Abstract
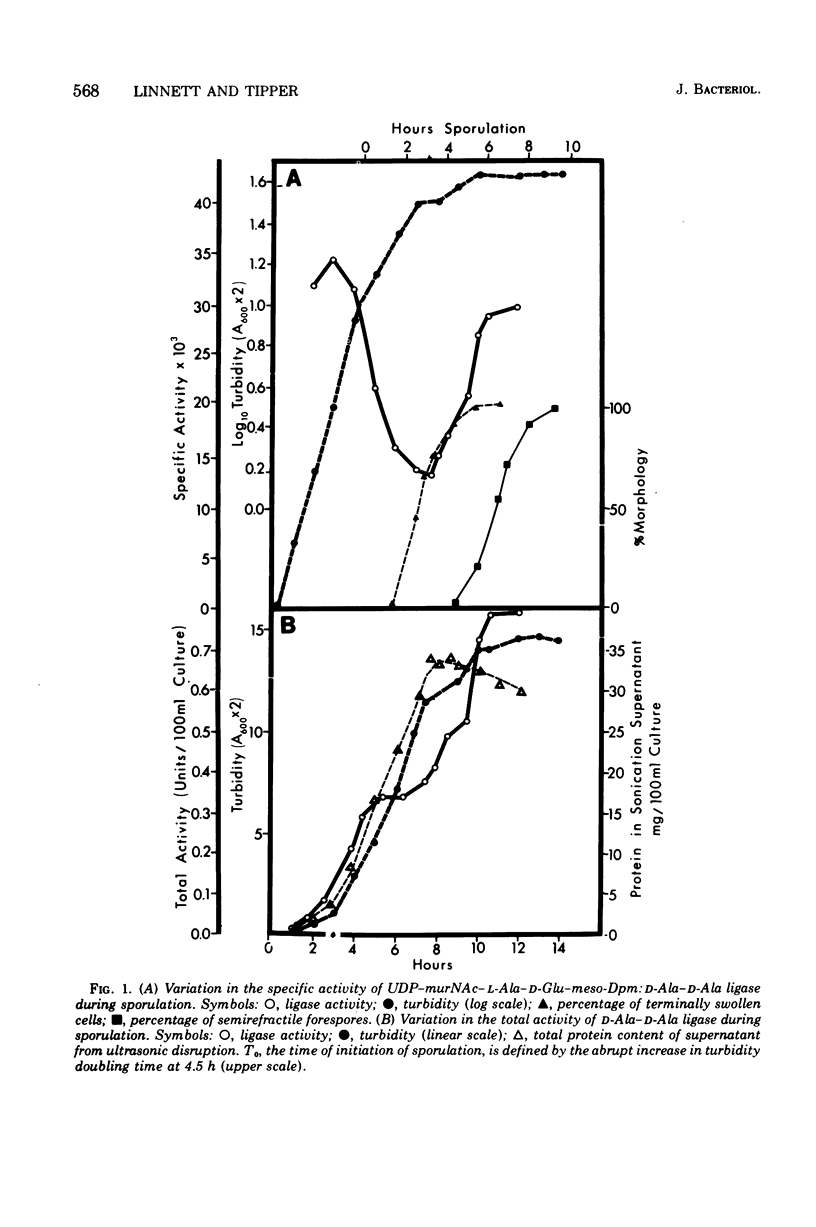
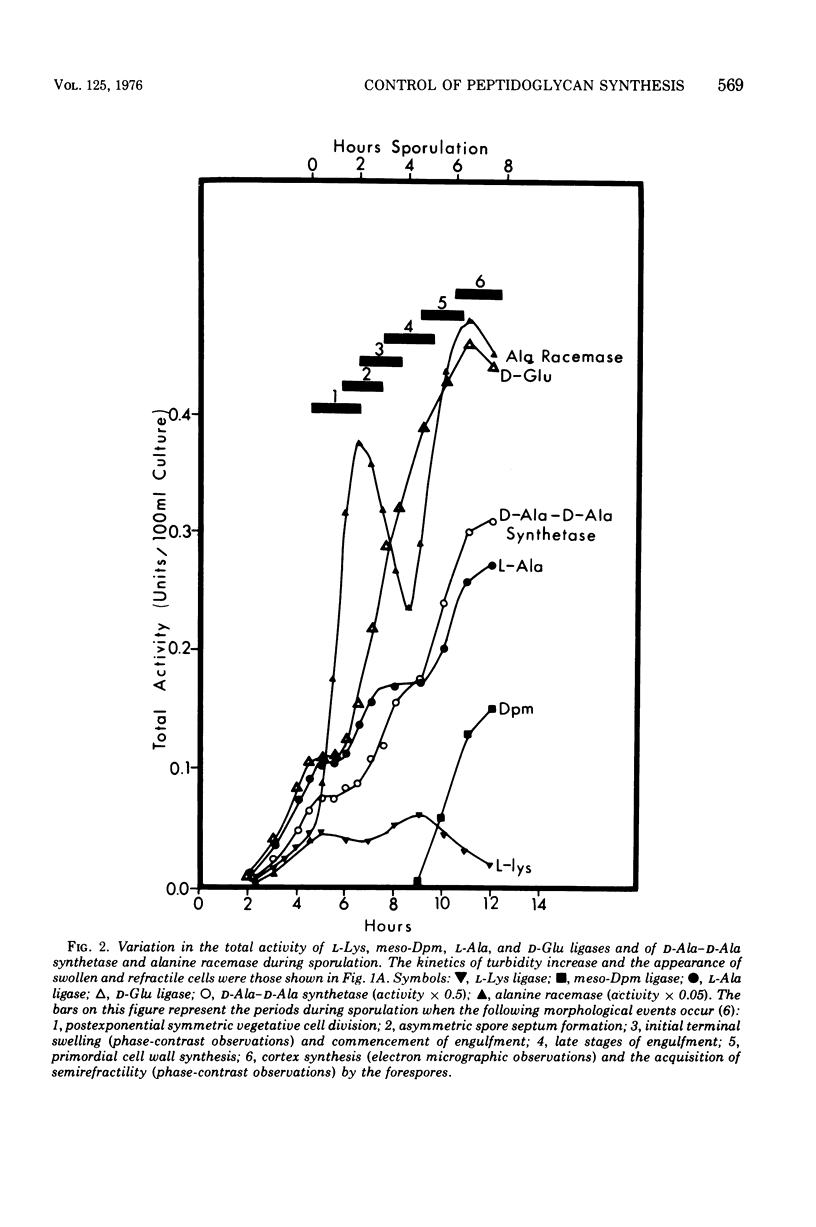
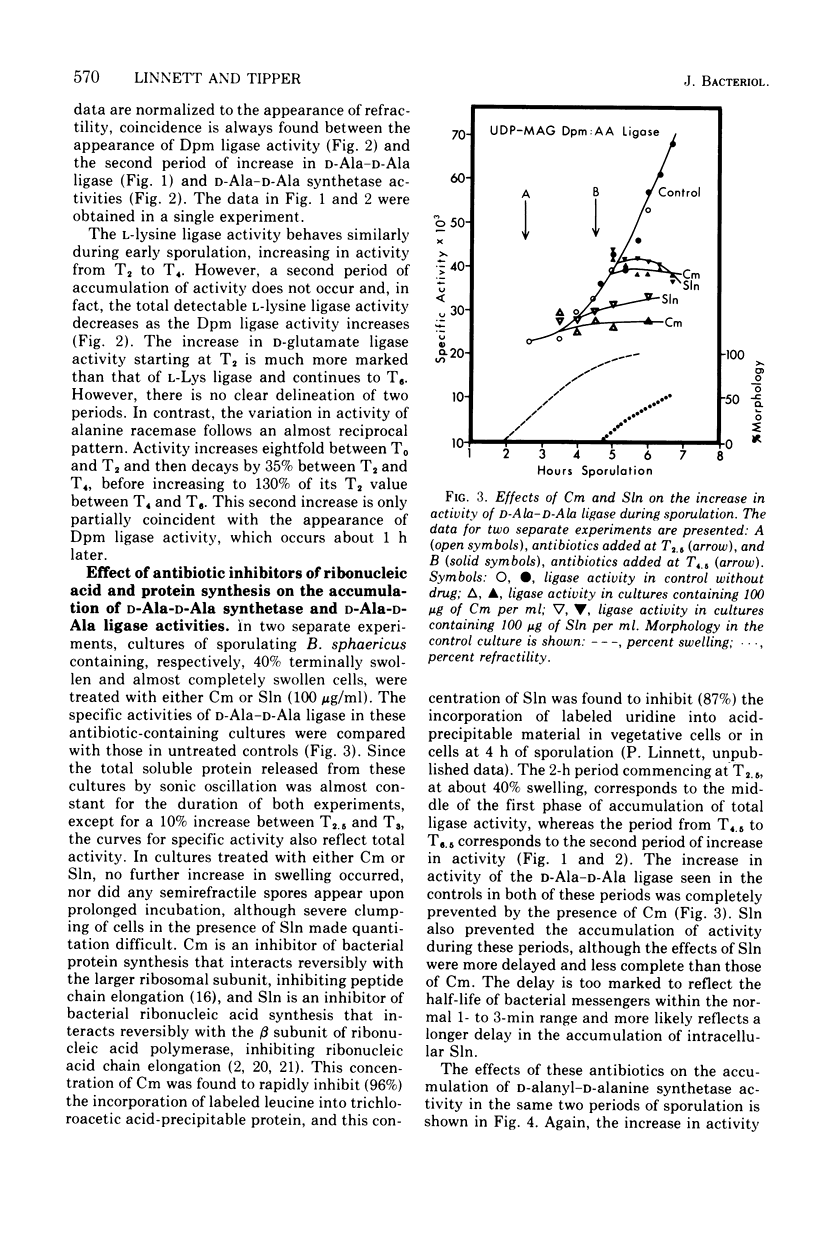
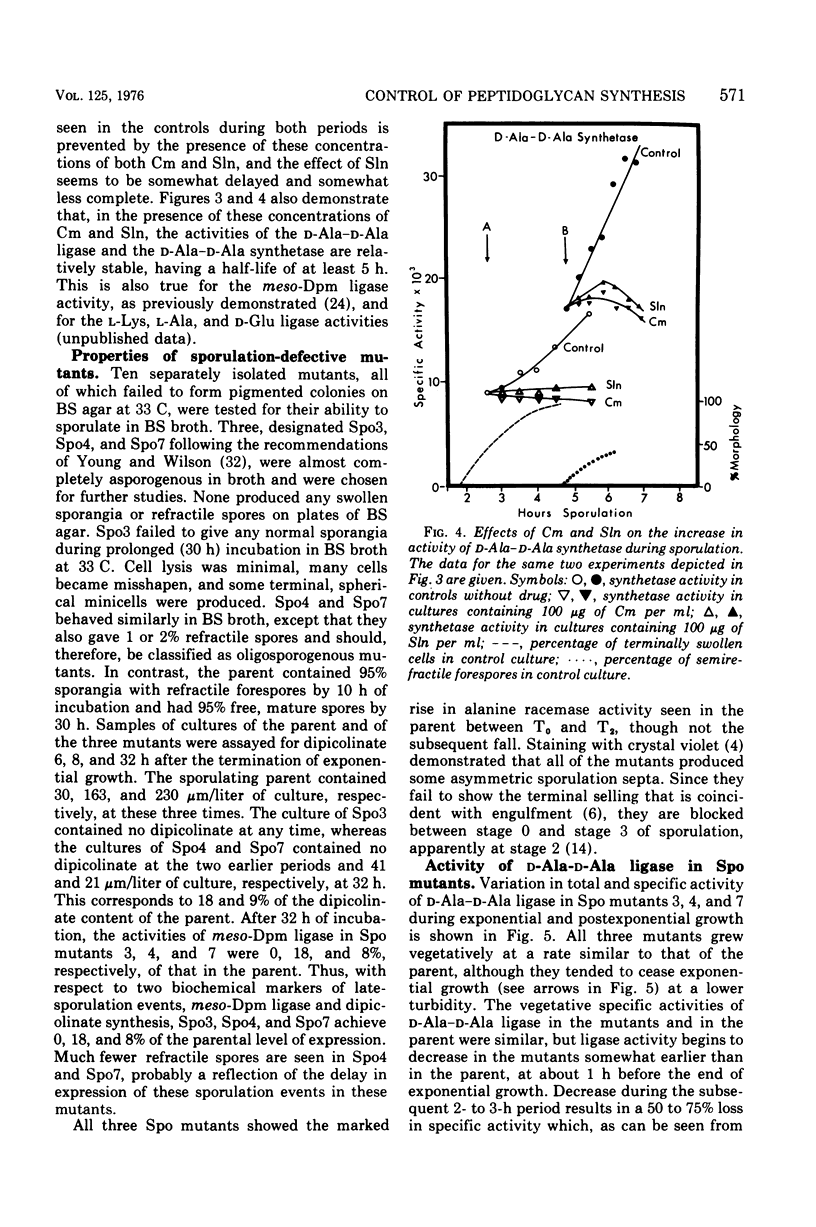
Synthesis of enzymes functional in the synthesis of nucleotide precursors of peptidoglycan ceases upon initiation of sporulation in Bacillus sphaericus. During sporulation, two periods of synthesis of these enzymes occur. The first starts at spore septum formation and is conincident with forespore engulfment; it involves the synthesis of those enzymes required for making the precursor of vegetative-type peptidoglycan, including L-lysyl ligase but no mesodiaminopimelyl ligase. The second period occurs shortly before the appearance of cortex. It involves the synthesis of diaminopimelyl ligase and the other enzymes needed for making the precursor of cortical peptidoglycan, but not lysyl ligase. Both events are a consequence of derepression at the level of transcription. Neither period of synthesis occurs in asporogenous mutants whose morphological block is at the point of spore septum formation.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Gordon R. A., Murrell W. G. Simple method of detecting spore septum formation and synchrony of sporulation. J Bacteriol. 1967 Jan;93(1):495–496. doi: 10.1128/jb.93.1.495-496.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guinand M., Michel G., Tipper D. J. Appearance of gamma-D-glutamyl-(L) meso-diaminopimealate peptidoglycan hydrolase during sporulation in Bacillus sphaericus. J Bacteriol. 1974 Oct;120(1):173–184. doi: 10.1128/jb.120.1.173-184.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt S. C., Gauther J. J., Tipper D. J. Ultrastructural studies of sporulation in Bacillus sphaericus. J Bacteriol. 1975 Jun;122(3):1322–1338. doi: 10.1128/jb.122.3.1322-1338.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hungerer K. D., Tipper D. J. Cell wall polymers of Bacillus sphaericus 9602. I. Structure of the vegetative cell wall peptidoglycan. Biochemistry. 1969 Sep;8(9):3577–3587. doi: 10.1021/bi00837a013. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lewis J. C. Determination of dipicolinic acid in bacterial spores by ultraviolet spectrometry of the calcium chelate. Anal Biochem. 1967 May;19(2):327–337. doi: 10.1016/0003-2697(67)90168-6. [DOI] [PubMed] [Google Scholar]
- Linnett P. E., Tipper D. J. Cell wall polymers of Bacillus sphaericus: activities of enzymes involved in peptidoglycan precursor synthesis during sporulation. J Bacteriol. 1974 Oct;120(1):342–354. doi: 10.1128/jb.120.1.342-354.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lugtenberg E. J., v Schijndel-van Dam A. Temperature-sensitive mutants of Escherichia coli K-12 with low activities of the L-alanine adding enzyme and the D-alanyl-D-alanine adding enzyme. J Bacteriol. 1972 Apr;110(1):35–40. doi: 10.1128/jb.110.1.35-40.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lugtenberg E. J., v Schijndel-van Dam A. Temperature-sensitive mutants of Escherichia coli K-12 with low activity of the diaminopimelic acid adding enzyme. J Bacteriol. 1972 Apr;110(1):41–46. doi: 10.1128/jb.110.1.41-46.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuzawa H., Matsuhashi M., Oka A., Sugino Y. Genetic and biochemical studies on cell wall peptidoglycan synthesis in Escherichia coli K-12. Biochem Biophys Res Commun. 1969 Aug 15;36(4):682–689. doi: 10.1016/0006-291x(69)90360-x. [DOI] [PubMed] [Google Scholar]
- Pearce S. M., Fitz-James P. C. Sporulation of a cortexless mutant of a variant of Bacillus cereus. J Bacteriol. 1971 Jan;105(1):339–348. doi: 10.1128/jb.105.1.339-348.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pestka S. Inhibitors of ribosome functions. Annu Rev Microbiol. 1971;25:487–562. doi: 10.1146/annurev.mi.25.100171.002415. [DOI] [PubMed] [Google Scholar]
- Pitel D. W., Gilvarg C. Mucopeptide metabolism during growth and sporulation in Bacillus megaterium. J Biol Chem. 1970 Dec 25;245(24):6711–6717. [PubMed] [Google Scholar]
- Pitel D. W., Gilvarg C. Timing of mucopeptide and phospholipid synthesis in sporulating Bacillus megaterium. J Biol Chem. 1971 Jun 10;246(11):3720–3724. [PubMed] [Google Scholar]
- Sadoff H. L., Celikkol E., Engelbrecht H. L. Conversion of bacterial aldolase from vegetative to spore form by a sporulation-specific protease. Proc Natl Acad Sci U S A. 1970 Jul;66(3):844–849. doi: 10.1073/pnas.66.3.844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schleif R. Isolation and characterization of streptolydigin resistant RNA polymerase. Nature. 1969 Sep 6;223(5210):1068–1069. doi: 10.1038/2231068a0. [DOI] [PubMed] [Google Scholar]
- Siddhikol C., Erbstoeszer J. W., Weisblum B. Mode of action of streptolydigin. J Bacteriol. 1969 Jul;99(1):151–155. doi: 10.1128/jb.99.1.151-155.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer S., Goodman N. S., Rogoff M. H. Defined media for the study of bacilli pathogenic to insects. Ann N Y Acad Sci. 1966 Oct 7;139(1):16–23. doi: 10.1111/j.1749-6632.1966.tb41181.x. [DOI] [PubMed] [Google Scholar]
- Tipper D. J., Pratt I. Cell wall polymers of Bacillus sphaericus 9602. II. Synthesis of the first enzyme unique to cortex synthesis during sporulation. J Bacteriol. 1970 Aug;103(2):305–317. doi: 10.1128/jb.103.2.305-317.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WARTH A. D., OHYE D. F., MURRELL W. G. Location and composition of spore mucopeptide in Bacillus species. J Cell Biol. 1963 Mar;16:593–609. doi: 10.1083/jcb.16.3.593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waites W. M., Kay D., Dawes I. W., Wood D. A., Warren S. C., Mandelstam J. Sporulation in Bacillus subtilis. Correlation of biochemical events with morphological changes in asporogenous mutants. Biochem J. 1970 Jul;118(4):667–676. doi: 10.1042/bj1180667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warth A. D., Strominger J. L. Structure of the peptidoglycan from spores of Bacillus subtilis. Biochemistry. 1972 Apr 11;11(8):1389–1396. doi: 10.1021/bi00758a010. [DOI] [PubMed] [Google Scholar]
- Warth A. D., Strominger J. L. Structure of the peptidoglycan of bacterial spores: occurrence of the lactam of muramic acid. Proc Natl Acad Sci U S A. 1969 Oct;64(2):528–535. doi: 10.1073/pnas.64.2.528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickus G. G., Warth A. D., Strominger J. L. Appearance of muramic lactam during cortex synthesis in sporulating cultures of Bacillus cereus and Bacillus megaterium. J Bacteriol. 1972 Aug;111(2):625–627. doi: 10.1128/jb.111.2.625-627.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood D. A. Sporulation in Bacillus subtilis. The appearance of sulpholactic acid as a marker event for sporulation. Biochem J. 1971 Jul;123(4):601–605. doi: 10.1042/bj1230601. [DOI] [PMC free article] [PubMed] [Google Scholar]



