Abstract
We evaluated the expression and amplification of cyclin L1 (CCNL1) gene, a potential oncogene localised in the commonly amplified 3q25–28 region, in human head and neck squamous cell carcinomas (HNSCCs). Overexpression was observed in 55 out of 96 cases (57%) and amplification in nine out of 35 tumours (26%) with no relationships to the clinico-pathological parameters. The Cyclin L1 antibody we developed labels nuclear speckles in tumour cells compatible with a role for CCNL1 in RNA splicing.
Keywords: head and neck, squamous cell carcinoma, cyclin L1, CCNL1, chromosome 3q, HNSCC
Definition of the genetic changes associated with head and neck squamous cell carcinoma (HNSCC), in particular by comparative genomic hybridisation (CGH), pinpointed gain of the 3q chromosome as one of the most frequent abnormalities (Bockmuhl et al, 2000). We demonstrated that the 3q25–28 region is a consistent site of gain in early HNSCC (Redon et al, 2001), and suggested that the gain is associated with shortened disease survival (Redon et al, 2001). This association was confirmed in other larger studies underlying the importance of this region in the development of HNSCC (Bockmuhl et al, 2000; Ashman et al, 2003, and publications therein). Several putative oncogenes from the 3q25–28 region have been proposed to be potential targets of amplification, including PIK3CA (Estilo et al, 2003), SCCRO (Estilo et al, 2003), p63 (Thurfjell et al, 2005), and cyclin L1 (CCNL1), which we showed by microarray-based CGH to be amplified in an HNSSC cell line (Redon et al, 2002). The main α isoform of Cyclin L1 contains an RS domain (Ser–Arg rich proteins) that may confer splicing activity to cyclin L1 (Dickinson et al, 2002). CCNL1 is an immediate early gene induced by several growth factors (Berke et al, 2001) that may regulate G0–G1 cell-cycle progression (Redon et al, 2002 and publications herein).
In the present study, we measured CCNL1 gene expression in 96 human HNSCCs by real-time quantitative PCR. CCNL1 gene copy number was evaluated to examine the relationship between RNA expression and DNA copy number alterations. The relationship between CCNL1 gene alterations and pathological features as well as clinical outcome is reported. Finally, the localisation of the cyclin L1 protein was determined by immunohistochemistry on normal and neoplastic head and neck tissues.
MATERIALS AND METHODS
Patients and samples
A total of 96 HNSCC samples were selected from consenting patients who underwent surgery as first treatment, without previous radiation or chemotherapy. Matched normal mucosa samples were obtained for 82 cases by resection at least 5 cm from the tumour. They were immediately frozen and stored in liquid nitrogen. Clinico-pathological features are summarised in Table 1 using the UICC TNM system. The mean clinical follow-up was 62 months (range 0.5–199).
Table 1. Relation between Cyclin L1 gene expression and clinico-pathological features of tumours.
| Number of cases | Overexpression Number of cases (%) | P-value * | |
|---|---|---|---|
| Localisation | |||
| All | 96 | 55 (57%) | |
| Hypopharynx | 64 | 38 (59%) | |
| Oropharynx | 20 | 13 (65%) | 0.245 |
| Oral cavity | 12 | 4 (33%) | |
| Tumour size | |||
| T1 | 12 | 7 (58%) | |
| T2 | 49 | 33 (67%) | 0.065 |
| T34 | 35 | 15 (43%) | |
| Lymph node involvement | |||
| N0 | 44 | 22 (50%) | 0.59 |
| N+ | 52 | 33 (63%) | |
| Tumour differentiation | |||
| Well | 30 | 13 (43%) | 0.34 |
| Moderately | 34 | 22 (65%) | |
| Poorly to undifferentiated | 32 | 20 (62%) | |
ANOVA test on relative expression values.
Cyclin L1 gene expression and copy number
Total RNA isolation and cDNA preparation were performed as described previously (Redon et al, 2001). Genomic DNA was isolated by phenol/chloroform extraction. CCNL1 gene amplification and expression were assessed by quantitative PCR performed with the LightCycler system, using LC Fast start DNA master SYBR green I mixture, and version 3.0 software (Roche Diagnostics, Meylan, France).
The specific oligonucleotide primers, designed by Primer3 software, were: (a) for gene expression analysis: CCNL1, 5′-ACTCCAAGCCCCCTGATCCT-3′ and 5′-TGGCAACGGAATCTGAAGTG-3′, which amplify the α and β isoforms; the ubiquitous gene RPLP0, 5′-GAAGGCTGTGGTGCTGATGG-3′ and 5′-CCGGATATGAGGCAGCAGTT-3′; (b) for gene amplification analysis: CCNL1, 5′-TAGGCGGAGTCGATCTGGAA-3′ and 5′-CCATGGTGCTTGCTTTTATGG-3′; and two genes located on respectively chromosome 15q15–21 and 11p15, CAPN3 (calpain3/p94), 5′-GCTGGTAGGAGACCCCCAAG-3′ and 5′-CCACAGATGCGCTAATGACG-3′, HBB (beta-globin), 5′-GAAGAGCCAAGGACAGGT-3′ and 5′-TGGTGTCTGTTTGAGGAAGC-3′.
In order to obtain for each gene results in ng for expression and copy number, respectively, calibration curves were constructed using pools of cDNAs from 10 normal head and neck tissue samples and DNA from peripheral blood samples from 10 healthy individuals. CCNL1 gene expression was evaluated for 96 tumours and 82 corresponding normal tissues using the mean value from three independent experiments. The relative CCNL1 gene expression level was calculated by successive normalisation to the RPLP0 (Ribosomal phospho-protein P0) internal control and then to the mean expression of all the normal tissue samples. A relative value ⩾1.7 was considered to represent overexpression. Cyclin L1 copy number changes were analysed for 35 tumours and 14 normal samples by independent duplicate PCR reactions with two DNA inputs, 6 and 2 ng. Copy number values for a tumour sample were calculated in relative units adjusted to the mean values of CAPN3 and HBB internal controls. The results were expressed as fold differences in target gene copy number in tumours relative to normal samples. A relative value ⩾1.5 was considered to represent amplification.
Cyclin L1 protein expression
Cyclin L1 protein expression was assessed by immunohistochemistry, using a mouse monoclonal antibody raised against the peptide SKHHGGRSGHCRHRR. This sequence is found only at the C-terminal of the isoform α. For retrieval, 3 μm de-waxed sections were pressure-cooked for 3 min in 0.1 M citrate buffer pH 6.0. They were incubated overnight at 4°C with a 1/1000 dilution of the primary antibody, and revealed by the avidin-biotin peroxydase complex method (DAKO labelled streptavidin-biotin LSAB). Negative controls lacked the primary antibody.
Statistics
Associations with clinico-pathological features and CCNL1 expression or amplification were analysed at the 5% significance level using the ANOVA or Kruskal Wallis tests where appropriate. Survival was analysed by the Kaplan–Meier method and the log-rank test with MedCalc software (MedCalc, Belgium).
RESULTS
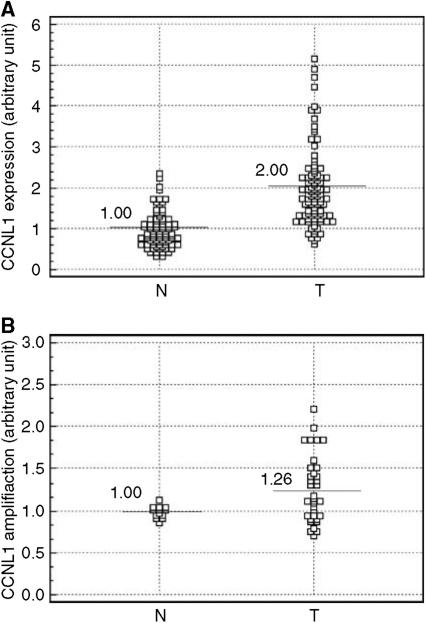
Real-time quantitative PCR analysis of 96 HNSCCs and 82 of their counterpart normal tissues showed that Cyclin L1 gene expression levels were significantly different between tumours and normal tissue samples (T-test; P<0.0001) (Figure 1A). Overexpression was found in 55 tumours (57%). Expression levels in tumours were not significantly associated with their clinico-pathological features (tumour site, size or differentiation, lymph node involvement, Table 1).
Figure 1.
CyclinL1 gene alterations assessed by quantitative real-time PCR. (A) Relative cyclin L1 gene expression level in 96 head and neck tumours (T) and 82 normal tissues (N); T-test, P<0.0001, mean value for N: 1.00 by construction (95% CI=0.9–1.09), for T: 2.01 (95% CI=1.8–2.2), (B) Relative cyclin L1 gene copy level in 14 normal (N) and 35 tumour tissues (T); T-test, P=0.02, mean value for N: 1.00 by construction (95% CI=0.95–1.05), for T: 1.26 (95% CI=1.12–1.39).
CCNL1 gene amplification, evaluated in 35 HNSCCs, was observed in 26% of the tumours and does not exceed three-fold (relative to the mean of 14 normal tissue samples) (Figure 1B). Overexpression was observed in almost all tumours with amplification (seven out of nine cases with amplification), while lack of overexpression in tumours without amplification was observed in 65% (17 out of 26) of the cases, suggesting correlation between CCNL1 overexpression and amplification (Table 2, Fisher's test P=0.049). Amplification was not statistically related to the clinico-pathological features of the tumours (not shown).
Table 2. Relationship between cyclin L1 gene amplification and expression in head and neck SCC.
| Not amplified | Amplified | Total | |
|---|---|---|---|
| No overexpression | 17 | 2 | 19 |
| Overexpression | 9 | 7 | 16 |
| Total | 26 | 9 | 35 |
Fisher's test, P=0.0497.
SCC, squamous cell carcinoma.
Disease progression was observed for 60 patients. No statistically significant relationship was observed between either CCNL1 overexpression or amplification and crude survival or relapse-free survival of the patients, even for tumours with high expression levels (highest quartile, data not shown). Gene amplification evaluation was mainly performed on tumours of pharyngeal origin (86%). For the tumours with concomitant overexpression and amplification, no association with either histo-clinical features or survival outcome was observed.
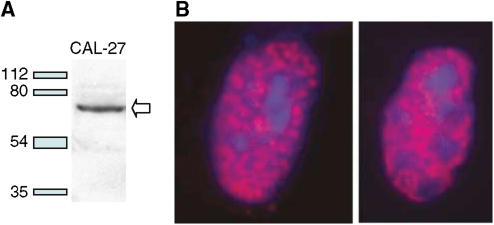
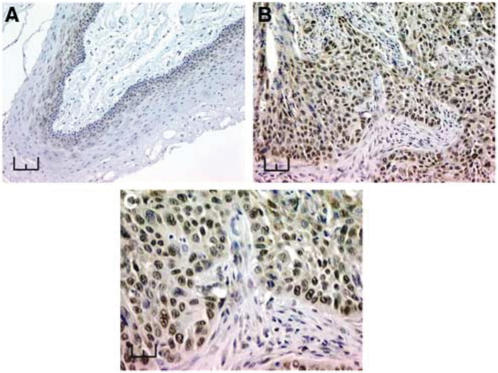
We developed a monoclonal antibody against the long form of the protein (α isoform) and used it for Western blotting (Figure 2A). The nuclear staining (Figure 2B) observed by immunocytofluorescence is similar to that observed in COS cells transfected with flagged CCNL1 and revealed by an anti-FLAG antibody (Berke et al, 2001). In tumours, Cyclin L1 staining is mostly restricted to the nuclei of the epithelial cells with discrete additional cytoplasmic staining in some cells (Figure 3). No staining was observed in stromal cells. Heterogeneous staining was observed throughout the tumour sections and between different tumours. Normal epithelium displays weak nuclear staining of the parabasal cells (arrow).
Figure 2.
Validation of anti Cyclin L1 alpha mouse monoclonal antibody. (A) Western blot validation. The monoclonal antibody was validated with a Cal-27 HNSCC cell line protein extract. It detects a protein (indicated by the arrow) of size between 54 and 80 kDa, compatible with predicted size of CCNL1 alpha (526 aa, around 60 kDa). Molecular marker sizes are indicated on the left. (B) Immunocytofluorescence validation. The CCNL1 alpha antibody (red colour) labels nuclear speckles of the two transfected COS cells presented. No cytoplasmic staining was observed. Blue colour corresponds to DAPI staining of nuclei. This pattern is identical to a previously published localisation using an anti-FLAG labelling on transfected COS cells (Berke et al, 2001).
Figure 3.
Immunohistochemical detection of cyclin L1 protein in normal and neoplastic head and neck tissues from HNSCC patients. (A) Slight positive staining of parabasal cells (arrow) of normal epithelium from the upper aero-digestive tract (Bar represents 200 μm). (B) Strong nuclear staining of tumour cells in a moderately differentiated head and neck tumour tract (bar represents 50 μm); (C) enlargement of image B, positive staining of speckles in the nuclei tract (bar represents 25 μm).
DISCUSSION
In this large series of HNSCCs, we observed a high prevalence of CCNL1 gene overexpression, occurring in more than half of the tumours. CCNL1 gene copy number was increased in 26% of the cases, in accordance with the 34% of gains reported by FISH analysis with a CCNL1 gene probe (Sticht et al, 2005). Furthermore, we demonstrated gene overexpression in most HNSCCs with amplification of the CCNL1 gene, supporting the hypothesis that its expression level partly follows a gene dosage effect. However, CCNL1 overexpression was also detected in 34% (nine out of 26) of tumours without CCNL1 gene amplification, showing that other transcriptionnal regulation factors are involved. Similar discrepancy between overexpression and amplification has been reported for other oncogenes. For example, overexpression of cyclin D1 in oral squamous cell carcinomas is twice as frequent as CCND1 amplification (Miyamoto et al, 2003).
A recent study, comparing DNA copy number to gene expression levels over large chromosomal regions in HNSCCs, concludes that chromosomal alterations affect the expression of many genes (Masayesva et al, 2004). The discrimination between genes that are important for tumour evolution from bystander ones within regions of copy number changes remains complex. Only some of these overexpressed genes may be important for tumorigenesis, and identifying them replies on several clues such as gene amplification/expression correlations, functional characterisation or association with clinical features. Multiple genes within the commonly gained 3q region appear to be relevant for head and neck cancer progression. The gain of chromosomal region 3q25–28 is associated with aggressive clinical behavior (Bockmuhl et al, 2000; Singh et al, 2002; Ashman et al, 2003). Overexpression of several genes at 3q26 have prognostic value, including SCCRO in oral tongue (Estilo et al, 2003) and ZASC1 in oesophageal (Imoto et al, 2003) tumours. Recently, high levels of CCNL1 gene amplification, assessed by FISH (>8 signals/cell) have been found in 3% of a large HNSCC series and were shown to be associated with shorter survival (Sticht et al, 2005). In our series, high-level amplification was not found, and neither CCNL1 gene amplification nor overexpression was associated with unfavourable tumour phenotype. Difference concerning amplification between both studies may be due to sampling differences since our population is mainly composed of tumours derived from pharynx (86 %) while Sticht et al investigated mostly oral cavity tumours.
Inappropriate expression of multiple genes at the 3q25–28 chromosomal locus, including CCNL1, could contribute to disease progression, providing growth advantage by additive effects in different cellular pathways. CCNL1 (isoform α) could be a regulator of the G0 to G1 cell-cycle transition. Analysis of the relationships with cell cycle regulators specially relevant in head and neck cancers, such as Cyclin D1 or growth factors, would be of interest. CCNL1 may have also a role in transcription or RNA splicing, since it colocalises within nuclear speckles with splicing factors SC-35 and 9G8 in cells transfected by cyclin L1 isoform α (Yang et al, 2004). Interestingly, cyclin L1 was also observed in the nuclei of malignant cells of head and neck tumours, by histological immunodetection using our antibody. CCNL1 overexpression may modify the splicing pattern of specific genes, in particular genes involved in apoptosis for which splicing variants have been shown to have agonist or antagonist effects (Schwerk and Schulze-Osthoff, 2005). Altogether, these findings suggest that cyclin L1 might have a role in the RNA processing complex and could participate to tumour progression of HNSCC. It thus remains of interest to investigate further the physiological functions of cyclin L1 and its link to head and neck carcinogenesis.
Acknowledgments
We are indebted to Christine Macabre for technical support. We are grateful to Mustapha Oulad from the IGBMC for the preparation of the cyclin L1 antibody and technical advice. This study was supported by grants from the ‘Comité Régional du Haut-Rhin de la Ligue contre le Cancer’ (Equipe Labellisée), and by funds from INSERM and the CNRS. Thomas Hussenet is recipient of a bursary from ‘Association pour la Recherche sur le Cancer’.
References
- Ashman JN, Patmore HS, Condon LT, Cawkwell L, Stafford ND, Greenman J (2003) Prognostic value of genomic alterations in head and neck squamous cell carcinoma detected by comparative genomic hybridisation. Br J Cancer 89: 864–869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berke JD, Sgambato V, Zhu PP, Lavoie B, Vincent M, Krause M, Hyman SE (2001) Dopamine and glutamate induce distinct striatal splice forms of Ania-6, an RNA polymerase II-associated cyclin. Neuron 32: 277–287 [DOI] [PubMed] [Google Scholar]
- Bockmuhl U, Schluns K, Kuchler I, Petersen S, Petersen I (2000) Genetic imbalances with impact on survival in head and neck cancer patients. Am J Pathol 157: 369–375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickinson LA, Edgar AJ, Ehley J, Gottesfeld JM (2002) Cyclin L is an RS domain protein involved in pre-mRNA splicing. J Biol Chem 277: 25465–25473 [DOI] [PubMed] [Google Scholar]
- Estilo CL, O-Charoenrat P, Ngai I, Patel SG, Reddy PG, Dao S, Shaha AR, Kraus DH, Boyle JO, Wong RJ, Pfister DG, Huryn JM, Zlotolow IM, Shah JP, Singh B (2003) The role of novel oncogenes squamous cell carcinoma-related oncogene and phosphatidylinositol 3-kinase p110alpha in squamous cell carcinoma of the oral tongue. Clin Cancer Res 9: 2300–2306 [PubMed] [Google Scholar]
- Imoto I, Yuki Y, Sonoda I, Ito T, Shimada Y, Imamura M, Inazawa J (2003) Identification of ZASC1 encoding a Kruppel-like zinc finger protein as a novel target for 3q26 amplification in esophageal squamous cell carcinomas. Cancer Res 63: 5691–5696 [PubMed] [Google Scholar]
- Masayesva BG, Ha P, Garrett-Mayer E, Pilkington T, Mao R, Pevsner J, Speed T, Benoit N, Moon CS, Sidransky D, Westra WH, Califano J (2004) Gene expression alterations over large chromosomal regions in cancers include multiple genes unrelated to malignant progression. Proc Natl Acad Sci USA 101: 8715–8720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamoto R, Uzawa N, Nagaoka S, Hirata Y, Amagasa T (2003) Prognostic significance of cyclin D1 amplification and overexpression in oral squamous cell carcinomas. Oral Oncol 39: 610–618 [DOI] [PubMed] [Google Scholar]
- Redon R, Hussenet T, Bour G, Caulee K, Jost B, Muller D, Abecassis J, du Manoir S (2002) Amplicon mapping and transcriptional analysis pinpoint cyclin L as a candidate oncogene in head and neck cancer. Cancer Res 62: 6211–6217 [PubMed] [Google Scholar]
- Redon R, Muller D, Caulee K, Wanherdrick K, Abecassis J, du Manoir S (2001) A simple specific pattern of chromosomal aberrations at early stages of head and neck squamous cell carcinomas: PIK3CA but not p63 gene as a likely target of 3q26-qter gains. Cancer Res 61: 4122–4129 [PubMed] [Google Scholar]
- Schwerk C, Schulze-Osthoff K (2005) Regulation of apoptosis by alternative pre-mRNA splicing. Mol Cell 19: 1–13 [DOI] [PubMed] [Google Scholar]
- Singh B, Stoffel A, Gogineni S, Poulri A, Pfister DG, Shaha AR, Pathak A, Bosl G, Cordon-Cardo C, Sha JP, Rao PH (2002) Amplification of the 3q26.3 locus is associated with progression to invasive cancer and is a negative prognostic factor in head and neck squamous cell carcinomas. Am J Pathol 161: 365–371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sticht C, Hofele C, Flechtenmacher C, Bosch FX, Freier K, Lichter P, Joos S (2005) Amplification of Cyclin L1 is associated with lymph node metastases in head and neck squamous cell carcinoma (HNSCC). Br J Cancer 92: 770–774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thurfjell N, Coates PJ, Boldrup L, Lindgren B, Backlund B, Uusitalo T, Mahani D, Dabelsteen E, Dahlqvist A, Sjostrom B, Roos G, Vojtesek B, Nenutil R, Nylander K (2005) Function and importance of p63 in normal oral mucosa and squamous cell carcinoma of the head and neck. Adv Otorhinolaryngol 62: 49–57 [DOI] [PubMed] [Google Scholar]
- Yang L, Li N, Wang C, Yu Y, Yuan L, Zhang M, Cao X (2004) Cyclin L2, a novel RNA polymerase II-associated cyclin, is involved in pre-mRNA splicing and induces apoptosis of human hepatocellular carcinoma cells. J Biol Chem 279: 11639–11648 [DOI] [PubMed] [Google Scholar]