Abstract
Deleterious mutations in the BRCA1 gene predispose women to an increased risk of breast and ovarian cancer. Many functional studies have suggested that BRCA1 has a role in DNA damage repair and failure in the DNA damage response pathway often leads to the accumulation of chromosomal aberrations. Here, we have compared normal lymphocytes with those heterozygous for a BRCA1 mutation. Short-term cultures were irradiated (8Gy) using a high dose rate and subsequently metaphases were analysed by 24-colour chromosome painting (M-FISH). We scored the chromosomal rearrangements in the metaphases from five BRCA1 mutation carriers and from five noncarrier control samples 6 days after irradiation. A significantly higher level of chromosomal damage was detected in the lymphocytes heterozygous for BRCA1 mutations compared with normal controls; the average number of aberrations per mitosis was 3.48 compared with 1.62 in controls (P=0.0001). This provides new evidence that heterozygous mutation carriers have a different response to DNA damage compared with noncarriers and that BRCA1 has a role in DNA damage surveillance. Our finding has implications for treatment and screening of BRCA1 mutation carriers using modalities that involve irradiation.
Keywords: breast cancer, BRCA1 , chromosomal damage, irradiation, M-FISH
It is estimated that 5–10% of breast cancer patients develop the disease due to the presence of a highly penetrant breast cancer predisposition gene. A significant proportion of these patients (20–45%) have a mutation in the breast cancer genes, BRCA1 or BRCA2 (Peto et al, 1999). It has long been debated whether women developing BRCA-associated breast cancer have a different response to irradiation. This would be clinically important since, if true, irradiation treatments could lead to more severe acute and late radiotoxicity and an increased carcinogenic risk for these individuals. Hence, the optimum management of BRCA1 mutation carriers remains unclear (Haffty et al, 2002). Elevated chromatid break frequency and various chromosomal abnormalities in cells from individuals with many cancer-prone genetic disorders have been long recognised (reviewed in Eyfjord and Bodvarsdottir, 2005). These abnormalities result from an alteration in chromatin structure, a higher rate of conversion of double-strand breaks (DSB) to chromatid breaks or from deficient DNA repair (Radford, 2004). Following DNA damage, cells have a complex response that may involve cell cycle checkpoint activation, DNA repair mechanisms and programmed cell death (apoptosis). So far, there is evidence that BRCA1 has a role in all these interdependent events (Kote-Jarai and Eeles, 1999). BRCA1 is a target for phosphorylation by ATM, ATR and Chk2 triggered by DNA damage and is required for cell cycle checkpoint activation (Shiloh, 2003). BRCA1 interacts with the MRE11-Rad50-NBS1 complex (Zhong et al, 1999), which is involved in the homologous recombination pathway of DSB DNA repair. A role for BRCA1 in chromatin remodelling and activation of transcription have also been described (Bochar et al, 2000).
The phenotypes of cells heterozygous for BRCA1 mutation have been studied both in vivo and in vitro. Clinical studies of women with BRCA1 mutations treated with radiation for breast cancer have not demonstrated increased acute or late toxicity (Pierce, 2005). The in vitro studies, however, are suggestive of differences between BRCA1 heterozygous cells and controls, but no clear evidence as yet has emerged for the impaired efficiency of DNA damage response mechanisms in these cells. Human fibroblasts and lymphoblastoid cells with heterozygous BRCA1 mutations seem to have heightened radiosensitivity in some assays (Buchholz et al, 2002) but not in others (Nieuwenhuis et al, 2002). Lymphocytes from BRCA1/2 carriers have shown increased radiosensitivity as measured by cell survival (Rothfuss et al, 2000). Here, we have quantified irradiation-induced chromosome damage in the lymphocytes of BRCA1 carriers and controls in order to identify a phenotypic effect of BRCA1 heterozygous mutations.
METHODS
Individuals heterozygous for BRCA1 germline mutations were identified from the BRCA1 and BRCA2 predictive testing programme in the Institute of Cancer Research/Royal Marsden Foundation NHS Trust, Cancer Genetics Carrier Clinic. Fresh blood samples were collected from five unaffected BRCA1 heterozygous gene mutation carriers and five healthy age-matched control women with no individual or family history of cancer, and short-term lymphocyte cultures were established. Written informed consent was obtained from all participating individuals prior to inclusion, and the study protocol was approved by the Royal Marsden Locoregional Ethics Committee. Cells were cultured for 24 h and 6 days following irradiation with 8 Gray (Gy) at a high dose rate (0.86 Gy/min) using a Co60 source. Metaphase spreads were prepared according to standard methods using 50 μl colcemid for 2 h. Multicolour FISH for karyotyping was performed using the SpectraVision™ Assay system (Abbott Laboratories, Maidenhead, UK). SpectraVision™ probes were hybridised to the chromosome preparations for 48 h, then slides were washed and counterstained with DAPI. Images of metaphase spreads were captured using a Zeiss epifluorescent microscope with a six position filter wheel and were analysed using the Quips SpectraVision™ software (Abbott, as above). An average of 30 metaphases per sample was analysed by an investigator blinded to the genetic status of the cells. A second cytogeneticist (BS), again blinded, independently scored a subset of four samples (two carriers and two controls) to assess interobserver variation. There was a complete concordance between the observations. Chromosomal translocations and breakages were counted per mitosis in BRCA1 mutation carriers and controls and compared by using the unpaired Student's t-test.
RESULTS
In this study, we evaluated a total of 288 metaphases and chromosomal aberrations were scored per mitosis. At 6 days postirradiation, aberrations were detected. A representative metaphase is shown in Figure 1. Analysis of the average number of aberrations per mitosis revealed an increased level of chromosomal aberrations in heterozygous BRCA1 mutation carriers compared with controls. In mutation carriers, the average number of chromosomal aberrations was 3.48±0.24 per mitosis, whereas in the control samples, this number was 1.62±0.33 at 6 days post-irradiation. The difference between controls and carriers is highly significant, P=0.0001 (Table 1). All chromosomes were equally involved in translocations or breaks when the data were corrected for chromosomal length. No breakage ‘hotspots’ were identified.
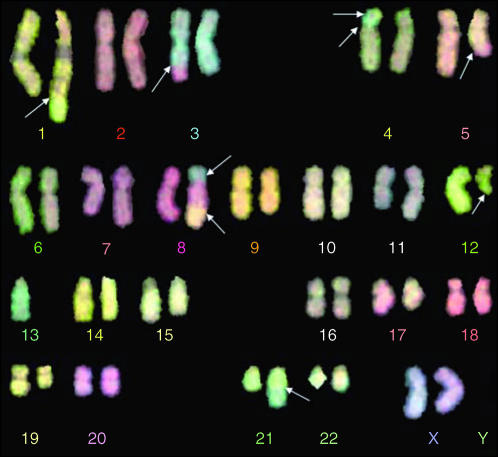
Figure 1.
M-FISH analysis of a metaphase spread from a BRCA1 carrier lymphocyte 6 days after high dose ionizing irradiation (8 Gy total). White arrows show the chromosomal aberrations.
Table 1. Average number of chromosomal aberrations per metaphase in BRCA1 mutation carriers and controls.
| Sample no. | BRCA1 Genotype | Aberration/metaphase | Sample no. | BRCA1 Genotype | Aberration/metaphase | |
|---|---|---|---|---|---|---|
| 1 | 187_188delAG | 3.80 | 6 | Wild type | 2.20 | |
| 2 | 2313G>T | 3.67 | 7 | Wild type | 1.60 | |
| 3 | Exon 13 duplication | 3.36 | 8 | Wild type | 1.39 | |
| 4 | 4184_4187delTCAA | 3.37 | 9 | Wild type | 1.55 | |
| 5 | 1014delGT | 3.17 | 10 | Wild type | 1.40 | |
| Average no of aberration/metaphase | 3.48±0.24 | 1.62±0.33 | P=0.0001 | |||
At the earlier time point of 24 h post-radiation, based on the analysis of four samples (two carriers, two controls), we did not find a difference between BRCA1 mutation carriers and controls. The number of average chromosome aberrations/mitosis varied between 0.7 and 2.0 independent of genotype. This suggests that the substantially higher level of chromosomal damage at 6 days reflects the heterozygous cells' lack of ability to recover or apoptose from irradiation-induced damage. This has not been previously described.
Our data indicate that after high dose irradiation, lymphocytes which are heterozygous for a BRCA1 mutation have inefficient DNA repair and/or apoptotic mechanisms leading to survival of cells with complex chromosomal aberrations. This observation confirms the DNA damage surveillance role of BRCA1 and could also have an impact on the clinical management of patients carrying a BRCA1 mutation.
DISCUSSION
Our data provide evidence that 6 days after high dose irradiation, normal cells (lymphocytes) heterozygous for a BRCA1 mutation develop a significantly higher level of chromosomal aberrations when compared with controls. Although the 8 Gy dose given here to lymphocytes is higher than that which would be used in a single screening mammogram or fraction of therapeutic radiation, these data emphasise that there is a distinct heterozygous phenotype in normal human cells harbouring a BRCA1 mutation. It is manifest in the development of twice the number of chromosomal aberrations after irradiation that is only seen after 6 days and is not seen acutely (within 24 h).
It has been shown that gross chromosomal changes are more likely to occur in cancers occurring in individuals with germline mutations in BRCA1 and BRCA2 compared with sporadic cancers. CGH analysis has identified a pattern of genetic imbalances that can differentiate familial BRCA1 tumours from unselected sporadic tumours (van Beers et al, 2005). This indicates that some changes might be of functional significance in tumour progression or might represent damage-prone fragile sites.
Recently, it has been shown that BRCA1 is required for common fragile-site stability. Cells lacking BRCA1 show an increased expression of specific common fragile sites (Arlt et al, 2004). This provides further evidence that cells lacking BRCA1 are likely to be prone to genomic alterations that can lead to deletion of associated genes and this consequently could promote tumourigenesis.
Our findings might also have some important clinical implications. It raises the possibility that there may be long-term risks of the development of chromosomal instabilities after irradiation of BRCA1 mutation carriers.
Even though the 8 Gy dose used in our experimental system exceeded the 2 Gy single fraction size commonly used in a breast cancer radiotherapy regimes, we have to consider that subsequent and repeated exposure to radiation might have a long-term and additive DNA damage effect. Cells with different genetic backgrounds might respond differently to this. We have used lymphocytes, rather than breast epithelial cells, in our study as these are easily collected and the primary aim was to investigate whether a distinct functional heterozygous phenotype for BRCA1 carriers exists which may lead to the development of a clinically useful assay. However, there is also experimental evidence that ionising radiation induces various molecular changes in breast epithelial cells and that a BRCA1-mutated breast cancer cell line shows deficient repair and increased chromosomal aberration (Mamon et al, 2003). We have also previously reported finding a differential gene expression profile in normal breast fibroblasts in response to DNA damage between BRCA1 mutation carriers and controls (Kote-Jarai et al, 2004), supporting the hypothesis that response to DNA damage due to irradiation differs in BRCA1 mutation carriers. Consequently, we suggest that clinical follow-up of the screening and treatment of individuals harbouring BRCA1 mutations is imperative. Studies are urgently needed to elucidate the applicability of these data to the clinical use of irradiation in screening and treatment for the management of BRCA1 gene mutation carriers. Concerns about the potential mutagenicity of ionising radiation, both radiotherapy and surveillance mammography in this group, have already driven the search for alternative tools such as breast magnetic resonance imaging in the UK MARIBS study (The MARIBS Advisory Group, 2005). Based on cohort studies of long-term mortality and risk estimation models for the induction of secondary cancers following radiotherapy at least 15–20 years follow-up will be required to fully assess whether there is an increased long-term treatment induced cancer risk (Dasu et al, 2005; Darby et al, 2005) and subtle late toxicity from irradiation in BRCA1 mutation carriers.
References
- Arlt MF, Xu B, Durkin SG, Casper AM, Kastan MB, Glover TW (2004) BRCA1 is required for common-fragile-site stability via its G2/M checkpoint function. Mol Cell Biol 24: 6701–6709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bochar DA, Wang L, Beniya H, Kinev A, Xue Y, Lane WS, Wang W, Kashanchi F, Shiekhattar R (2000) BRCA1 is associated with a human SWI/SNF-related complex: linking chromatin remodeling to breast cancer. Cell 102: 257–265 [DOI] [PubMed] [Google Scholar]
- Buchholz TA, Wu X, Hussain A, Tucker SL, Mills GB, Haffty B, Bergh S, Story M, Geara FB, Brock WA (2002) Evidence of haplotype insufficiency in human cells containing a germline mutation in BRCA1 or BRCA2. Int J Cancer 97: 557–561 [DOI] [PubMed] [Google Scholar]
- Darby SC, McGale P, Taylor CW, Peto R (2005) Long-term mortality from heart disease and lung cancer after radiotherapy for early breast cancer: prospective cohort study of about 300 000 women in US SEER cancer registries. Lancet Oncol 6: 557–565 [DOI] [PubMed] [Google Scholar]
- Dasu A, Toma-Dasu I, Oloffson J, Karlsson M (2005) The use of risk estimation models for the induction of secondary cancers following radiotherapy. Acta Oncol 44: 339–347 [DOI] [PubMed] [Google Scholar]
- Eyfjord JE, Bodvarsdottir SK (2005) Genomic instability and cancer: networks involved in response to DNA damage. Mutat Res, in press [DOI] [PubMed]
- Haffty BG, Harrold E, Khan AJ (2002) Outcome of conservatively managed early-onset breast cancer by BRCA1/2 status. Lancet 359: 1471–1477 [DOI] [PubMed] [Google Scholar]
- Kote-Jarai Z, Eeles RA (1999) BRCA1, BRCA2 and their possible function in DNA damage response. Br J Cancer 81: 1099–1102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kote-Jarai Z, Williams R, Cattini N, Copeland M, Giddings I, Wooster R, tePoele RH, Workman P, Gusterson B, Peacock J, Gui G, Campbell C, Eeles R (2004) Gene expression profiling after radiation induced DNA damage is highly predictive of BRCA1 mutation carrier status. Clin Canc Res 10: 958–963 [DOI] [PubMed] [Google Scholar]
- Mamon HJ, Dahlberg W, Azzam EJ, Nagasawa H, Muto MG, Little JB (2003) Differing effects of breast cancer 1, early onset (BRCA1) and ataxia talengectasia mutated (ATM) mutations on cellular responses to ionising radiation. Int J Radiat Biol 79: 817–829 [DOI] [PubMed] [Google Scholar]
- Nieuwenhuis B, Van Assen-Bolt AJ, Van Waarde-Verhagen MA, Sijmons RH, Van der Hout AH, Bauch T, Streffer C, Kampinga HH (2002) BRCA1 and BRCA2 heterozygosity and repair of X-ray-induced DNA damage. Int J Radiat Biol 78: 285–295 [DOI] [PubMed] [Google Scholar]
- Peto J, Collins N, Barfoot R, Seal S, Warren W, Rahman N, Easton DF, Evans C, Deacon J, Stratton MR (1999) Prevalence of BRCA1 and BRCA2 gene mutations in patients with early-onset breast cancer. J Natl Cancer Inst 91: 943–949 [DOI] [PubMed] [Google Scholar]
- Pierce L (2005) The use of radiotherapy after mastectomy: a review of the literature. J Clin Oncol 23: 1706–1717, Review [DOI] [PubMed] [Google Scholar]
- Radford IR (2004) Chromosomal rearrangement as the basis for human tumourigenesis. Int J Radiat Biol 8: 543–557, Review [DOI] [PubMed] [Google Scholar]
- Rothfuss A, Schutz P, Bochum S, Volm T, Eberhardt E, Kreienberg R et al. (2000) Induced micronucleus frequencies in peripheral lymphocytes as a screening test for carriers of a BRCA1 mutation in breast cancer families. Cancer Res 60: 390–394 [PubMed] [Google Scholar]
- Shiloh Y (2003) ATM and related protein kinases: safeguarding genome integrity. Nat Rev Cancer 3: 155–168, Review [DOI] [PubMed] [Google Scholar]
- The MARIBS Advisory Group (2005) Screening with magnetic resonance imaging and mammography of a UK population at high familial risk of breast cancer: a prospective multicentre cohort study (MARIBS). Lancet 365: 1769–1778 [DOI] [PubMed] [Google Scholar]
- van Beers EH, van Welsem T, Wessels LF, Li Y, Oldenburg RA, Devilee P, Cornelisse CJ, Verhoef S, Hogervorst FB, van't Veer LJ, Nederlof PM (2005) Comparative genomic hybridization profiles in human BRCA1 and BRCA2 breast tumors highlight differential sets of genomic aberrations. Cancer Res 65: 822–827 [PubMed] [Google Scholar]
- Zhong Q, Chen CF, Li S, Chen Y, Wang CC, Xiao J, Chen PL, Sharp ZD, Lee WH (1999) Association of BRCA1 with the hRad50-hMre11-p95 complex and the DNA damage response. Science 285: 747–750 [DOI] [PubMed] [Google Scholar]