Abstract
In a pioneer study, we showed 10 years ago that enhanced tissue levels of the matrix metalloproteinases (MMPs) MMP-2 and MMP-9 in gastric cancers, as determined by zymography, were related with worse overall survival of the patients. To corroborate these observations, we now assessed MMP-2 and MMP-9 with new techniques in an expanded group of gastric cancer patients (n=81) and included for comparison MMP-7, MMP-8 and the tissue inhibitors of MMPs, TIMP-1 and -2. All MMPs and TIMP-1 were significantly increased in tumour tissue compared to normal gastric mucosa. Matrix metalloproteinase-7, -8 and -9, and the TIMPs showed some correlations with the clinicopathologic parameters TNM, WHO and Laurén classification, but their levels were not related with survival. Regardless of the determination method used, that is, enzyme-linked immunosorbent assay or bioactivity assay, an enhanced tumour MMP-2 level did not show a significant correlation with any of the clinicopathological parameters, but was confirmed to be an independent prognostic factor in gastric cancer.
Keywords: gelatinases, MMP-7, MMP-8, MMP-9, TIMP, tissue inhibitor of metalloproteinases
A decade ago, we were the first to report that the levels of matrix metalloproteinase (MMP)-2 and MMP-9 in human gastric carcinoma tissues were enhanced and related to the survival of the patients, using a simple but laborious zymography technique in a relatively small group of patients (Sier et al, 1996). Matrix metalloproteinases are believed to play an important role in carcinogenesis via the degradation and remodelling of tumour surrounding extracellular matrix, which could explain the association with survival (Zucker et al, 2000; McCawley and Matrisian, 2001; Polette et al, 2004). We concluded that measuring MMPs could have clinical value as indicators for gastric carcinoma patients who needed adjuvant therapy and that inhibitors of MMPs might be useful for therapeutic intervention. Several accomplishments have been made since. The prognostic value of MMPs for gastric carcinoma patients has been confirmed in several other studies (Allgayer et al, 1998; Zhang et al, 2003), and clinical trials testing the effect of MMP inhibitors for patients with various types of cancer were performed, with variable success (Zucker et al, 2000; Bramhall et al, 2002).
In general, MMPs are secreted as inactive pro-enzymes, activated by proteolytic cleavage, and controlled in their activity by interaction with inhibitors. Disturbances in these processes are of eminent importance in tumour invasion and metastasis (McCawley and Matrisian, 2001; Polette et al, 2004). In the present more comprehensive study, we extended our MMP analyses in the same group of patients and compared the results with those obtained with a new and more recent group of patients. Furthermore, instead of zymography, which identifies isoforms, we now used recently established quantitative bioactivity assays (BIAs) and specific antigen enzyme-linked immunosorbent assays (ELISAs) for MMP-2 and MMP-9. Moreover, we compared the prognostic value of MMP-2 and MMP-9 with those of MMP-7 and MMP-8 and expanded the study by determination of the inhibitors TIMP-1 and TIMP-2. In addition, because of the increasing age of the patients and the length of the follow-up, we now used tumour-associated survival.
PATIENTS, MATERIALS AND METHODS
Patients and study design
Fresh tissue specimens of 81 patients (21 female and 60 male subjects, mean age 65.9 years, range 35.10–91.33), who underwent resection for primary gastric adenocarcinoma at the Department of Oncologic Surgery of the Leiden University Medical Centre, were collected prospectively. Immediately after resection, fresh samples from the mid-central, non-necrotic part of the carcinoma and/or from distant normal mucosa, taken approximately 10 cm from the tumour, were snap frozen and stored at −70°C until extraction, to be used for research purposes. Various clinicopathological data were evaluated or collected from patient files. All carcinomas were classified according to the TNM classification (Hermanek and Sobin, 1992) and localisation and also diameter of the tumour, differentiation grade, WHO, Borrman, and Laurén classification, as well as the presence of intestinal metaplasia in the normal gastric mucosa, as revised by a gastroenterologist (FK) and a pathologist (JvK). All patients entered the study at operation date, and the patient's time experience ended in the event of death or, when still alive, at the common closing date. The minimal follow-up was 33 months with a decreasing overall survival according to TNM stage, that is, from TNM I (52.2%, n=23), to TNM II (26.9%, n=26), to TNM III (28%, n=25), and to TNM IV (0%, n=7).
Tissue preparation and protein concentration
Homogenisation of tissue specimens and determination of protein concentrations were performed as described previously (Sier et al, 1996).
Metalloproteinase-2 and -9 activity assays
Quantitative gelatin zymography and BIAs for MMP-2 and MMP-9 were carried out as described before (Sier et al, 1996; Hanemaaijer et al, 2000). Active and activatable (pro) MMP-2 and MMP-9 were determined with the BIA in 96-well plates, coated with monospecific antibodies to the MMPs, sample/standard incubation overnight and detection by modified MMP-sensitive pro-urokinase in combination with peptide substrate S-2444 and measurement of absorbance change at 405 nm over time. Activation of pro-MMPs was achieved by incubation with p-aminophenyl-mercuric acetate.
Enzyme-linked immunosorbent assay for MMP-2, MMP-7, MMP-8, MMP-9, TIMP-1 and TIMP-2
Antigen levels of MMP-2 and MMP-9 were determined using previously described ELISAs (Hanemaaijer et al, 1998). In brief, the same catching antibodies were used as for the BIAs. Next, appropriate dilutions of tissue homogenates were incubated overnight at 4°C. Immunodetection of MMP-2 and MMP-9 was performed directly or indirectly with in-house anti-MMP-2 and -MMP-9 biotinylated-polyclonal antibodies. Avidin–horseradish peroxidase and 3,3′,5,5′ tetramethyl benzidine were used for the colouration reaction. The respective amounts of MMP-2 and MMP-9 were calculated from standard curves. The concentrations of MMP-7, TIMP1 and TIMP-2 antigens were determined using commercial ELISAs according to the manufacturer's instructions (R&D Systems Europe, Abingdon, UK). The amount of MMP-8 was measured using a previously described ELISA (Bergmann et al, 1989).
Statistical analysis
Differences between normal and tumour values for all parameters were calculated using the Wilcoxon signed rank test. For the survival analyses, the clinicopathological parameters were dichotomised as described previously unless indicated elsewhere. Cutoff points for MMP data were optimised or medians were used. Univariate and multivariate survival analyses were performed with the Cox proportional hazards model, using the SPSS Windows Release 12.0.1. statistical package (2004, SPSS Inc., Chicago, IL, USA). Multivariate survival analyses were performed using the Cox proportional hazards method by separately adding the significant MMP variables to the dichotomised clinicopathological parameters. Overall and tumour-related survival curves were constructed using the method of Kaplan and Meier including the Log-rank test. Differences were considered significant when P⩽0.05.
RESULTS
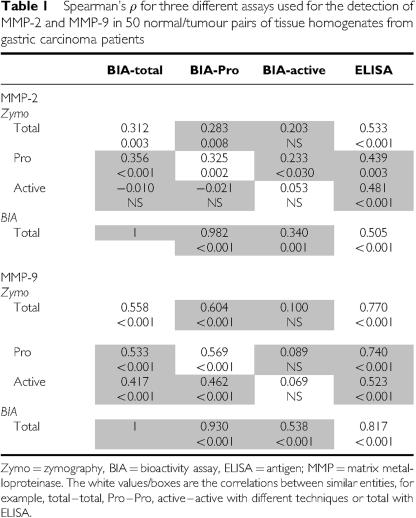
Although quantitative zymography is a reliable and sensitive technique to identify active and latent isoforms of MMP-2 and MMP-9, it is a laborious assay to perform. Therefore, we compared the previously obtained zymography data for MMP-2 and MMP-9 with the results from more practicable and sophisticated immunoassays, that is, BIAs and ELISAs. Table 1 shows an overview of the correlation coefficients and P-values for the different assays (samples n=100). The total zymography data, which consist of the sum of active and pro-form bands, correlated significantly with the total BIA and ELISA levels for both MMP-2 (0.312<ρ<0.533, P⩽0.003) and even better for MMP-9 (0.558<ρ<0.817, P<0.001). The latent pro-forms of MMP-2 and MMP-9, separately detected by zymography and BIA, also correlated significantly. No correlation between both assays was found, however, for active MMP-2 or MMP-9, indicating that the active isoform as identified by the very sensitive zymography is not necessarily functionally active in the less-sensitive BIA, probably through interaction with inhibitors.
Table 1. Spearman's ρ for three different assays used for the detection of MMP-2 and MMP-9 in 50 normal/tumour pairs of tissue homogenates from gastric carcinoma patients.
The levels of MMP-2 and MMP-9 as detected with the BIAs and ELISAs in normal mucosa and tumour tissue in the expanded group of 81 gastric carcinoma patients are shown in Table 2. Carcinomas contained significantly higher MMP-2 and MMP-9 levels in antigen as well as activity than adjacent normal tissue. Particularly remarkable is the presence of more active MMP-2, but not of active MMP-9, in the tumour tissue homogenates. The most impressive enhancement (>20-fold) in carcinomas compared to normal tissue, however, was noted for MMP-7 (Table 2). Matrix metalloproteinase-8 and TIMP-1 were also significantly increased, whereas tumour TIMP-2 levels were found not to be enhanced. Interestingly, a striking difference was observed in the correlation between the primary MMP–TIMP interactor antigen levels, that is, MMP-9 with TIMP-1 (ρ=0.358, P<0.0005) and MMP-2 with TIMP-2 (ρ=0.085, NS).
Table 2. Antigen levels (ng mg−1 protein) of MMP-2, MMP-7, MMP-8 and MMP-9 and of inhibitors TIMP-1 and TIMP-2 in normal mucosa and carcinoma of 81 patients with gastric cancer.
| Mucosa | Carcinoma | P-value | |
|---|---|---|---|
| MMP-2 | |||
| Antigen | 4.7±0.4 | 17.0±2.0 | ⩽0.001 |
| Total activitya | 81.1±23.6 | 185.7±45.5 | ⩽0.001 |
| Pro-forma | 78.9±23.6 | 181.1±45.3 | 0.001 |
| Activea | 2.3±0.5 | 4.7±1.1 | 0.02 |
| MMP-9 | |||
| Antigen | 9.0±0.9 | 24.7±2.3 | ⩽0.001 |
| Total activitya | 67.5±6.0 | 128.8±11.3 | ⩽0.001 |
| Pro-forma | 59.9±5.6 | 117.1±10.1 | ⩽0.001 |
| Activea | 7.6±1.5 | 9.5±2.1 | NS |
| MMP-7 | 2.0±0.5 | 47.1±12.4 | 0.002 |
| MMP-8 | 95±12 | 319±47 | ⩽0.001 |
| TIMP-1 | 8.0±0.8 | 16.9±1.3 | ⩽0.001 |
| TIMP-2 | 5.9±0.2 | 6.3±0.4 | NS |
Mean±s.e.m.
Bioactivity assay levels of MMP-2 and MMP-9 are expressed as units per mg protein.
As determined by BIA.
MMP=matrix metalloproteinase; BIA=bioactivity assay; TIMP=tissue inhibitors of MMP.
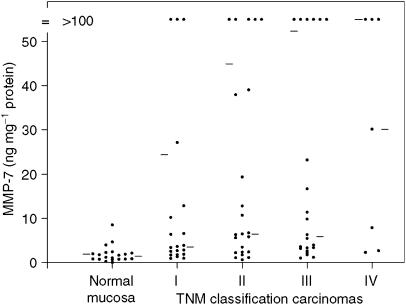
The levels of MMPs and TIMPs were also evaluated for correlation with all the clinicopathological parameters. Tumour levels of MMP-2, TIMP-1 and TIMP-2 did not show significant correlations with any of these parameters. The mean MMP-7 levels increased stepwise with TNM classification (Figure 1) and were significantly enhanced in Laurén's intestinal-type carcinomas compared to diffuse or mixed types (56±16 vs 34±22, P<0.02). Matrix metalloproteinase-8 levels were enhanced in Laurén's intestinal-type tumours (402±72 vs 178±29 ng mg−1 protein, P<0.006) and differentiated tumours (393±67 vs 163±29 ng mg−1 protein, P<0.002) according to the WHO classification. Matrix metalloproteinase-9 levels showed a similar enhancement for Laurén's intestinal-type carcinomas (BIA total activity 140 vs 99 U mg−1 protein, P<0.02; ELISA 29 vs 17 ng mg−1 protein, P<0.01) and differentiated tumours (BIA total activity 133±13 vs 104±16 U mg−1 protein, NS; ELISA 28±3 vs 17±3 ng mg−1 protein, P<0.02). Matrix metalloproteinase-9 total activity showed a stepwise decrease with TNM classification, which did not reach significance (I, 145±27; II 127±18; III, 120±20; IV, 114±28 U mg−1 protein).
Figure 1.
Relation between MMP-7 antigen levels and TNM classification in gastric carcinomas. The mean and median for the subgroups are indicated by bars on, respectively, the left- and right-hand side of each column.
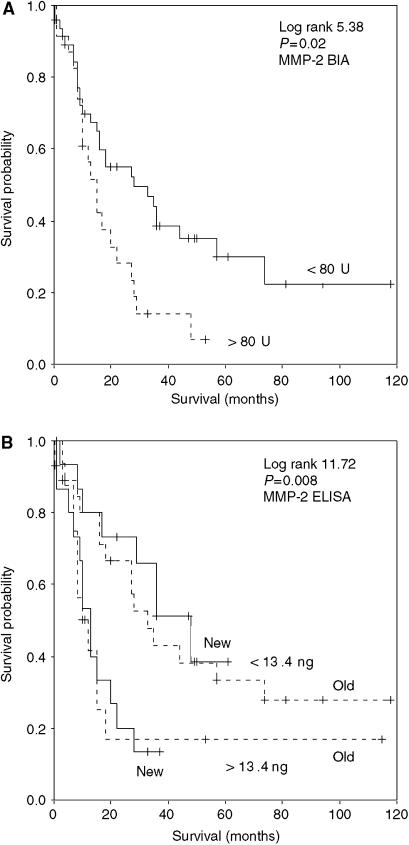
For tumour-associated survival analyses, all MMP and TIMP parameters in tumour homogenates were evaluated for optimal cutoff points using the log rank test. Significant cutoffs were only found for the MMP-2 levels by BIA and ELISA (Figures 2A and B). No significant association for MMP-7, MMP-8, MMP-9, TIMP-1 and TIMP-2 with tumour-associated survival was found according to stepwise univariate Cox analyses and thus the medians were used (hazard ratio and 95% confidence interval ranges of the median levels varied from 0.801 to 1.257 and from 0.445 to 2.307, respectively). High MMP-2 levels determined by BIA as well as ELISA were significantly associated with worse survival, but in multivariate analyses with the clinicopathological parameters, only the MMP-2 ELISA kept its independent prognostic value (Table 3). The consistent prognostic relevance of MMP-2 is underlined by Figure 2B, in which the old group of patients (n=50) and the more recent patients group (n=31) are independently subdivided based on a low or high MMP-2 antigen content of the carcinoma, using the same cutoff value. Similar results were obtained with the BIA data (not shown).
Figure 2.
Kaplan–Meier tumour-related overall survival curves for (A) MMP-2 BIA total activity, (B) MMP-2 ELISA old vs new gastric cancer patient groups, with the cutoff levels from the Cox analyses.
Table 3. Uni- and multivariate Cox's proportional hazards analyses of MMP-2, determined by ELISA and BIA, and clinicopathological parameters in relation to the overall tumour-related survival of 81 patients with gastric cancer.
|
Univariate
|
Multivariate
|
||||||
|---|---|---|---|---|---|---|---|
| Parameter | n | HR | CI 95% | P | HR | CI 95% | P |
| Gender | |||||||
| Male vs female | 60/21 | 1.384 | 0.768–2.494 | NS | 1.767 | 0.935–3.342 | NS |
| Age | |||||||
| Median (66 years) | 40/41 | 1.313 | 0.764–2.255 | NS | 1.467 | 0.775–2.774 | NS |
| TNM | |||||||
| I | 23/81 | 1 | — | 1 | — | ||
| II | 26/81 | 3.133 | 1.360–7.222 | 0.007 | 4.001 | 1.510–10.60 | 0.005 |
| III | 25/81 | 3.021 | 1.305–6.991 | 0.010 | 3.557 | 1.290–9.813 | 0.014 |
| IV | 7/81 | 7.387 | 2.495–21.86 | 0.000 | 20.416 | 4.992–83.49 | 0.000 |
| Laurén | |||||||
| Diffuse/mixed vs intestinal | 30/50 | 0.889 | 0.516–1.531 | NS | 1.152 | 0.353–3.756 | NS |
| WHO differentiation | |||||||
| Well vs poor | 54/26 | 1.133 | 0.650–1.975 | NS | 1.270 | 0.370–4.363 | NS |
| Borrmann | |||||||
| I+II vs III+IV | 55/24 | 1.118 | 0.609–2.053 | NS | 0.761 | 0.386–1.502 | NS |
| Localisation | |||||||
| Cardia vs rest | 36/45 | 0.573 | 0.330–0.993 | 0.034 | 0.330 | 0.159–0.682 | 0.003 |
| Diameter tumour | |||||||
| <5 vs >5 cm | 47/34 | 1.048 | 0.608–1.808 | NS | 0.622 | 0.337–1.149 | NS |
| Eosinophils | |||||||
| Few vs many | 56/24 | 1.035 | 0.568–1.886 | NS | 1.743 | 0.806–3.766 | NS |
| Intestinal metaplasia | |||||||
| Absent vs present | 39/42 | 0.490 | 0.280–0.858 | 0.013 | 0.706 | 0.379–1.315 | NS |
| Carcinoma | |||||||
| MMP-2 ELISA | |||||||
| <13.4 vs >13.4 ng mg−1 protein | 45/31 | 2.611 | 1.455–4.686 | 0.001 | 2.620 | 1.249–5.494 | 0.011 |
| MMP-2 BIA | |||||||
| <80 vs >80 U mg−1 protein | 49/23 | 1.974 | 1.089–3.577 | 0.025 | 1.493 | 0.655–3.404 | NS |
HR=hazard ratio; CI=confidence interval.
DISCUSSION
The present study corroborates our previous finding of increased MMP-2 in gastric cancer. The high MMP-2 antigen and activity levels were significantly associated with worse survival according to univariate Cox proportional hazards analysis. In the multivariate analysis, including a broad selection of clinical parameters, the MMP-2 antigen level kept its independent prognostic value, but the significance for the MMP-2 BIA activity level of the carcinomas was lost. The optimal cutoff point for MMP-2 antigen calculated for survival prognosis in the old group of patients was similarly predictive in the new group of patients, indicating the strength of MMP-2 as a prognostic indicator for gastric carcinoma patients. The notion that MMP-2 is a valuable indicator of gastric cancer progression and prognosis is supported by immunohistochemical, zymography and mRNA studies showing that MMP-2 is associated with tumour invasion, lymph node metastasis and survival (Allgayer et al, 1998; Mönig et al, 2001; Chuanzhong et al, 2002; Kabashima et al, 2002; Liu et al, 2002a; Elnemr et al, 2003; Yokoyama et al, 2004; Ji et al, 2005). The value of MMP-2 as an independent prognostic marker for gastric carcinomas is underscored by our observation that MMP-9, MMP-7, MMP-8, TIMP-1 and TIMP-2 have no prognostic relevance.
Matrix metalloproteinase-9 levels were enhanced in some clinicopathological subgroups of gastric cancer, that is, according to the Laurén classification and for WHO differentiation grade. The association between MMP-9 and early stages of gastric carcinoma, as shown before (Torii et al, 1998; Kabashima et al, 2000), was also present in our study. In contrast to our previous findings, high MMP-9 levels did not show a significant correlation with survival and also not for the ratio MMP-9/TIMP-1 (data not shown) as recently suggested (Zhang et al, 2003). One obvious explanation for the discrepancy with our previous data is the small number of patients in the study. However, the relatively high MMP-9 levels in early gastric carcinomas also might affect the relation between MMP-9 and prognosis, especially in our extended follow-up study using tumour-related survival.
Matrix metalloproteinase-7, MMP-8, TIMP-1 and TIMP-2 were included in the present study as comparisons to evaluate the prognostic strength of MMP-2 and MMP-9. Matrix metalloproteinase-7 was selected because MMP-7 production in various types of carcinomas has predominantly been found in tumour cells and because MMP-7 was recently suggested as potential marker for gastric carcinoma (Liu et al, 2002b). Although enhanced levels were found in the different carcinoma subgroups, for example, TNM stage and Laurén's intestinal type, there was no correlation between high MMP-7 levels and patients survival. This contrasts in part with several other studies reporting not only a clear association between MMP-7 expression and gastric cancer progression but also with survival (Liu et al, 2002b; Ajisaka et al, 2004). Essential differences with our study are, however, that the latter studies were carried out using immunohistochemistry, focusing on MMP-7-expressing carcinoma cells at the invasive front, whereas our ELISA antigen values were derived from representative overall parts of the tumours. Matrix metalloproteinase-8, like MMP-9, is mainly present in neutrophils in carcinomas. Therefore, the expected correlation in presence of MMP-8 and MMP-9 was confirmed by the high correlation between both antigen levels (ρ 0.810, P⩽0.001, n=158), and the similar distribution according to the different cancer subgroups. The lack of correlation with survival was, therefore, not surprising in this study, as described by others before (Yokoyama et al, 2004).
The levels of TIMP-1 were significantly enhanced in cancer tissue, but the previously found association between TIMP-1 levels in sections or homogenates from gastric cancer tissue with survival (Joo et al, 2000; Yoshikawa et al, 2001) was not observed in our group of patients. However, our group contained relatively less patients with advanced TNM stages, which could account for the different results compared with these former studies. In contrast to what was expected from in vitro studies (Koyama, 2004), we did not find differences between TIMP-2 levels in normal and cancerous tissue. Also, the levels between different tumour subgroups did not vary, indicating a rather constitutive expression of this inhibitor. As TIMP-2 immunohistochemical staining combined with in situ hybridisation experiments detected the expression of TIMP-2 in gastric cancer tissue, primarily in peritumoral stromal cells rather than in malignant cells (Joo et al, 2000), we conclude that the localisation of TIMP-2 within the cancerous tissue might be of crucial importance but apparently not the total amount of the inhibitor. The recently suggested role for TIMP-2 in the activation of pro-MMP-2 (Itoh et al, 2001), combined with the different cell types involved in the expression of MMP-2 and its main inhibitor TIMP-2 in gastric carcinoma, indicate the importance of local cell–cell and molecule–molecule interactions in the activation process. This is particularly noticeable from our finding that there is no correlation between MMP-2 and TIMP-2 levels in the tissue homogenates, where the increase in MMP-2 outbalances that of TIMP-2, resulting in an increased net MMP-2 activity in the tumours, an observation which can only be made by using the BIA. This process was not observed with MMP-9 and TIMP-1, where a more balanced increase was found in the tumours.
Although many in vitro studies, animal models and clinical studies clearly showed that MMPs are indeed involved in a number of critical steps during tumour growth and invasion, most synthetic MMP inhibitors, designed as anticancer agents, failed to improve patients outcome in clinical trials (Zucker et al, 2000), showing that our understanding of the working mechanisms of MMPs in tumour biology is still poor. Coincidentally, gastric cancer appeared to be one of the few cancers for which a significant survival benefit from therapy with a matrix metalloproteinase inhibitor has been described (Bramhall et al, 2002). Recent studies indicate that proteolytic MMP activity is involved in the uncovering or release of specific sites from macromolecules in the extracellular matrix (McCawley and Matrisian, 2001; Polette et al, 2004), which at least in vitro leads to various biological activities. Our study shows that an enhanced MMP-2 level is consistently and more strongly associated with prognosis of gastric cancer patients than other MMPs or TIMPs. This association might be caused by the noninvasion-related activities of MMPs, like cytokine release/activation, which makes MMP-2 in our opinion an important player in gastric cancer, deserving further investigation. Finally, differences in the association of the other MMPs and TIMPs with gastric cancer survival between our study and other reports, as mentioned above, might be related to differences in genetic background, that is, Caucasian vs Asian, which is currently under study.
Acknowledgments
We thank Professor Dr H Tschesche and A Oberpichler (Department of Biochemistry, University of Bielefeld, Germany) for kindly performing the MMP-8 ELISA.
References
- Ajisaka H, Yonemura Y, Miwa K (2004) Correlation of lymph node metastases and expression of matrix metalloproteinase-7 in patients with gastric cancer. Hepatogastroenterology 51: 900–905 [PubMed] [Google Scholar]
- Allgayer H, Babic R, Beyer BC, Grutzner KU, Tarabichi A, Schildberg FW, Heiss MM (1998) Prognostic relevance of MMP-2 (72-kDa collagenase IV) in gastric cancer. Oncology 55: 152–160 [DOI] [PubMed] [Google Scholar]
- Bergmann U, Michaelis J, Oberhoff R, Knauper V, Beckmann R, Tschesche H (1989) Enzyme linked immunosorbent assays (ELISA) for the quantitative determination of human leukocyte collagenase and gelatinase. J Clin Chem Clin Biochem 27: 351–359 [DOI] [PubMed] [Google Scholar]
- Bramhall SR, Hallissey MT, Whiting J, Scholefield J, Tierney G, Stuart RC, Hawkins RE, McCulloch P, Maughan T, Brown PD, Baillet M, Fielding JW (2002) Marimastat as maintenance therapy for patients with advanced gastric cancer: a randomised trial. Br J Cancer 86: 1864–1870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuanzhong Y, Ming G, Fanglin Z, Haijiao C, Zhen L, Shiping C, YongKang Z (2002) Real-time quantitative reverse transcription–PCR assay for renal cell carcinoma-associated antigen G250. Clin Chim Acta 318: 33–40 [DOI] [PubMed] [Google Scholar]
- Elnemr A, Yonemura Y, Bandou E, Kinoshita K, Kawamura T, Takahashi S, Tochiori S, Endou Y, Sasaki T (2003) Expression of collagenase-3 (matrix metalloproteinase-13) in human gastric cancer. Gastric Cancer 6: 30–38 [DOI] [PubMed] [Google Scholar]
- Hanemaaijer R, Verheijen JH, Maguire TM, Visser H, Toet K, McDermott E, O'Higgins N, Duffy MJ (2000) Increased gelatinase-A and gelatinase-B activities in malignant vs benign breast tumors. Int J Cancer 86: 204–207 [DOI] [PubMed] [Google Scholar]
- Hanemaaijer R, Visser H, Konttinen YT, Koolwijk P, Verheijen JH (1998) A novel and simple immunocapture assay for determination of gelatinase-B (MMP-9) activities in biological fluids: saliva from patients with Sjogren's syndrome contain increased latent and active gelatinase-B levels. Matrix Biol 17: 657–665 [DOI] [PubMed] [Google Scholar]
- Hermanek P, Sobin LH (1992) UICC TNM classification of malignant tumors, 4th edn. London: Springer [Google Scholar]
- Itoh Y, Takamura A, Ito N, Maru Y, Sato H, Suenaga N, Aoki T, Seiki M (2001) Homophilic complex formation of MT1-MMP facilitates proMMP-2 activation on the cell surface and promotes tumor cell invasion. EMBO J 20: 4782–4793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji F, Chen YL, Jin EY, Wang WL, Yang ZL, Li YM (2005) Relationship between matrix metalloproteinase-2 mRNA expression and clinicopathological and urokinase-type plasminogen activator system parameters and prognosis in human gastric cancer. World J Gastroenterol 11: 3222–3226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joo YE, Seo KS, Kim HS, Rew JS, Park CS, Kim SJ (2000) Expression of tissue inhibitors of metalloproteinases (TIMPs) in gastric cancer. Dig Dis Sci 45: 114–121 [DOI] [PubMed] [Google Scholar]
- Kabashima A, Maehara Y, Kakeji Y, Baba H, Koga T, Sugimachi K (2000) Clinicopathological features and overexpression of matrix metalloproteinases in intramucosal gastric carcinoma with lymph node metastasis. Clin Cancer Res 6: 3581–3584 [PubMed] [Google Scholar]
- Kabashima A, Yao T, Sugimachi K, Tsuneyoshi M (2002) Relationship between biologic behavior and phenotypic expression in intramucosal gastric carcinomas. Hum Pathol 33: 80–86 [DOI] [PubMed] [Google Scholar]
- Koyama S (2004) Enhanced cell surface expression of matrix metalloproteinases and their inhibitors, and tumor-induced host response in progression of human gastric carcinoma. Dig Dis Sci 49: 1621–1630 [DOI] [PubMed] [Google Scholar]
- Liu LX, Liu ZH, Jiang HC, Qu X, Zhang WH, Wu LF, Zhu AL, Wang XQ, Wu M (2002a) Profiling of differentially expressed genes in human gastric carcinoma by cDNA expression array. World J Gastroenterol 8: 580–585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu XP, Kawauchi S, Oga A, Tsushimi K, Tsushimi M, Furuya T, Sasaki K (2002b) Prognostic significance of matrix metalloproteinase-7 (MMP-7) expression at the invasive front in gastric carcinoma. Jpn J Cancer Res 93: 291–295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCawley LJ, Matrisian LM (2001) Matrix metalloproteinases: they're not just for matrix anymore!. Curr Opin Cell Biol 13: 534–540 [DOI] [PubMed] [Google Scholar]
- Mönig SP, Baldus SE, Hennecken JK, Spiecker DB, Grass G, Schneider PM, Thiele J, Dienes HP, Holscher AH (2001) Expression of MMP-2 is associated with progression and lymph node metastasis of gastric carcinoma. Histopathology 39: 597–602 [DOI] [PubMed] [Google Scholar]
- Polette M, Nawrocki-Raby B, Gilles C, Clavel C, Birembaut P (2004) Tumour invasion and matrix metalloproteinases. Crit Rev Oncol Hematol 49: 179–186 [DOI] [PubMed] [Google Scholar]
- Sier CF, Kubben FJ, Ganesh S, Heerding MM, Griffioen G, Hanemaaijer R, van Krieken JH, Lamers CB, Verspaget HW (1996) Tissue levels of matrix metalloproteinases MMP-2 and MMP-9 are related to the overall survival of patients with gastric carcinoma. Br J Cancer 74: 413–417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torii A, Kodera Y, Ito M, Shimizu Y, Hirai T, Yasui K, Morimoto T, Yamamura Y, Kato T, Hayakawa T, Fujimoto N, Kito T (1998) Matrix metalloproteinase 9 in mucosally invasive gastric cancer. Gastric Cancer 1: 142–145 [DOI] [PubMed] [Google Scholar]
- Yokoyama T, Nakamura H, Otani Y, Kubota T, Fujimoto N, Seiki M, Kitajima M, Okada Y (2004) Differences between scirrhous and non-scirrhous human gastric carcinomas from the aspect of proMMP-2 activation regulated by TIMP-3. Clin Exp Metast 21: 223–233 [DOI] [PubMed] [Google Scholar]
- Yoshikawa T, Tsuburaya A, Kobayashi O, Sairenji M, Motohashi H, Yanoma S, Noguchi Y (2001) Intratumoral concentrations of tissue inhibitor of matrix metalloproteinase 1 in patients with gastric carcinoma: a new biomarker for invasion and its impact on survival. Cancer 91: 1739–1744 [DOI] [PubMed] [Google Scholar]
- Zhang S, Li L, Lin JY, Lin H (2003) Imbalance between expression of matrix metalloproteinase-9 and tissue inhibitor of metalloproteinase-1 in invasiveness and metastasis of human gastric carcinoma. World J Gastroenterol 9: 899–904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zucker S, Cao J, Chen WT (2000) Critical appraisal of the use of matrix metalloproteinase inhibitors in cancer treatment. Oncogene 19: 6642–6650 [DOI] [PubMed] [Google Scholar]