
Two mutant strains of Bacillus subtilis Marburg, NIG43 and NIG45, were isolated. They showed high sensitivities to gamma rays, ultraviolet light (UV), and chemicals. Deficiencies in genetic recombination of these two mutants were shown by the experiments on their capacity in transformation. SPO2 transfection, and PBS1 phage transduction, as well as on their radiation and drug sensitivities and their Hcr+ capacity for UV-exposed phage M2. Some of these characteristics were compared with those of the known strains possessing the recA1 or recB2 alleles. Mapping studies revealed that the mutation rec-43 of strain NIG43 lies in the region of chromosome replication origin. The order was purA dna-8132 rec-43. Another mutation, rec-45, of strain NIG45 was found to be tightly linked to recA1. The mutation rec-43 reduced mainly the frequency of PBS1 transduction. On the other hand, the mutation rec-45 reduced the frequency of recombination involved both in transformation and PBS1 transduction. The mutation rec-43 of strain NIG43 is conditional, but rec-45 of strain NIG45 is not. The UV impairment in cellular survival of strain NIG43 was gradually reverted at higher salt or sucrose concentrations, suggesting cellular possession of a mutated gene produce whose function is conditional. In contrast to several other recombination-deficient strains, SPO2 lysogens of strain NIG43 and NIG45 were not inducible, indicating involvement of rec-43+ or rec-45+ gene product in the development of SPO2 prophage to a vegetative form. The UV-induced deoxyribonucleic acid degradation in vegetative cells was higher in rec-43 and rec-45 strains.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BARAT M., ANAGNOSTOPOULOS C., SCHNEIDER A. M. LINKAGE RELATIONSHIPS OF GENES CONTROLLING ISOLEUCINE, VALINE, AND LEUCINE BIOSYNTHESIS IN BACILLUS SUBTILIS. J Bacteriol. 1965 Aug;90:357–369. doi: 10.1128/jb.90.2.357-369.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BURTON K. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J. 1956 Feb;62(2):315–323. doi: 10.1042/bj0620315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark A. J. Toward a metabolic interpretation of genetic recombination of E. coli and its phages. Annu Rev Microbiol. 1971;25:437–464. doi: 10.1146/annurev.mi.25.100171.002253. [DOI] [PubMed] [Google Scholar]
- Coote J. G. Sporulation in Bacillus subtilis. Genetic analysis of oligosporogenous mutants. J Gen Microbiol. 1972 Jun;71(1):17–27. doi: 10.1099/00221287-71-1-17. [DOI] [PubMed] [Google Scholar]
- Davidoff-Abelson R., Dubnau D. Fate of transforming DNA after uptake by competent Bacillus subtilis: failure of donor DNA to replicate in a recombination-deficient recipient. Proc Natl Acad Sci U S A. 1971 May;68(5):1070–1074. doi: 10.1073/pnas.68.5.1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doly J., Sasarman E., Anagnostopoulos C. ATP-dependent deoxyribonuclease in Bacillus subtilis and a mutant deficient in this activity. Mutat Res. 1974 Jan;22(1):15–23. doi: 10.1016/0027-5107(74)90003-7. [DOI] [PubMed] [Google Scholar]
- Dubnau D., Cirigliano C. Genetic characterization of recombination-deficient mutants of Bacillus subtilis. J Bacteriol. 1974 Feb;117(2):488–493. doi: 10.1128/jb.117.2.488-493.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubnau D., Davidoff-Abelson R., Scher B., Cirigliano C. Fate of transforming deoxyribonucleic acid after uptake by competent Bacillus subtilis: phenotypic characterization of radiation-sensitive recombination-deficient mutants. J Bacteriol. 1973 Apr;114(1):273–286. doi: 10.1128/jb.114.1.273-286.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubnau D., Davidoff-Abelson R., Smith I. Transformation and transduction in Bacillus subtilis: evidence for separate modes of recombinant formation. J Mol Biol. 1969 Oct 28;45(2):155–179. doi: 10.1016/0022-2836(69)90097-7. [DOI] [PubMed] [Google Scholar]
- HAWTHORNE D. C., FRIIS J. OSMOTIC-REMEDIAL MUTANTS. A NEW CLASSIFICATION FOR NUTRITIONAL MUTANTS IN YEAST. Genetics. 1964 Nov;50:829–839. doi: 10.1093/genetics/50.5.829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara H., Yoshikawa H. Asymmetric bidirectional replication of Bacillus subtilis chromosome. Nat New Biol. 1973 Aug 15;244(137):200–203. doi: 10.1038/newbio244200a0. [DOI] [PubMed] [Google Scholar]
- Hoch J. A., Anagnostopoulos C. Chromosomal location and properties of radiation sensitivity mutations in Bacillus subtilis. J Bacteriol. 1970 Aug;103(2):295–301. doi: 10.1128/jb.103.2.295-301.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoch J. A., Barat M., Anagnostopoulos C. Transformation and transduction in recombination-defective mutants of Bacillus subtilis. J Bacteriol. 1967 Jun;93(6):1925–1937. doi: 10.1128/jb.93.6.1925-1937.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard-Flanders P. DNA repair. Annu Rev Biochem. 1968;37:175–200. doi: 10.1146/annurev.bi.37.070168.001135. [DOI] [PubMed] [Google Scholar]
- Joys T. M. Correlation between susceptibility to bacteriophage PBS1 and motility in Bacillus subtilis. J Bacteriol. 1965 Dec;90(6):1575–1577. doi: 10.1128/jb.90.6.1575-1577.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kada T., Moriya M., Shirasu Y. Screening of pesticides for DNA interactions by "rec-assay" and mutagenesis testing, and frameshift mutagens detected. Mutat Res. 1974 Aug;26(4):243–248. doi: 10.1016/s0027-5107(74)80021-7. [DOI] [PubMed] [Google Scholar]
- Kada T., Tutikawa K., Sadaie Y. In vitro and host-mediated "rec-assay" procedures for screening chemical mutagens; and phloxine, a mutagenic red dye detected. Mutat Res. 1972 Oct;16(2):165–174. doi: 10.1016/0027-5107(72)90177-7. [DOI] [PubMed] [Google Scholar]
- Lepesant J. A., Kunst F., Lepesant-Kejzlarová J., Dedonder R. Chromosomal location of mutations affecting sucrose metabolism in Bacillus subtilis Marburg. Mol Gen Genet. 1972;118(2):135–160. doi: 10.1007/BF00267084. [DOI] [PubMed] [Google Scholar]
- Munakata N., Ikeda Y. Inactivation of transforming DNA by ultraviolet irradiation: a study with ultraviot-sensitive mutants of Bacillus subtilis. Mutat Res. 1969 Mar-Apr;7(2):133–139. doi: 10.1016/0027-5107(69)90025-6. [DOI] [PubMed] [Google Scholar]
- Munakata N., Saito H., Ikeda Y. Inactivation of transforming DNA by ultraviolet irradiation. Mutat Res. 1966 Apr;3(2):93–100. doi: 10.1016/0027-5107(66)90022-4. [DOI] [PubMed] [Google Scholar]
- OKUBO S., STRAUSS B., STODOLSKY M. THE POSSIBLE ROLE OF RECOMBINATION IN THE INFECTION OF COMPETENT BACILLUS SUBTILIS BY BACTERIOPHAGE DEOXYRIBONUCLEIC ACID. Virology. 1964 Dec;24:552–562. doi: 10.1016/0042-6822(64)90207-7. [DOI] [PubMed] [Google Scholar]
- Okubo S., Nakayama H. DNA synthesis after ultraviolet light irradiation in uv-sensitive mutants of Bacillus subtilis. Mutat Res. 1967 Sep-Oct;4(5):533–541. doi: 10.1016/0027-5107(67)90039-5. [DOI] [PubMed] [Google Scholar]
- Okubo S., Romig W. R. Comparison of ultraviolet sensitivity of Bacillus subtilis bacteriophage SPO2 and its infectious DNA. J Mol Biol. 1965 Nov;14(1):130–142. doi: 10.1016/s0022-2836(65)80235-2. [DOI] [PubMed] [Google Scholar]
- Okubo S., Romig W. R. Impaired transformability of Bacillus subtilis mutant sensitive to mitomycin C and ultraviolet radiation. J Mol Biol. 1966 Feb;15(2):440–454. doi: 10.1016/s0022-2836(66)80120-1. [DOI] [PubMed] [Google Scholar]
- Ricard M., Hirota Y. Effet des sels et autres composés sur le phénotype de mutants thermosensibles de Escherichia coli. Ann Microbiol (Paris) 1973 Jan;124(1):29–43. [PubMed] [Google Scholar]
- SAITO H., MIURA K. I. PREPARATION OF TRANSFORMING DEOXYRIBONUCLEIC ACID BY PHENOL TREATMENT. Biochim Biophys Acta. 1963 Aug 20;72:619–629. [PubMed] [Google Scholar]
- Sadaie Y., Kada T. Radiation inactivation and recombination repair in Bacillus subtilis spores. Mutat Res. 1973 Jan;17(1):138–141. doi: 10.1016/0027-5107(73)90262-5. [DOI] [PubMed] [Google Scholar]
- Shimazu Y., Morimyo M., Suzuki K. Temperature-sensitive recovery of a mutant of Escherichia coli K-12 irradiated with ultraviolet light. J Bacteriol. 1971 Sep;107(3):623–632. doi: 10.1128/jb.107.3.623-632.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha R. P., Iyer V. N. Isolation and some distinctive properties of a new type of recombination-deficient mutant of Bacillus subtilis. J Mol Biol. 1972 Dec 30;72(3):711–724. doi: 10.1016/0022-2836(72)90186-6. [DOI] [PubMed] [Google Scholar]
- TAKAHASHI I. Transducing phages for Bacillus subtilis. J Gen Microbiol. 1963 May;31:211–217. doi: 10.1099/00221287-31-2-211. [DOI] [PubMed] [Google Scholar]
- Yamagishi H., Takahashi I. Transducing particles of PBS 1. Virology. 1968 Dec;36(4):639–645. doi: 10.1016/0042-6822(68)90194-3. [DOI] [PubMed] [Google Scholar]
- Zadrazil S., Fucík V. Fate of transforming DNA in Bacillus subtilis strain sensitive to methyl methanesulfonate. Biochem Biophys Res Commun. 1971 Feb 19;42(4):676–683. doi: 10.1016/0006-291x(71)90541-9. [DOI] [PubMed] [Google Scholar]



