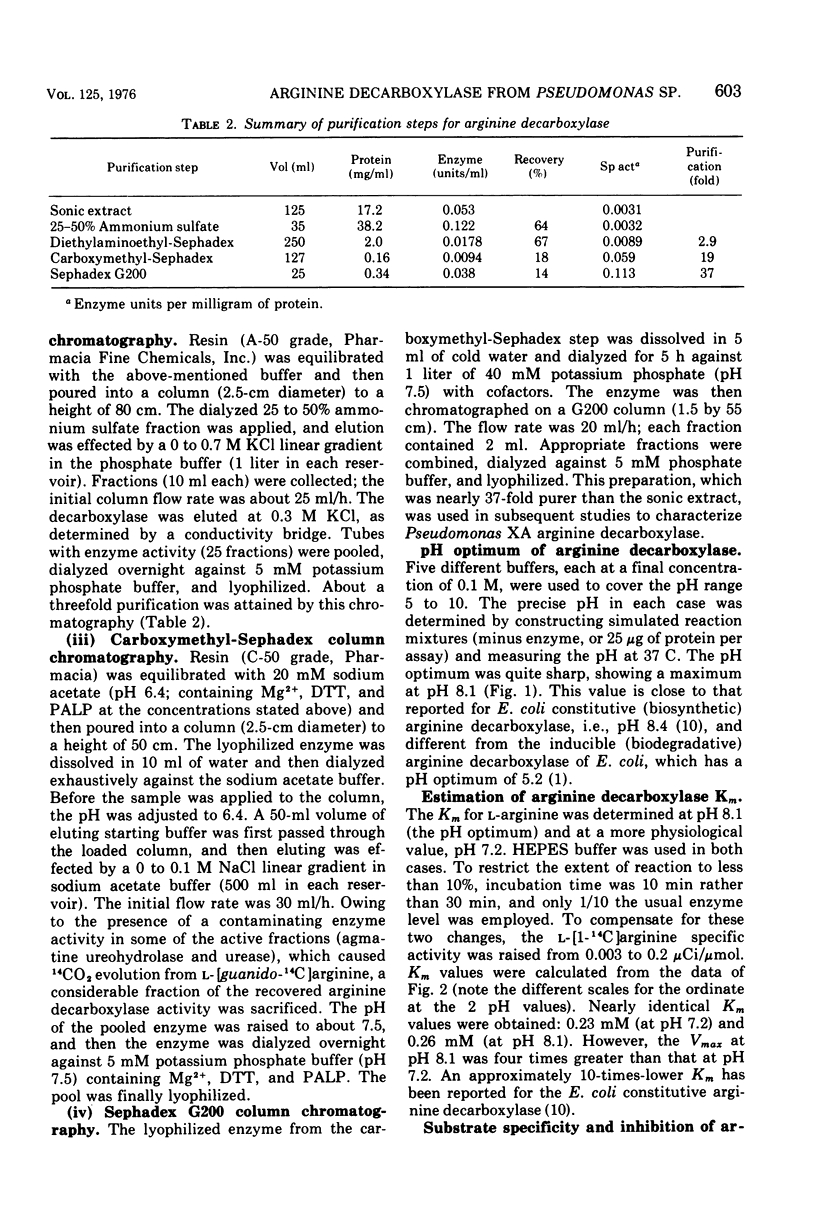
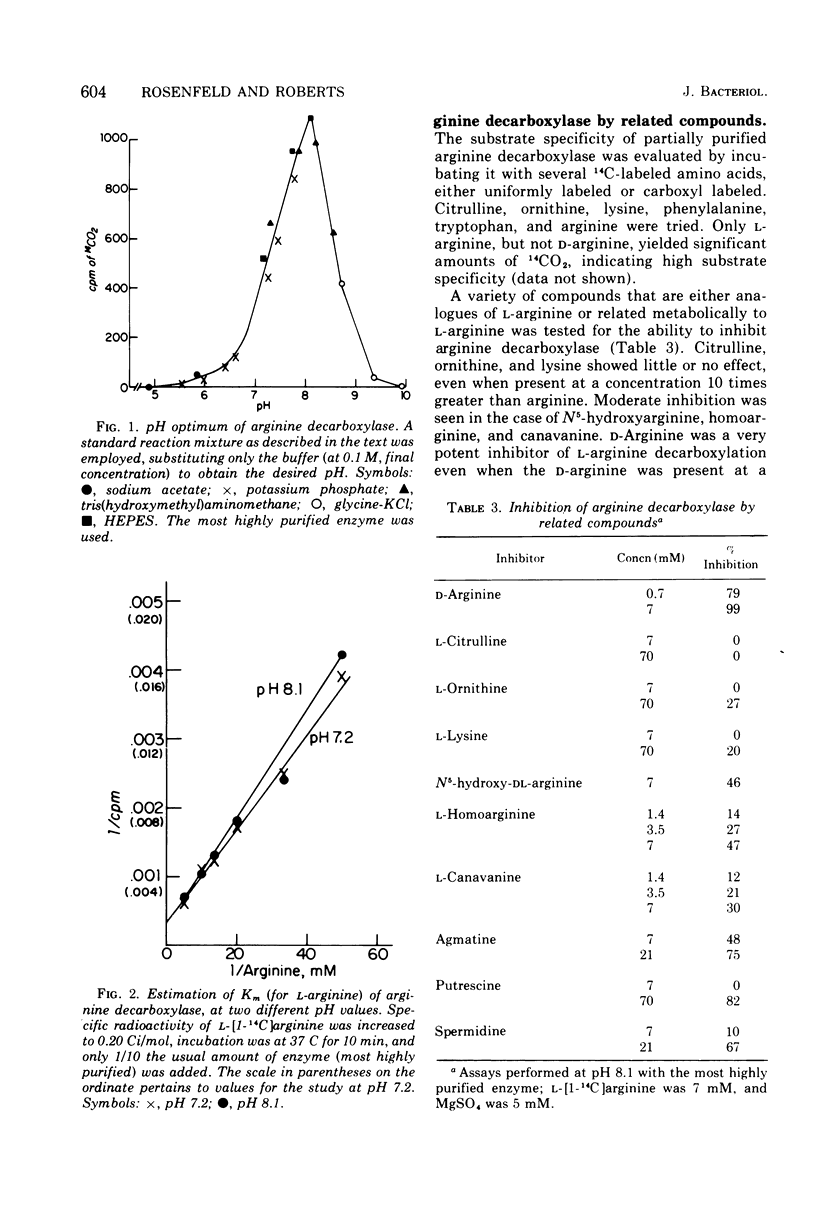
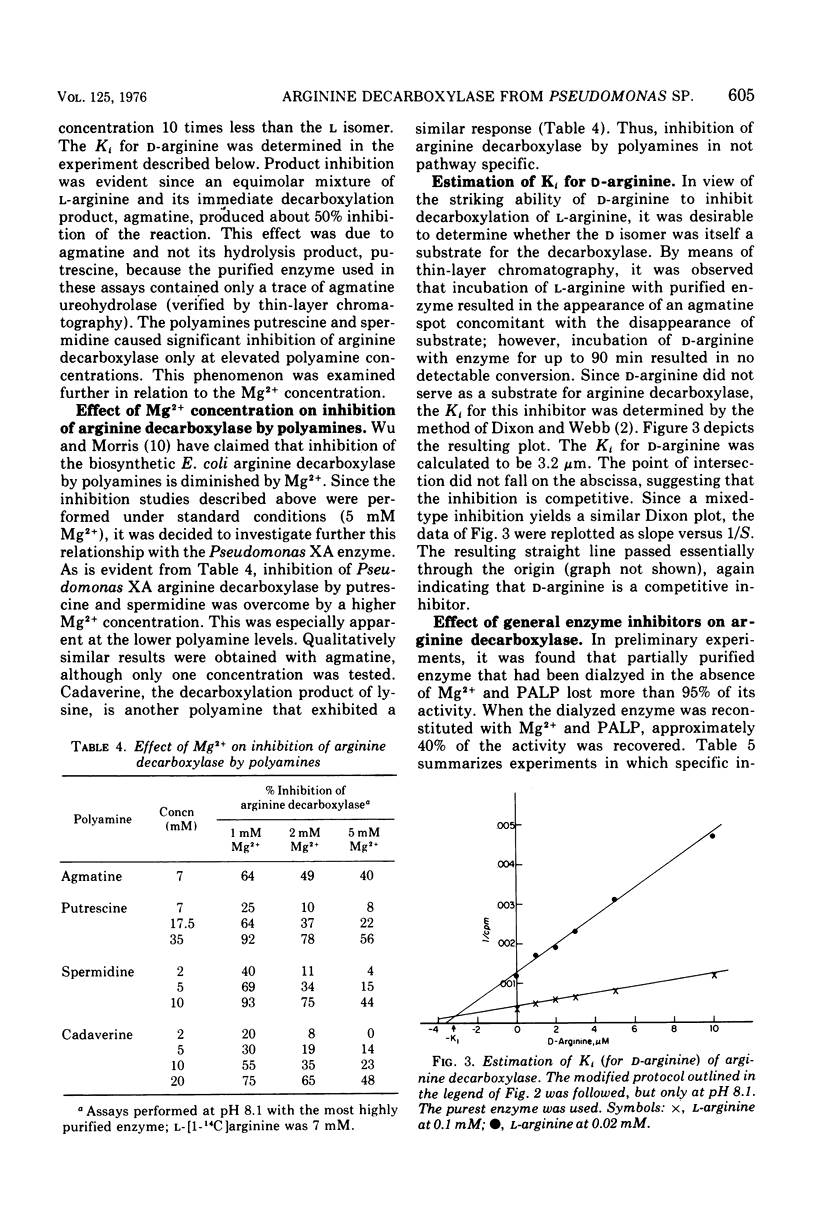
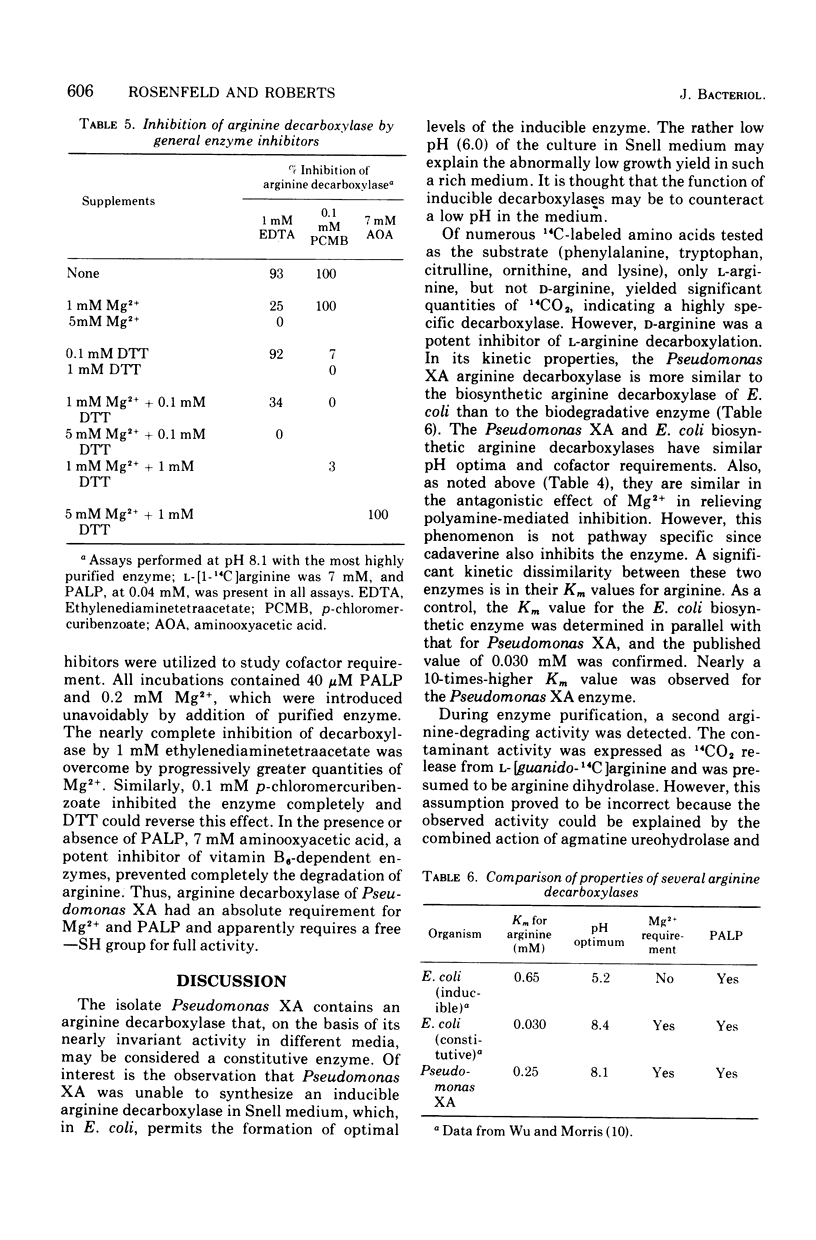
Abstract
An arginine decarboxylase has been isolated from a Pseudomonas species. The enzyme is constitutive and did not appear to be repressed by a variety of carbon sources. After an approximately 40-fold purification, the enzyme appeared more similar in its properties to the Escherichia coli biosynthetic arginine decarboxylase than to the E. coli inducible (biodegradative) enzyme. The Pseudomonas arginine decarboxylase exhibited a pH optimum of 8.1 and an absolute requirement of Mg2+ and pyridoxal phosphate, and was inhibited significantly at lower Mg2+ concentrations by the polyamines putrescine, spermidine, and cadaverine. The Km for L-arginine was about 0.25 mM at pH 8.1 AND 7.2. The enzyme was completely inhibited by p-chloromercuribenzoate. The inhibition was prevented by dithiothreitol, a feature that suggests the involvement of an -SH group. Of a variety of labeled amino acids tested, only L-arginine, but not D-arginine was decarboxylated. D-Arginine was a potent inhibitor of arginine decarboxylase with a Ki of 3.2 muM.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blethen S. L., Boeker E. A., Snell E. E. Argenine decarboxylase from Escherichia coli. I. Purification and specificity for substrates and coenzyme. J Biol Chem. 1968 Apr 25;243(8):1671–1677. [PubMed] [Google Scholar]
- Hirshfield I. N., Rosenfeld H. J., Leifer Z., Maas W. K. Isolation and characterization of a mutant of Escherichia coli blocked in the synthesis of putrescine. J Bacteriol. 1970 Mar;101(3):725–730. doi: 10.1128/jb.101.3.725-730.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Roberts J., Holcenberg J. S., Dolowy W. C. Isolation, crystallization, and properties of Achromobacteraceae glutaminase-asparaginase with antitumor activity. J Biol Chem. 1972 Jan 10;247(1):84–90. [PubMed] [Google Scholar]
- Storr J. M., Burton A. F. The effects of arginine deficiency on lymphoma cells. Br J Cancer. 1974 Jul;30(1):50–59. doi: 10.1038/bjc.1974.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu W. H., Morris D. R. Biosynthetic arginine decarboxylase from Escherichia coli. Purification and properties. J Biol Chem. 1973 Mar 10;248(5):1687–1695. [PubMed] [Google Scholar]


