Abstract
Gastric adenocarcinoma (GA) is a significant cause of mortality worldwide. The molecular mechanisms of GA remain poorly characterised. Our aim was to characterise the functional activity of the computationally identified genes, NET 1 and MYEOV in GA. Digital Differential Display was used to identify genes altered expression in GA-derived EST libraries. mRNA levels of a subset of genes were quantitated by qPCR in a panel of cell lines and tumour tissue. The effect of pro- and anti-inflammatory stimuli on gene expression was investigated. Cell proliferation and invasion were measured using in an in-vitro GA model following inhibition of expression using siRNA. In all, 23 genes not previously reported in association with GA were identified. Two genes, Net1 and Myeov, were selected for further analysis and increased expression was detected in GA tissue compared to paired normal tissue using quantitative PCR. siRNA-mediated downregulation of Net1 and Myeov resulted in decreased proliferation and invasion of gastric cancer cells in vitro. These functional studies highlight a putative role for NET1 and Myeov in the development and progression of gastric cancer. These genes may provide important targets for intervention in GA, evidenced by their role in promoting invasion and proliferation, key phenotypic hallmarks of cancer cells.
Keywords: Net1, Myeov, gastric cancer, DDD
Gastric cancer is the second most common cause of cancer-related mortality worldwide, with highest incidence in Japan and China (Parkin et al, 2001). While the incidence of gastric cancer has been falling in recent decades, the overall 5-year survival for gastric cancer remains poor, with European rates varying from 12 to 28% (Newnham et al, 2003). This poor survival, despite advances in both diagnostic and therapeutic modalities, is primarily due to the advanced stage of disease at presentation. Adenocarcinomas represent the majority of gastric cancers and histologically, can be classified into two distinct types – intestinal and diffuse (Faivre et al, 1998). Intestinal type gastric adenocarcinoma (GA) is the most common and is believed to arise as a result of chronic inflammation inducing intestinal metaplasia, subsequent dysplasia and finally cancer (Lauren, 1965). Diffuse-type GA is characterised by both solitary or poorly organised clusters of mucin-rich cells and a diffuse infiltrating growth pattern. Unlike intestinal type GA, no identifiable precancerous lesions occur. Several environmental factors have been linked to the development of GA, the most significant being the presence of chronic Helicobacter Pylori infection (Correa, 1992). While the molecular events underlying the pathogenesis of GA have not been fully elucidated, mutations in, and altered expression of several genes involved in several important cellular processes have been identified, such as the oncogenes bcl-2, β-Catenin, c-erbB-2, cyclinE, K-ras, K-sam, c-Met; the tumour suppressor genes APC, DCC, p53, E-Cadherin, p16, p53; and growth factors such as VEGF and EGF (Ming, 1998). Furthermore, high-level microsatellite instability, as a result of alteration in DNA mismatch repair genes, is seen in approximately 15% of diffuse and familial gastric cancer (El-Omar et al, 2000; Engel et al, 2003; Nardone, 2003).
Recent advances in the field of molecular biology, genomics and bioinformatics have ushered in a new era in cancer research. One can now study global gene expression patterns in specific tissues or cell types. This approach can be used to identify novel cancer markers, oncogenes, tumour suppressor genes, and potential therapeutic targets. Digital Differential Display (DDD) is a web-based bioinformatics tool for generating differential gene expression profiles between cDNA tissue libraries (http://www.ncbi.nlm.nih.gov/UniGene/ddd.cgi). Using the EST profiles of normal and disease cDNA libraries represented in the NCBI UniGene database, DDD compares the number of times that ESTs from different libraries, or pools of libraries, are assigned to a specific UniGene cluster (Scheurle et al, 2000; Dennis et al, 2002; Pontius et al, 2003).
Herein, we describe the expression and functional characterisation of the DDD identified gastric cancer-associated genes Net 1 and Myeov. We verified the expression of these genes in an in-vitro GA model and a panel of ex-vivo GA and adjacent normal tissue using real-time PCR. Having determined the changes in expression of these genes in GA, their functional importance was assessed using a gene knockdown-based approach. Specifically, siRNA duplexes were used to knockdown mRNA expression of Net1 and Myeov. This approach induced alterations in the cellular phenotype as evidenced by perturbed cell invasion and proliferation, key endpoints in the molecular phenotype of GA.
MATERIALS AND METHODS
Cell culture and cell treatments
An in vitro model of gastric cancer was established using three GA cell lines: AGS (ECACC, UK), Kato III (ECACC, UK) and 23132/87 (DSMZ, Germany). The AGS and 23132/87 cell lines were established from primary tumours while the KatoIII was derived from metastatic tissue (Sekiguchi et al, 1978; Barranco et al, 1983; Vollmers et al, 1993). AGS cells were cultured in Hams F12 medium containing 10% fetal bovine serum (FBS). KatoIII cells were grown in RPMI 1640 containing 20% FBS and 23132/87 cells were cultured in RPMI 1640 medium containing 10% FBS. All medium was supplemented with 2 mM L-glutamine, 1 U ml−1 penicillin and 1 μg ml−1 streptomycin. All cells were incubated in a humid 5% CO2 atmosphere at 37°C. To investigate the effect of pro- and anti-inflammatory stimuli on gene expression, AGS cells were treated separately with 0, 0.3, 1, 3 and 10 ng ml−1 interleukin-1β, TNF-α and dexamethasone for 4 h under the growth conditions described above. AGS cells were also treated with or without 10 ng ml−1 of each treatment for 0, 2, 4, 6 and 8 h. Total RNA was obtained from normal human alveolar epithelial cells (AEC) (Promocell).
Human tissue
With informed patient consent according to a protocol approved by the local ethics committee, representative samples of GA and adjacent normal gastric mucosa were collected at endoscopy and surgery and immediately stored in an RNAse inhibitor (RNAlater, Sigma-Aldrich, Ireland) at −20°C. Details of the tumour histology, stage, patient age and gender were noted. In addition to these samples total RNA derived from both GA tissue and matched adjacent normal mucosa was obtained (Ambion, UK; Biochain Institute Inc, Hayward, CA, USA; and Stratagene Europe, Amsterdam, The Netherlands). Details of all tissue used in this study are listed in Table 1.
Table 1. Matched gastric adenocarcinoma and normal tissue specimens used in this study.
| Sample | Gender | Age (years) | Histology | Clinical stage |
|---|---|---|---|---|
| 1 | Male | 57 | Adenocarcinoma | Ia |
| 2 | Male | 63 | Poorly differentiated adenocarcinoma | Not available |
| 3 | Female | 69 | Adenocarcinoma | Not available |
| 4 | Male | 76 | Moderately differentiated adenocarcinoma | IV |
| 5 | Male | 70 | Poorly differentiated adenocarcinoma | Not available |
| 6 | Female | 73 | Moderately differentiated adenocarcinoma | IV |
| 7 | Male | 71 | Poorly differentiated adenocarcinoma | Not available |
RNA extraction and PCR
TRIzol™ (Sigma-Aldrich, Ireland) was used to extract RNA as previously described (Murray et al, 2004). For extraction from tissue, 1 ml TRIzol™ was added and the tissue was homogenised at 15 000 r.p.m. using a POLYTRON PT 2100 rotor-stator (Kinematica Inc, Newark, NJ, USA). For extraction from cells in culture, cells were washed in PBS and 1 ml of TRIzol™ was added to cells and left for 10 min at room temperature with occasional shaking. A measure of 2 μg of total RNA was treated with DNAse I and reverse transcribed using random hexamers and SuperScript II reverse transcriptase (Invitrogen Ltd, UK). Primers were designed using Primer3 software (http://frodo.wi.mit.edu/cgi-bin/primer3/primer3_www.cgi) and synthesised by Sigma-Aldrich, Ireland. The sequences of primers used for PCR were β-actin forward: 5′-GTC ACC TTC ACC GTT CCA G-3′, reverse: 5′-CTC TTC CAG CCT TCC TTC CT-3′, Net1 forward: 5′-CTG TTC ACC TCG GGA CAT TT-3′, reverse: 5′-TGG AGC TGT CAG ACG TTT TG-3′, Myeov forward: 5′-GGG CTC AGT GAA GAG TCT GG-3′, reverse: 5′-CACACC ACG GAG ACA ATG AC-3′, TNFa forward: 5′-TGG TGT GGG TGA GGA GTA CA-3′, TNFa reverse: 5′-AGC CCA TGT TGT AGC AAA CA-3′, MKP1 forward: 5′-TCC TGC CCT TTC TGT ACC TG-3′, MKP1 reverse: 5′-ATG AAG TCA ATG GCC TCG TT-3′. polymerase chain reaction was carried out in a 50 μl mix containing 0.5 μl of Taq polymerase (Invitrogen, UK) and 1 μl of cDNA. Polymerase chain reaction products were run on a 2% agarose gel with a parallel 100 bp DNA ladder (Promega, UK). Real-time PCR was carried out according to the manufacturers' instructions using the LightCycler RNA SYBR Green 1 Amplification Kit (Roche Applied Science) together with the Light Cycler instrument. All measurements were independently repeated three times. The maximum concentration of total RNA template used was 0.5 μg μl−1. The following components were added to 1 μl of total RNA in a 20 μl capillary tube: 10.2 μl PCR-grade H2O, 4 μl SYBR Green 1 reaction mix, 2.4 μl 25 mM MgCl2, 0.4 μl RT–PCR enzyme mix and 1 μl each of forward and reverse primers. The reaction conditions were as follows: reverse transcription: 55°C for 10 min followed by denaturation: 95°C for 30 s. Forty-five PCR cycles were then run at denaturation: 95°C for 10 s; annealing: (β-actin: 54°C, Net1: 52°C, Myeov 56°C) for 10 s and extension: 72°C for 13 s. Data analysis using the delta Ct method was performed using the LightCycler version 4.0 software. β-actin expression levels were used to normalise Net1 and Myeov expression.
Gene silencing by RNA interference
Two siRNA duplexes were designed and synthesised for silencing each of the genes Net1 and Myeov (Qiagen Inc. CA, USA). The duplexes had the following sequences: Net1(1) sense, 5′-GGA GGA UGC UAU AUU GAU A-3′; Net1(1) antisense, 5′-UAU CAA UAU AGC AUC CUC C-3′; Net1(2) sense, 5′-GGU GUG GAU UGA UUG GAA A-3′; Net1(2) antisense 5′-UUU CCA AUC AAU CCA CAC C-3′; Myeov(1) sense, 5′-GGA UGU AAG UUA UCA ACU A-3′; Myeov(1) antisense, 5′-UAG UUG AUA ACU UAC AUC C-3′; Myeov(2) sense, 5′-CCA UGA GGU AGC UAC UAA A-3′ and Myeov(2) antisense, 5′-UUU AGU AGC UAC CUC AUG G-3′. A chemically synthesised non-silencing siRNA duplex with the following sequence; sense, 5′-UUC UCC GAA CGU GUC ACG U-3′; antisense, 5′-ACG UGA CAC GUU CGG AGA A-3′ that had no known homology with any mammalian gene was used to control for nonspecific silencing events. A total of 6 × 104 AGS cells were added to each well of a 24-well plate in 500 μl growth media and incubated under the standard conditions of 37°C and 5% CO2 in a humid incubator for 24 h. A volume of 98 μl growth medium was mixed with 0.5 μg siRNA (or a combination of 0.25 μg of each) and 3 μl RNAifect (Qiagen). Following incubation, media was removed from the cells and this mix was added dropwise. A volume of 300 μl growth medium was added and the cells were incubated for 48 h under standard conditions before either being assayed as described below of before RNA was extracted as described.
Cell proliferation assay
A colorimetric MTS assay (Promega) was used to assess the effect of Net1 and Myeov knockdown on AGS cell proliferation. Following siRNA knockdown, 5 × 104 of either control or siRNA-treated cells were added, in triplicate, to each well of a 96-well micro-plate. The total volume was adjusted to 100 μl with growth medium and 20 μl of MTS reagent was added to each well. The micro-plate was incubated for 2 h at 37°C and 5% CO2 and absorbance at 492 nm was read using a Rosys Anthos 2010 plate reader (http://www.promega.com/tbs/tb169/tb169.pdf).
In vitro invasion assay
Biocoat Matrigel invasion chambers (BD Biosciences, USA) were used to investigate the effect of siRNA-mediated Myeov and Net1 gene silencing on the in vitro invasiveness of the AGS gastric cell line as previously described (Murray et al, 2004). Briefly, 5 × 104 AGS cells were seeded into the upper chamber of the 24-well invasion chamber in medium containing 1% serum. A volume of 750 μl of medium containing 20% FBS was added to the outer chambers to act as a chemoattractant for the cells. The plates were then incubated for 48 h in a 5% CO2 humidified 37°C incubator. Following incubation cells that had invaded through the membrane were fixed and stained before the membrane was removed and mounted on a slide for microscopic assesment. Invasive cells were visualised at × 40 magnification and the number of cells in five random fields were counted and an average calculated.
Statistical methods
A student's t-test was used to compare the expression profiles, based on RT–PCR mRNA levels, of selected genes in matched GA and normal tissues, and in the 3 GA cell lines. A P-value of <0.05 was taken to indicate significant alterations.
RESULTS
Differential expression of Net1 and Myeov in gastric cancer cell lines and human tissue
Digital differential display (DDD) was used to compare 185 pooled normal and 201 pooled adult cancer tissue EST libraries with 11 pooled gastric cancer tissue libraries, details of which are listed in Table 2. All gastric cancer tissue libraries analysed were derived from primary tumours. This comparison identified 23 genes whose expression was altered in gastric cancer-derived material (Table 3). Digital differential display was used to calculate the fold difference in the representation of these genes between libraries. Two genes (Net1 and Myeov) not previously studied in gastric cancer were selected for further investigation using an in vitro GA model and a panel of matched ex vivo GA tissue and adjacent normal tissue. Our group has shown elevated expression of Myeov, a putative oncogene, initially described in Multiple Myeloma (Janssen et al, 2000), in colorectal adenomas and cancer compared to normal colonic mucosa (Moss et al, 2004). Net1 is a member of the guanine nucleotide exchange factor (GEF) family which are involved, through their regulation of RhoA activity, in a range of biological processes including cell proliferation, apoptosis, differentiation and cytoskeletal reorganisation (Rossman et al, 2005).
Table 2. Details of pooled gastric cancer tissue EST libraries studied.
| dbEST library ID | Title | Tissue | Protocol | Histology | Tumour site | Cell morphology |
|---|---|---|---|---|---|---|
| 10299 | S4SNU1 | Cell line | Uncharacterised | Adenocarcinoma, poorly differentiated | Primary | Lympoblast-like |
| 10301 | S5SNU484 | Cell line | Uncharacterised | Adenocarcinoma, poorly differentiated | Primary | Epithelial |
| 10302 | S5SNU484s1 | Cell line | Subtracted | Adenocarcinoma, poorly differentiated | Primary | Epithelial |
| 10305 | S7SNU719 | Cell line | Uncharacterised | Adenocarcinoma, moderately differentiated | Primary | Epithelial |
| 10306 | S7SNU719s1 | Cell line | Subtracted | Adenocarcinoma, moderately differentiated | Primary | Epithelial |
| 10310 | S10SNU1 | Cell line | Uncharacterised | Adenocarcinoma, poorly differentiated | Primary | Lympoblast-like |
| 10311 | S11SNU1 | Cell line | Uncharacterised | Adenocarcinoma, poorly differentiated | Primary | Lympoblast-like |
| 10324 | S21SNU520 | Cell line | Uncharacterised | Adenocarcinoma, poorly differentiated | Primary | Floating aggregates |
| 10325 | S21SNU520s1 | Cell line | Subtracted | Adenocarcinoma, poorly differentiated | Primary | Floating aggregates |
| 1449 | NCI_CGAP_Gas4 | Bulk | Non-normalised | Adenocarcinoma, poorly differentiated | Primary | Bulk tissue |
| 3637 | ST0240 | Bulk | Uncharacterised | Carcinoma | Primary | Bulk tissue |
Table 3. Novel genes identified by DDD.
| Symbol | Name | UniGene ID | GAa | Non cancera | Cancera | Function |
|---|---|---|---|---|---|---|
| AGR2 | Arginase, type II | Hs.226391 | 0.00242 | 0.00012 | 0.00017 | Urea cycle |
| ANKRD9 | Ankyrin repeat domain 9 | Hs.432945 | 0.00098 | 0.00002 | 0.00004 | Function unknown |
| ARHGEF16 | Rho guanine exchange factor 16 | Hs.87435 | 0.00032 | 0.00002 | 0.00002 | Function unknown |
| BENE | BENE protein | Hs.185055 | 0.00059 | 0.00003 | 0.00004 | Function unknown |
| C8G | Complement component 8, gamma polypeptide | Hs.1285 | 0.00016 | 0.00000 | 0.00000 | Complement activation |
| CLDN18 | Claudin 18 | Hs.278966 | 0.00043 | 0.00003 | 0.00001 | Tight junction component |
| CLDN2 | Claudin 2 | Hs.16098 | 0.00023 | 0.00002 | 0.00002 | Tight junction component |
| EPS8L3 | EPS8-like 3 | Hs.5366 | 0.00021 | 0.00001 | 0.00002 | Receptor activity |
| FBXL6 | F-box and leucine-rich repeat protein 6 | Hs.12271 | 0.00037 | 0.00002 | 0.00003 | Ubiquitin cycle |
| GEFT | RAC/CDC42 exchange factor | Hs.61581 | 0.00027 | 0.00002 | 0.00003 | Cell proliferation |
| KIAA1706 | KIAA1706 protein | Hs.412318 | 0.00034 | 0.00003 | 0.00001 | DNA binding |
| KREMEN2 | Kringle containing transmembrane protein 2 | Hs.73452 | 0.00043 | 0.00000 | 0.00004 | Wnt signaling pathway |
| MEIS4 | Likely ortholog of mouse myeloid ecotropic viral integration site-related gene 2 | Hs.356135 | 0.00043 | 0.00002 | 0.00004 | Transcription regulation |
| MYEOV | Myeloma overexpressed gene | Hs.436000 | 0.00048 | 0.00000 | 0.00004 | Nucleic acid binding |
| NET1 | Neuroepithelial cell transforming gene 1 | Hs.25155 | 0.00100 | 0.00008 | 0.00008 | Regulation of cell growth |
| NOS3 | Nitric-oxide synthase activity | Hs.511603 | 0.00027 | 0.00002 | 0.00001 | Nitric-oxide synthase activity |
| PARD6G | Par-6 partitioning defective 6 homolog gamma (Caenorhabditis elegans) | Hs.223584 | 0.00037 | 0.00001 | 0.00001 | Cytokinesis |
| PTK2B | Protein tyrosine kinase 2 beta | Hs.438975 | 0.00094 | 0.00004 | 0.00005 | Signal transduction |
| S100A14 | S100 calcium binding protein A14 | Hs.288998 | 0.00041 | 0.00001 | 0.00003 | Calcium ion binding |
| SMCX | Smcx homolog, X chromosome (mouse) | Hs.103381 | 0.00066 | 0.00004 | 0.00004 | Transcription regulation |
| THBS3 | Thrombospondin 3 | Hs.169875 | 0.00046 | 0.00004 | 0.00003 | Cell-matrix adhesion |
| UNC93A | Unc-93 homolog A (C. elegans) | Hs.267749 | 0.00027 | 0.00000 | 0.00000 | Function unknown |
| WBSCR21 | Williams beuren syndrome chromosome region 21 | Hs.182476 | 0.00043 | 0.00002 | 0.00004 | Aromatic compound metabolism |
Fraction of sequences mapping to UniGene cluster.
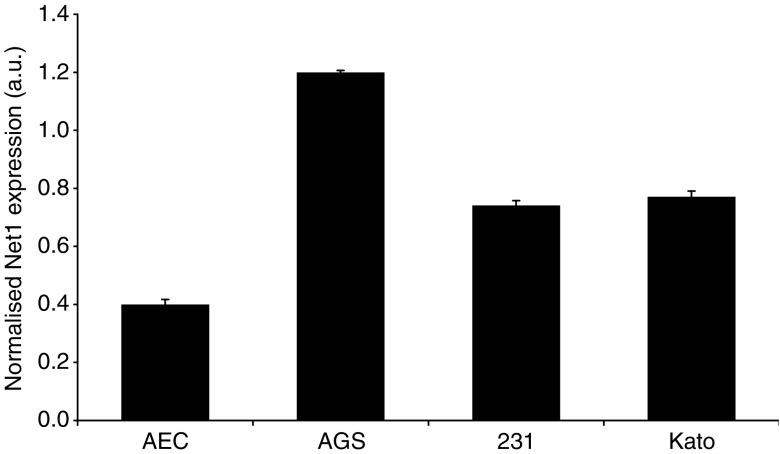
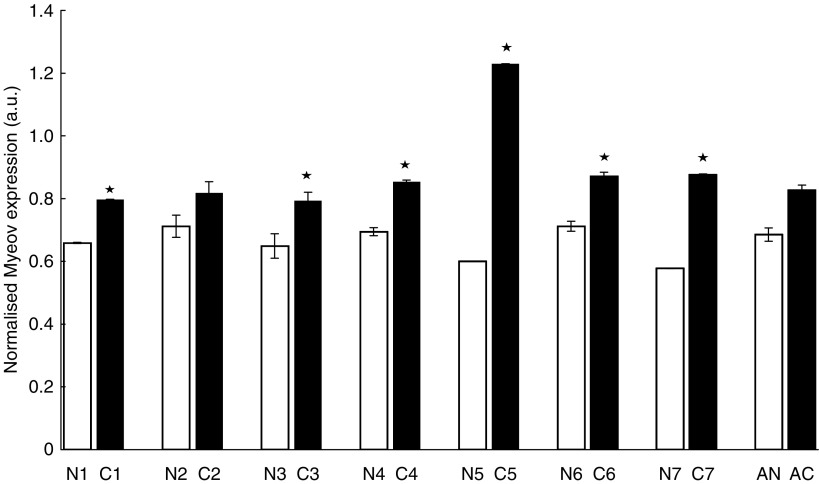
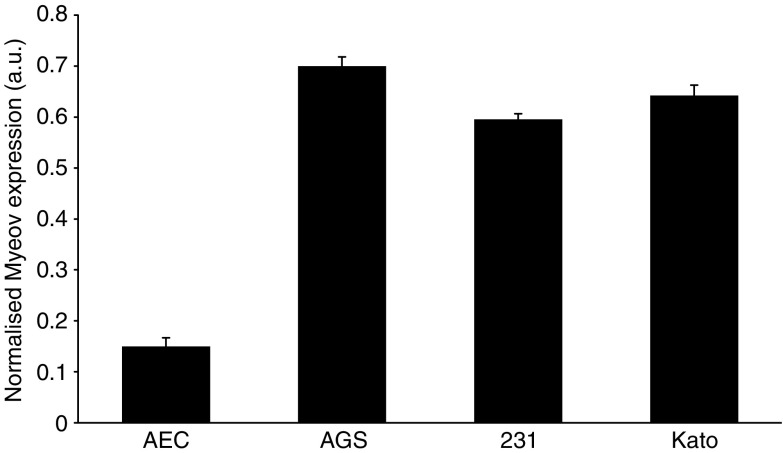
To investigate the expression of Net1 and Myeov in gastric cancer, real-time PCR was used to determine mRNA levels in tissue and cells lines. Using β-actin mRNA expression to normalise, quantitative PCR confirmed elevated levels of Net1 expression in all cancer tissue specimens studied, in comparison with adjacent normal tissue (Figure 1). Net1 expression was significantly increased by 34, 32, 58, 25 and 30% in five of the seven paired tumour tissue samples studied (P<0.05). This confirmed the enhanced expression identified using DDD. Expression of Net1 was shown in three separate gastric cancer cell lines, namely AGS, 23132 and KatoIII (Figure 2). Net1 expression in a non-cancerous airway alveolar epithelial cell line (AEC) was less than that in the gastric cancer cell lines. Similarly, increased Myeov expression in gastric cancer tissue in comparison with normal tissue from the same patient was confirmed using real-time PCR (Figure 3). All gastric cancer tissue studied, expressed higher levels of Myeov mRNA than adjacent normal tissue. Myeov expression was significantly increased by 17, 18, 19, 51, 18 and 34% in six of the seven tumour tissue samples studied in comparison with adjacent normal tissue (P<0.05). The increased levels of Myeov in gastric cancer tissue confirmed the observations made using DDD. Myeov expression was also detected in all gastric cancer cell lines studied (Figure 4). The expression of Myeov in AEC cells was not as high as its expression in gastric cancer cell lines.
Figure 1.
Net1 expression in paired gastric normal and tumour tissue. Real-time PCR was used to investigate the levels of Net1 expression in matched gastric cancer (C) and normal (N) tissue. Each tissue specimen was analysed in triplicate for mRNA levels. β-actin expression was used to normalise the data. *(P<0.05). Average Net1 expression in cancer (AC) and normal (AN) tissue is displayed.
Figure 2.
Net1 expression in normal epithelial and gastric cancer cell lines. Real-time PCR was used to determine the relative expression levels of Net1 mRNA in normal alveolar epithelial cells (AEC) and in three gastric cancer cell lines (AGS, 23132/87 (231) and KatoIII (Kato). Each sample was analysed in triplicate for mRNA levels. β-actin expression was used to normalise the data.
Figure 3.
Myeov expression in paired gastric normal and tumour tissue. Real-time PCR was used to investigate the levels of Myeov expression in paired gastric cancer (C) and normal (N) tissue. Each tissue specimen was analysed in triplicate for mRNA levels. β-actin expression levels were used to normalise the data. β-actin expression was used to normalise all data. *(P<0.05). Average Myeov expression in cancer (AC) and normal (AN) tissue is displayed.
Figure 4.
Myeov expression in normal epithelial and gastric cancer cell lines. Real-time PCR was used to determine the relative expression levels of Myeov mRNA in normal alveolar epithelial cells (AEC) and in three gastric cancer cell lines (AGS, 23132/87 (231) and KatoIII (Kato). Each sample was analysed in triplicate and β-actin expression was used to normalise the data.
The expression of Net1 and Myeov in response to inflammatory stimuli
Inflammation is a key process underpinning the progression of GA (Kai et al, 2005), thus the putative role of inflammatory mediators in driving Net1 and Myeov expression was further investigated. Cells were treated with specific pro- and anti-inflammatory stimuli to investigate to effect on Net1 and Myeov expression in response to inflammation. Net1 mRNA expression increased in a dose- and time-responsive manner to treatment with TNFα (Figure 5). Treatment with 10 ng ml−1 TNFα resulted in a threefold increase in Net1 expression after 4 h (P<0.05). Treatment with IL-1b or dexamethasone did not have a significant effect on Net1 expression (Figure 5). Treatment with either TNFα, IL1β or dexamethasone did not have a significant effect on Myeov expression (Figure 5). This data suggests that these genes respond in a stimulus-specific manner.
Figure 5.
The effect of pro- and anti-inflammatory stimuli on Net1 and Myeov expression in gastric cancer. (A) The effect of 10 ng ml−1 interleukin-1β (IL-1B), TNF-alpha (TNFa) and Dexamethasone (DEX) on Net1 and Myeov expression in AGS gastric cancer cells was monitored using real-time PCR. Cells were treated for 4 h. Enhanced TNFa expression was used as a positive response to TNFa and IL1B treatment (Gallagher et al, 2003). Enhanced MKP1 expression was used a positive response to DEX treatment (Lasa et al, 2002). Apart from the positive controls, the only trteatment to have a significant effect was TNFα, which resulted in a threefold increase in Net1 mRNA expression *(P<0.05). Enhanced TNFa-induced Net1 expression resulted in a dose-dependent (B) and time-dependent (C) manner *(P<0.05).
Effect of Net1 and Myeov knockdown on cellular proliferation
Having identified enhanced expression of Net1 and Myeov in GA, the functional consequence of their expression was studied. Specifically, the functional effect of Net1 and Myeov gene knockdown using a silencing RNA approach was determined. Using AGS gastric cancer cells, siRNA was used to suppress gene expression. Two siRNA duplexes were designed to target each transcript and gene silencing was confirmed using real-time PCR. Using two separate siRNA duplexes, Net1 expression was reduced by 49 and 41% (Figure 6A) (P<0.05). Combining both duplexes resulted in a 55% reduction in Net1 expression. Cells in which Net1 expression was reduced had significantly lower proliferation rates in compassion with the same cells in which Net1 mRNA was unperturbed (Figure 6B). Using both siRNA duplexes seperataly, cell proliferation was decreased by 47 and 41% in comparison with control cells (P<0.05). Cell proliferation was decreased by 58% using both duplexes in combination. Similarly, two siRNA duplexes were used to supress Myeov expression by 100 and 30% (Figure 7A) (P<0.05) which in turn led to 68 and 36% reduction in gastric cancer-cell proliferation (Figure 7B) (P<0.05). Using both siRNA duplexes in combination resulted in a 75% decrease in Myeov expression (P<0.05) and a 73% decrease in AGS cell proliferation (P<0.05). These data demonstrate that the enhanced expression of Net1 and Myeov in the setting of GA has a significant effect on gastric epithelial tumour cell biology.
Figure 6.
The effect of Net1 knockdown on gastric adenocarcinoma cell proliferation and invasion. (A) Real-time PCR was used to confirm the significant siRNA-mediated reduction in Net1 expression. Using two siRNA duplexes; Net1(1) and Net1(2), Net 1 expression was reduced by 49 and 41%, respectively, in comparison with control cells. Using a combination of both siRNA duplexes; Net1(1+2) resulted in 55% decreased Net1 expression (P<0.05). (B) The proliferation of cells treated with both duplexes was compared with control cells using an MTS assay. siRNA mediated Net1 downregulation resulted in 47 and 41% decrease in cell proliferation. Using both Net1 siRNA duplexes in combination resulted in 58% decreased cell proliferation (P<0.05). (C) Downregulation of Net1 expression using both siRNA duplexes resulted in 100 and 96% reduction in cell invasion in comparison with control cells. A combination of both siRNA molecules resulted in 96% AGS cell invasion *(P<0.05).
Figure 7.
The effect of siRNA-mediated Myeov knockdown on AGS cell proliferation and invasion. (A) Confirmation of Myeov downregulation using real-time PCR. Myeov downregulation, using two separate siRNA duplexes, Myeov(1) and Myeov(2), resulted in 100 and 30% decreased expression when compared with control cells. A combination of both Myeov siRNA molecules resulted in 75% decreased Myeov expression (P<0.05). (B) Using siRNA duplexes, cell proliferation was reduced 68 and 36%, respectively, when compared with control cells. Using both duplexes in combination resulted in 73% decrease in gastric cancer cell proliferation (P<0.05). (C) Similarly, cell invasion was reduced by 85 and 90% and by 99% using a combination of both siRNA duplexes in comparison with control cells *(P<0.05).
Effect of Net1 and Myeov knockdown on in vitro invasion
To further demonstrate the putative role of Net1 and Myeov in gastric cancer, the effect of RNAi-mediated mRNA downregulation on the in vitro invasion of AGS cells was monitored. The Net1-targetted siRNA duplexes resulted in 49 and 41% knockdown in mRNA expression (Figure 6A) and the same duplexes caused 100 and 96% decrease in cell invasion (Figure 6C) (P<0.05). Using both siRNA suplexes in combination resulted in a 55% reduction in Net1 mRNA expression and a 96% reduction in in vitro cell invasion (P<0.05). Reduction in Myeov mRNA by 100 and 30% using two separate RNAi duplexes (Figure 7A) resulted in 85 and 90% decreased cell invasion (Figure 7C) (P<0.05). Using both siRNA molecules resulted in a 75% reduction in Myeov expression and a 99% decrease in AGS cell invasion (P<0.05).
DISCUSSION
Gastric adenocarcinoma is a significant global cause of morbidity and mortality, with no satisfactory therapy available. Therefore, the development of novel diagnostic and prognostic markers, coupled with enhanced therapeutic options is a key goal for the cancer research community. This goal can only be realistically achieved by further improving our knowledge of the gastric tumour at a molecular level. Examination of EST libraries from both gastric tumours and normal tissue identified a cohort of genes with differential representation in cancer-derived libraries.
Neuroepithelial transforming gene-1 (Net1) is a guanine nucleotide exchange factor (GEF) that activates Rho family proteins (Symons and Rusk, 2003). The Net1 gene was originally isolated in a tissue culture screen for novel oncogenes in NIH 3T3 fibroblasts (Chan et al, 1996; Alberts and Treisman, 1998). GEFs regulate Rho- GTPases, a main branch of the Ras superfamily of small (∼21 kDa) GTPases. Rho proteins, once activated, stimulate signalling in multiple pathways by binding to downstream effector proteins, modulating their activities and thereby regulating a range of cellular processes including cell proliferation, apoptosis, differentiation and cytoskeletal reorganisation. They are also thought to play a role in transformation and metastasis (Yoshioka et al, 1999; Etienne-Manneville and Hall, 2002; Jaffe and Hall, 2002; Sahai and Marshall, 2002). Other GEFs with established roles in various malignancies include Bcr (Advani and Pendergast, 2002) Tiam (Malliri et al, 2002) and Vav1 (Fernandez-Zapico et al, 2005). Owing to the importance of GEFs in normal cellular processes and in malignancies and also because there is no published evidence to support its role in the gastric cancer setting Net1 was chosen for further validation and investigation.
mRNA expression determination, using real-time PCR, confirmed elevated Net1 expression in gastric cancer tissue in comparison with normal tissue (Figure 1). Net1 was further shown to be expressed in three separate gastric cancer cell lines (Data not shown). In this study, Net1 expression was responsive in a dose- and time-dependent manner to the pro-inflammatory cytokine TNF-α (Figure 5). There was no significant effect on Net1 expression in response to treatment with IL1β or with the anti-inflammatory steroid dexamethasone. These data suggest that in the disease setting, Net1 expression is increased in response to inflammation, a common driving factor of GA. To delineate the functional role of Net1 in gastric cancer, RNAi technology was employed to knockdown expression and the effect on cellular proliferation and invasion was monitored. Net1 is a RhoA-specific GEF (Kawasaki et al, 2003). Elevated levels of Rho activity have been shown to downregulate the cell cycle regulator p21/Waf1 thus inducing cell proliferation in Swiss-3T3 cells (Sahai et al, 2001). Recently, it has been demonstrated that abolition of RhoA expression in AGS cells resulted in decreased proliferation (Liu et al, 2004). In this study a reduction in Net1 expression resulted in a significant decrease in the proliferation of GA cells (Figure 4B). Using two siRNA duplexes that targeted Net1 mRNA, AGS cells with Net1 knockdown showed 47 and 41% decreased proliferation, in comparison with control cells in which Net1 expression was unchanged. Net 1 is a GEF for RhoA, which has been previously shown to promote the cell cycle by inducing cyclin D1 expression via the AP-1 transcription factor (Hill et al, 1995; Marinissen et al, 2001). Increased cyclin D1 expression has been shown to be associated with uncontrolled gastric cancer cell proliferation (Song et al, 2003). Cyclin D1 is a critical regulator of normal progression of cells through G1-S transition via the activity of cyclin D1-dependent regulatory proteins (Shie et al, 2000; Yu et al, 2000). In addition, in a study utilising a mouse model in which hyperproliferation/hyperplasia of the colon was induced, cyclin D1 protein levels measured in tissue extracts were significantly enhanced (Sellin et al, 2001). Furthermore, it has been demonstrated in a clinical analysis that nearly 50% of tissue samples examined from 70 colorectal cancer patients expressed increased levels of cyclin D1 (Utsunomiya et al, 2001). These studies suggest that one of the potential mechanisms of tumour growth promotion may include the disregulation of cyclin D1.
The in vitro invasion of AGS gastric cancer cells following Net1 knockdown was also assessed. siRNA-mediated Net1 knockdown using two separate duplexes significantly reduced the invasive capacity of these cells by 100 and 96% in comparison with control cells in which the Net1 expression was unaltered (Figure 4C). Invasion is an essential event in the malignancy of gastric cancer and this finding further underpins the role of Net1 in mediating this process.
Given the importance of Rho proteins in the motility of normal cells and their aberrant regulation in transformed cells, it is likely that they are involved in the invasion of tumour cells. RhoA has been shown to induce the AP-1 transcription factor, which is known to regulate matrix metalloproteinase (MMP) expression (Benbow and Brinckerhoff, 1997; Marinissen et al, 2004). The roles of MMPs in malignant cancers are well established, where elevated expression occurs in areas of active invasion (Sternlicht and Bergers, 2000). Rho proteins have been shown to mediate MMP-2 activation and enhance cell invasion (Zhuge and Xu, 2001). It is therefore likely that the RhoA-mediated increase in AP-1 activity leads to increased MMP expression thus supporting the motile and invasive phenotype. As well as increased ECM turnover and proteolytic activity, cyoskeletal reorganisation is a crucial aspect of cellular locomotion and its disregulation is often a hallmark of invasive tumour cells. RhoA can regulate the function of the ERM (ezrin, radixin, moesin) family of adaptor proteins (Matsui et al, 1998). ERM proteins promote cell motility by functioning as molecular linkers between the plasma membrane and the actin cytoskeleton through their association with the cell adhesion molecule CD44 (Tsukita et al, 1994; Bretscher et al, 2002; Martin et al, 2003). CD44 upregulation has been reported in various invasive tumours and have previously demonstrated that CD44 activation leads to enhanced MMP expression and increased cell invasion in colorectal cancer (Murray et al, 2004). RhoA function therefore promotes both cell motility and ECM turnover, thus linking two key metastatic events. It is therefore likely that elevated levels of Net1 in gastric cancers favours tumour proliferation and invasion through RhoA activation. The specific mechanisms of Net1 activation remain to be established and the exact role of Net1 in controlling proliferation and invasion requires further investigation.
Myeov was initially identified as a transforming gene through the application of a NIH/3T3 tumorigenicity assay to DNA from a gastric carcinoma however its role in gastric cancer remains unclear (Janssen et al, 1999). Myeov maps to the 11q13 region. This region of chromosome 11 contains several genes, most notably CyclinD1 and ESM1/Cortactin, which are frequently overexpressed, mainly through amplification, in a variety of human cancers (Schuuring, 1995; Ormandy et al, 2003). Myeov has been shown to be activated concurrently with CyclinD1 in multiple myeloma, breast cancer and esophageal squamous cell carcinomas (Janssen et al, 2002a, 2002b). Sequence analysis predicted Myeov is a 313-amino-acid protein containing no known functional motifs except for an RNP1 motif typical of RNA-binding proteins (Kai et al, 2005). Herein we have evaluated the expression and biological significance of Myeov in human gastric cancer to determine its role in the disease process.
Real-time PCR confirmed elevated Myeov mRNA levels in gastric cancer tissue and cell lines in comparison with its expression in normal tissue (Figure 2A). AGS cells were treated with specific pro- and anti-inflammatory cytokines, yet neither had a significant effect on Myeov expression (Figure 5), suggesting that the role of Myeov in GA is independent of inflammation and not driven by it. The significance of enhanced Myeov expression in gastric cancer was then investigated. As GA cells are characterised by their increased proliferative and invasive capabilities, these two functional end points were monitored in vitro. Using two duplexes, RNAi-mediated knockdown of Myeov in human gastric cancer cells resulted in 68 and 36% decreased proliferation in comparison with control cells in which Myeov expression was unaltered (Figure 5B). The role of Myeov in the invasivness of gastric cancers was highlighted by the decreased in vitro invasion of gastric cancer cells in which Myeov expression had been suppressed using siRNA (Figure 5C). Using two siRNA duplexes that targeted Myeov mRNA, cell invasion was decreased by 85 and 90% in comparison with control cells.
As Myeov is co-expressed with Cyclin D1, we propose that both genes are co-regulated and may share activity in advancing the neoplastic process. Cyclin D1 upregulation has been implicated as a driver of gastric cancer, and its inhibition has been shown to reverse the transformed phenotype of human gastric cancer cells (Chen et al, 1999). The degree of cyclin D1 overexpression has been correlated with invasive stages of gastric cancer (Oda et al, 1999).
In this study we have employed comparison of EST libraries to identify genes whose expression is putatively altered in GA. DDD. Net1 and Myeov expression was confirmed in gastric cancer tissue and cell lines. siRNA-mediated knockdown of both genes resulted in significantly decreased cell proliferation and invasion. The data presented herein suggest regulatory roles for the in silico identified genes Net1 and Myeov in the setting of gastric cancer. The functional consequences of knockdown of the genes provide important clues to the activity of Net1 and Myeov in GA and further study may establish the roles for these genes in initiation and progression of this disease. Further investigations will doubtlessly lend more weight to the potential of these genes as therapeutic targets in GA.
Acknowledgments
This work was supported by the Irish Cancer Society, and the Irish Government's Programme for Research in Third Level Institutions.
References
- Advani AS, Pendergast AM (2002) Bcr–Abl variants: biological and clinical aspects. Leuk Res 26: 713–720 [DOI] [PubMed] [Google Scholar]
- Alberts AS, Treisman R (1998) Activation of RhoA and SAPK/JNK signalling pathways by the RhoA-specific exchange factor mNET1. EMBO J 17: 4075–4085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barranco SC, Townsend Jr CM, Casartelli C, Macik BG, Burger NL, Boerwinkle WR, Gourley WK (1983) Establishment and characterization of an in vitro model system for human adenocarcinoma of the stomach. Cancer Res 43: 1703–1709 [PubMed] [Google Scholar]
- Benbow U, Brinckerhoff CE (1997) The AP-1 site and MMP gene regulation: what is all the fuss about? Matrix Biol 15: 519–526 [DOI] [PubMed] [Google Scholar]
- Bretscher A, Edwards K, Fehon RG (2002) ERM proteins and merlin: integrators at the cell cortex. Nat Rev Mol Cell Biol 3: 586–599 [DOI] [PubMed] [Google Scholar]
- Chan AM, Takai S, Yamada K, Miki T (1996) Isolation of a novel oncogene, NET1, from neuroepithelioma cells by expression cDNA cloning. Oncogene 12: 1259–1266 [PubMed] [Google Scholar]
- Chen B, Zhang XY, Zhang YJ, Zhou P, Gu Y, Fan DM (1999) Antisense to cyclin D1 reverses the transformed phenotype of human gastric cancer cells. World J Gastroenterol 5: 18–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correa P (1992) Human gastric carcinogenesis: a multistep and multifactorial process – First American Cancer Society Award Lecture on Cancer Epidemiology and Prevention1. Cancer Res 52: 6735–6740 [PubMed] [Google Scholar]
- Dennis JL, Vass JK, Wit EC, Keith WN, Oien KA (2002) Identification from public data of molecular markers of adenocarcinoma characteristic of the site of origin. Cancer Res 62: 5999–6005 [PubMed] [Google Scholar]
- El-Omar EM, Carrington M, Chow WH, McColl KE, Bream JH, Young HA, Herrera J, Lissowska J, Yuan CC, Rothman N, Lanyon G, Martin M, Fraumeni Jr JF, Rabkin CS (2000) Interleukin-1 polymorphisms associated with increased risk of gastric cancer. Nature 404: 398–402 [DOI] [PubMed] [Google Scholar]
- Engel LS, Chow WH, Vaughan TL, Gammon MD, Risch HA, Stanford JL, Schoenberg JB, Mayne ST, Dubrow R, Rotterdam H, West AB, Blaser M, Blot WJ, Gail MH, Fraumeni Jr JF (2003) Population attributable risks of esophageal and gastric cancers. J Natl Cancer Inst 95: 1404–1413 [DOI] [PubMed] [Google Scholar]
- Etienne-Manneville S, Hall A (2002) Rho GTPases in cell biology. Nature 420: 629–635 [DOI] [PubMed] [Google Scholar]
- Faivre J, Forman D, Esteve J, Gatta G (1998) Survival of patients with oesophageal and gastric cancers in Europe. EUROCARE Working Group. Eur J Cancer 34: 2167–2175 [DOI] [PubMed] [Google Scholar]
- Fernandez-Zapico ME, Gonzalez-Paz NC, Weiss E, Savoy DN, Molina JR, Fonseca R, Smyrk TC, Chari ST, Urrutia R, Billadeau DD (2005) Ectopic expression of VAV1 reveals an unexpected role in pancreatic cancer tumorigenesis. Cancer Cell 1: 39–49 [DOI] [PubMed] [Google Scholar]
- Gallagher J, Howlin J, McCarthy C, Murphy EP, Bresnihan B, FitzGerald O, Godson C, Brady HR, Martin F (2003) Identification of Naf1/ABIN-1 among TNF-alpha-induced expressed genes in human synoviocytes using oligonucleotide microarrays. FEBS Lett 551: 8–12 [DOI] [PubMed] [Google Scholar]
- Hill CS, Wynne J, Treisman R (1995) The Rho family GTPases RhoA, Rac1, and CDC42Hs regulate transcriptional activation by SRF. Cell 81: 1159–1170 [DOI] [PubMed] [Google Scholar]
- Jaffe AB, Hall A (2002) Rho GTPases in transformation and metastasis. Adv Cancer Res 84: 57–80 [DOI] [PubMed] [Google Scholar]
- Janssen JW, Braunger J, Ballas K, Faust M, Siebers U, Steenvoorden AC, Bartram CR (1999) Spectrum of transforming sequences detected by tumorigenicity assay in a large series of human neoplasms. Int J Cancer 80: 857. [DOI] [PubMed] [Google Scholar]
- Janssen JW, Cuny M, Orsetti B, Rodriguez C, Valles H, Bartram CR, Schuuring E, Theillet C (2002a) MYEOV: a candidate gene for DNA amplification events occurring centromeric to CCND1 in breast cancer. Int J Cancer 102: 608–614 [DOI] [PubMed] [Google Scholar]
- Janssen JW, Imoto I, Inoue J, Shimada Y, Ueda M, Imamura M, Bartram CR, Inazawa J (2002b) MYEOV, a gene at 11q13, is coamplified with CCND1, but epigenetically inactivated in a subset of esophageal squamous cell carcinomas. J Hum Genet 47(9): 460–464 [DOI] [PubMed] [Google Scholar]
- Janssen JW, Vaandrager JW, Heuser T, Jauch A, Kluin PM, Geelen E, Bergsagel PL, Kuehl WM, Drexler HG, Otsuki T, Bartram CR, Schuuring E (2000) Concurrent activation of a novel putative transforming gene, myeov, and cyclin D1 in a subset of multiple myeloma cell lines with t(11;14)(q13;q32). Blood 95: 2691–2698 [PubMed] [Google Scholar]
- Kai H, Kitadai Y, Kodama M, Cho S, Kuroda T, Ito M, Tanaka S, Ohmoto Y, Chayama K (2005) Involvement of proinflammatory cytokines IL-1beta and IL-6 in progression of human gastric carcinoma. Anticancer Res 25: 709–713 [PubMed] [Google Scholar]
- Kawasaki Y, Sato R, Akiyama T (2003) Mutated APC and Asef are involved in the migration of colorectal tumour cells. Nature Cell Biol 5: 211–215 [DOI] [PubMed] [Google Scholar]
- Lasa M, Abraham SM, Boucheron C, Saklatvala J, Clark AR (2002) Dexamethasone causes sustained expression of mitogen-activated protein kinase (MAPK) phosphatase 1 and phosphatase-mediated inhibition of MAPK p38. Mol Cell Biol 22: 7802–7811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauren P (1965) The two histological main types of gastric carcinoma: diffuse and so-called intestinal-type carcinoma. An attempt at a histo-clinical classification. Acta Pathol Microbiol Scand 64: 31–49 [DOI] [PubMed] [Google Scholar]
- Liu N, Bi F, Pan Y, Sun L, Xue Y, Shi Y, Yao X, Zheng Y, Fan D (2004) Reversal of the malignant phenotype of gastric cancer cells by inhibition of RhoA expression and activity. Clin Cancer Res 10: 6239–6247 [DOI] [PubMed] [Google Scholar]
- Malliri A, van der Kammen RA, Clark K, van der Valk M, Michiels F, Collard JG (2002) Mice deficient in the Rac activator Tiam1 are resistant to Ras-induced skin tumours. Nature 417: 867–871 [DOI] [PubMed] [Google Scholar]
- Marinissen MJ, Chiariello M, Gutkind JS (2001) Regulation of gene expression by the small GTPase Rho through the ERK6 (p38-γ) MAP kinase pathway. Genes Dev 15: 535–553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marinissen MJ, Chiariello M, Tanos T, Bernard O, Narumiya S, Gutkind JS (2004) The small GTP-binding protein RhoA regulates c-jun by a ROCK-JNK signaling axis. Mol Cell 14: 29–41 [DOI] [PubMed] [Google Scholar]
- Martin TA, Harrison G, Mansel RE, Jiang WG (2003) The role of the CD44/ezrin complex in cancer metastasis. Crit Rev Oncol Hematol 46: 165–186 [DOI] [PubMed] [Google Scholar]
- Matsui T, Maeda M, Doi Y, Yonemura S, Amano M, Kaibuchi K, Tsukita S, Tsukita S (1998) Rho-kinase phosphorylates COOH-terminal threonines of ezrin/radixin/moesin (ERM) proteins and regulates their head-to-tail association. J Cell Biol 140: 647–657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ming SC (1998) Cellular and molecular pathology of gastric carcinoma and precursor lesions: a critical review. Gastric Cancer 1: 31–50 [DOI] [PubMed] [Google Scholar]
- Moss A, Madden S, Mulligan AM, O'Keane C, Henger A, Kretzler M, Brady HR, Doran P, MacMathuna P (2004) Validation of data-mining in identifying novel genes in the colonic polyp-cancer sequence. Endoscopy 36: 477 [Google Scholar]
- Murray D, Morrin M, McDonnell S (2004) Increased invasion and expression of MMP-9 in human colorectal cell lines by a CD44-dependent mechanism. Anticancer Res 24: 489–494 [PubMed] [Google Scholar]
- Nardone G (2003) Molecular basis of gastric carcinogenesis. Aliment Pharmacol Ther 17: 75–81 [DOI] [PubMed] [Google Scholar]
- Newnham A, Quinn MJ, Babb P, Kang JY, Majeed A (2003) Trends in the subsite and morphology of oesophageal and gastric cancer in England and Wales 1971–1998. Alimentary Pharmacol Therapeutics 17: 665–676 [DOI] [PubMed] [Google Scholar]
- Oda K, Okabayashi T, Kataoka M, Takeda A, Shibuya Y, Orita K, Tanaka N (1999) Evaluation of cyclin D1 mRNA expression in gastric and colorectal cancers. Res Commun Mol Pathol Pharmacol 105: 237–252 [PubMed] [Google Scholar]
- Ormandy CJ, Musgrove EA, Hui R, Daly RJ, Sutherland RL (2003) Cyclin D1, EMS1 and 11q13 amplification in breast cancer. Breast Cancer Res Treat 78: 323–335 [DOI] [PubMed] [Google Scholar]
- Parkin DM, Bray FI, Devesa SS (2001) Cancer burden in the year 2000. The global picture. Eur J Cancer 37: 4–66 [DOI] [PubMed] [Google Scholar]
- Pontius JU, Wagner L, Schuler GD (2003) UniGene: a unified view of the transcriptome. The NCBI Handbook, available at http://www.ncbi.nlm.nih.gov/books/bv.fcgi?rid=handbook.chapter.857
- Rossman KL, Der CJ, Sondek J (2005) GEF means go: turning on RHO GTPases with guanine nucleotide-exchange factors. Nat Rev Mol Cell Biol 6: 167–180 [DOI] [PubMed] [Google Scholar]
- Sahai E, Marshall CJ (2002) RHO-GTPases and cancer. Nat Rev Cancer 2: 133–142 [DOI] [PubMed] [Google Scholar]
- Sahai E, Olson MF, Marshall CJ (2001) Cross-talk between Ras and Rho signalling pathways in transformation favours proliferation and increased motility. EMBO J 20: 755–766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheurle D, DeYoung MP, Binninger DM, Page H, Jahanzeb M, Narayanan R (2000) Cancer gene discovery using digital differential display. Cancer Res 60: 4037–4043 [PubMed] [Google Scholar]
- Schuuring E (1995) The involvement of the chromosome 11q13 region in human malignancies: cyclin D1 and EMS1 are two new candidate oncogenes – a review. Gene 159: 83–96 [DOI] [PubMed] [Google Scholar]
- Sekiguchi M, Sakakibara K, Fujii G (1978) Establishment of cultured cell lines derived from a human gastric carcinoma. Jpn J Exp Med 48: 61–68 [PubMed] [Google Scholar]
- Sellin JH, Umar S, Xiao J, Morris AP (2001) Increased betacatenin expression and nuclear translocation accompany cellular hyperproliferation in vivo. Cancer Res 61: 2899–2906 [PubMed] [Google Scholar]
- Shie JL, Chen ZY, Fu M, Pestell RG, Tseng CC (2000) Gut enriched Kruppel-like factor represses cyclin D1 promoter activity through Sp1 motif. Nucleic Acids Res 28: 2969–2976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song DH, Rana B, Wolfe JR, Crimmins G, Choi C, Albanese C, Wang TC, Pestell RG, Wolfe MM (2003) Gastrin-induced gastric adenocarcinoma growth is mediated through cyclin D1. Am J Physiol Gastrointest Liver Physiol 285: 217–222 [DOI] [PubMed] [Google Scholar]
- Sternlicht MD, Bergers G (2000) Matrix metalloproteinases as emerging targets in anti-cancer therapy: status and prospects. Emerging Ther Targets 4: 609–633 [Google Scholar]
- Symons M, Rusk N (2003) Control of vesicular trafficking by Rho GTPases. Curr Biol 13: 409–418 [DOI] [PubMed] [Google Scholar]
- Tsukita S, Oishi K, Sato N, Sagara J, Kawai A, Tsukita S (1994) ERM family members as molecular linkers between the cell surface glycoprotein CD44 and actin-based cytoskeletons. J Cell Biol 126: 391–401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Utsunomiya T, Doki Y, Takemoto H, Shiozaki H, Yano M, Sekimoto M, Tamura S, Yasuda T, Fujiwara Y, Monden M (2001) Correlation of beta-catenin and cyclin D1 expression in colon cancers. Oncology 61: 226–233 [DOI] [PubMed] [Google Scholar]
- Vollmers HP, Stulle K, Dammrich J, Pfaff M, Papadopoulos T, Betz C, Saal K, Muller-Hermelink HK (1993) Characterization of four new gastric cancer cell lines. Virchows Arch B Cell Pathol Inc Mol Pathol 63: 335–343 [DOI] [PubMed] [Google Scholar]
- Yoshioka K, Nakamori S, Itoh K (1999) Overexpression of small GTPbinding protein RhoA promotes invasion of tumor cells. Cancer Res 59: 2004–2010 [PubMed] [Google Scholar]
- Yu B, Lane ME, Pestell RG, Albanese C, Wadler S (2000) Downregulation of cyclin D1 alters cdk 4- and cdk 2-specific phosphorylation of retinoblastoma protein. Mol Cell Biol Res Commun 3: 352–359 [DOI] [PubMed] [Google Scholar]
- Zhuge Y, Xu J (2001) Rac1 mediates type I collagen-dependent MMP-2 activation: role in cell invasion across collagen barrier. J Biol Chem 276: 16248–16256 [DOI] [PubMed] [Google Scholar]