Abstract
Overexpression of the transcriptional activator β-catenin, mostly owing to loss-of-function mutations of the adenomatous polyposis coli (APC) tumour suppressor gene, is crucial for the initiation and progression of human colorectal carcinogenesis. Securin is a regulator of chromosome separation and its overexpression has been shown to be involved in different tumour-promoting processes, like transformation, hyperproliferation and angiogenesis, and correlates with tumour cell invasion. However, the molecular mechanism leading to securin overexpression in human colorectal cancer is unknown. Here we show a correlated high expression of β-catenin and securin (hPTTG1) in colorectal adenomas and carcinomas and further demonstrate that securin is a target of β-catenin transcriptional activation. This implies that deregulation of the β-catenin/T-cell factor-signalling pathway leads to overexpression of securin in human colorectal cancer, which subsequently may contribute to tumour progression.
Keywords: colorectal carcinoma, β-catenin, human pituitary tumour transforming gene, hPTTG1, Wnt-signalling
Loss-of-function mutations in the adenomatosis polyposis coli (APC) tumour suppressor gene, resulting in deregulation of the WNT-signalling pathway, has been associated with the progression of the majority of colorectal carcinomas. The effect is an accumulation of the coactivator protein β-catenin, which translocates to the nucleus and functions as a composite transcription factor together with the DNA-binding proteins of the T-cell factor (TCF/LEF) family (Korinek et al, 1997). Several genes important for development and tumorigenesis have been reported to be regulated by β-catenin/TCF (see for review Brabletz et al, 2002).
The human securin (human pituitary tumour transforming gene, hPTTG1) is an oncogene with little expression in normal adult colon tissue (Zhang et al, 1999) in contrast to strong expression in colorectal cancer (Heaney et al, 2000) and other human tumours (Dominguez et al, 1998; Kakar, 1998; Shibata et al, 2002) and several carcinoma cell lines (Kakar, 1998; Zhang et al, 1999). Securin is essential for sister chromatid separation during mitosis thereby regulating cell proliferation and chromosome stability (Zou et al, 1999). Loss of securin function increases aneuploidy (Jallepalli et al, 2001; Wang et al, 2001) and apoptosis, in part by modulating p53 function (Yu et al, 2000; Bernal et al, 2002; Hamid and Kakar, 2004). When overexpressed, securin increases c-myc expression and results in elevated cell proliferation and non-tumour cell transformation (Zhang et al, 1999; Hamid and Kakar, 2004). Furthermore, high expression of securin has been shown to induce angiogenesis, possibly by activating basic fibroblast growth factor and vascular endothelial growth factor expression (Zhang et al, 1999; Ishikawa et al, 2001), and to correlate with tumour cell invasion and metastasis (Zhang et al, 1999; Heaney et al, 2000; Ramaswamy et al, 2003). Therefore, securin may contribute to three important ‘hallmarks of cancer’ (Hanahan and Weinberg, 2000): transformation (‘self-sufficient growth’), angiogenesis and invasion.
The molecular mechanism of securin regulation in colorectal cancer is unknown. We investigated whether deregulation of the β-catenin/TCF-signalling pathway leading to the accumulation of β-catenin in colorectal carcinoma causes high expression of securin in these tumours, which may contribute to tumour progression and poor prognosis.
MATERIALS AND METHODS
Tissue specimen and immunohistochemistry
Formalin-fixed, paraffin-embedded colorectal adenocarcinomas from patients who underwent surgery without additional treatments were retrieved from the archive of the Department of Pathology, University of Erlangen-Nürnberg. The study comprised 30 cases. Immunohistochemistry for β-catenin and Ki-67 was performed as described previously (Hlubek et al, 2004). For securin staining, a mouse anti-securin antibody (1 : 25; NovoCastra NCL-SECURIN clone DCS-280.2, Newcastle, UK) and microwave treatment for antigen retrieval was used.
RNA isolation and DNA microarray analysis
Detailed protocols and results will be published elsewhere (Hlubek et al, in preparation). This study comprised well characterised moderate-to-well differentiated colorectal adenocarcinomas and mucosa from six patients. Total RNA was extracted from each tissue sample using the RNeasy kit (Qiagen, Hilden, Germany) including an DNA digest according to the manufacturer's instructions. Linear amplification of RNA was carried out with the MessageAmp aRNA kit (Ambion, Huntingdon, UK). Biotin-labelled cRNA was hybridised to the HG-U133A oligonucleotide microarray (22283 probe sets, Affymetrix, Santa Clara, CA, USA). All reactions were performed according to the Affymetrix protocol.
For statistical analysis, the signals of each probe set were summarised to a single expression value according to the standard protocol of the chip manufacturer (MAS 5.0). The expression values were transformed to log-2 scale and normalised by a non-linear procedure based on a scatterplot smoother (Cleveland, 1977). All samples under investigation were normalised against one expression signature that was used as reference. We made use of Welch's two-sample t-test in order to detect differential expression between tumour tissue (T) and normal mucosa (N). P-values were calculated and corrected in the multiple testing context in order to obtain control over the false discovery rate (FDR), that is, the fraction of false positives in a candidate list of probe sets. Making use of the Benjamini–Hochberg procedure (Benjamini and Hochberg, 1995) we identified a list of 3018 candidates from a total number of 22 283 probe sets (set FDR=5%).
Special attention was paid to targets of the Wnt-signalling pathway. A list of known β-catenin/TCF target genes was obtained from the homepage of Joel Nusse (http://www.stanford.edu/~rnusse/wntwindow.html) and expanded by recent reports found in the literature (see for review Brabletz et al, 2005). Our list contained a total number of 45 β-catenin/TCF target genes of which 42 were represented by one or more probe sets on the GeneChip array. We identified a cluster of 13 target genes (including securin) that are (i) significantly regulated in the sense that they are represented by at least one probe set contained in the list of 3018 candidates and (ii) upregulated in tumour tissue compared to normal mucosa.
Electromobility shift assay
The following sense and corresponding antisense oligonucleotides were annealed and used as probes or for competition (500- and 100-fold molar excess): securin-TBE: 5′-ATAAATCACTATCAAAGGATAGAATTT-3′; securin-mutTBE: 5′-ATAAATCA CTGCCAAAGGAT AGAATTT-3′. Probes were end labelled and incubated with 0.5 μg of bacterially expressed GST-TCF-4 DBD (DNA-binding domain codon 265–496) or GST alone as described previously (Hlubek et al, 2004).
DNA clones
The securin promoter–reporter plasmid (pGL-Sec. fl.) was constructed by amplification of the primers U1 and D1 described previously (Kakar, 1999) using the bacterial artificial chromosome clone #RP11-35508 (BioCat, Heidelberg, Germany) as template. The PCR product (nt −1336 to +34; GenBank accession number AF167560) was cloned into the pGL3basic (Promega, Mannheim, Germany) reporter vector. For construction of the securin promoter fragment (nt −1336 to 625; pGL-Sec frag.), the primers U1 and PTTG1-625 (5′-TTAAAAAATAAATCGAGAGGCTTT-3′) were used. Plasmids provided by other reseachers are as follows: pGST-TCF4(DBD), pcDNA/hTCF4 and pcDNA/DN-TCF4 from Bert Vogelstein (Johns Hopkins University, Baltimore, MD, USA); pcDNAhβ-catenin from Hans Clevers (University Medical Center, Utrecht, The Netherlands).
Transfections and reporter assays
Human colon carcinoma cell lines SW480, DLD1 and HeLa (human cervix carcinoma) cells were obtained from the American Type Culture Collection (ATCC). Cells were cultivated under standard conditions in Dulbecco's modified Eagle's medium +10% fetal bovine serum. Transfections and reporter assays were carried out as described previously (Hlubek et al, 2004). Experiments were conducted in triplicate and repeated at least three times with similar results. Control plasmid (pcDNA3; Invitrogen, Karlsruhe, Germany) was used to equalise for the total amount of DNA and to keep the amount of background plasmid constant.
RNA interference and quantitative real-time reverse-transcription–PCR
Synthetic siRNA (Thermo Electron, Ulm, Germany) specific for β-catenin (CAGUUGU GGUUAAGCUCUUdTdT) or GFP (AAGCUACCUGUUCCAUGGCCAdTdT) as control and Oligofectamine (Invitrogen, Karlsruhe, Germany) was used for transient transfection of the colorectal cancer cells according to the instructions of the manufacturer. After 72 h incubation time, cells were harvested, total RNA isolated and real-time reverse transcription—PCR (RT–PCR) was performed as described (Hlubek et al, 2004). The relative quantities of securin-specific mRNA in β-catenin-siRNA- and control-siRNA-transfected cells were determined for each sample based on the Ct value from a standard curve generated for each primer/probe set and normalised to the corresponding values of the house-keeping gene β-actin.
RESULTS
Overexpression of securin correlates with nuclear β-catenin and Ki-67 expression in human colorectal adenoma and carcinoma
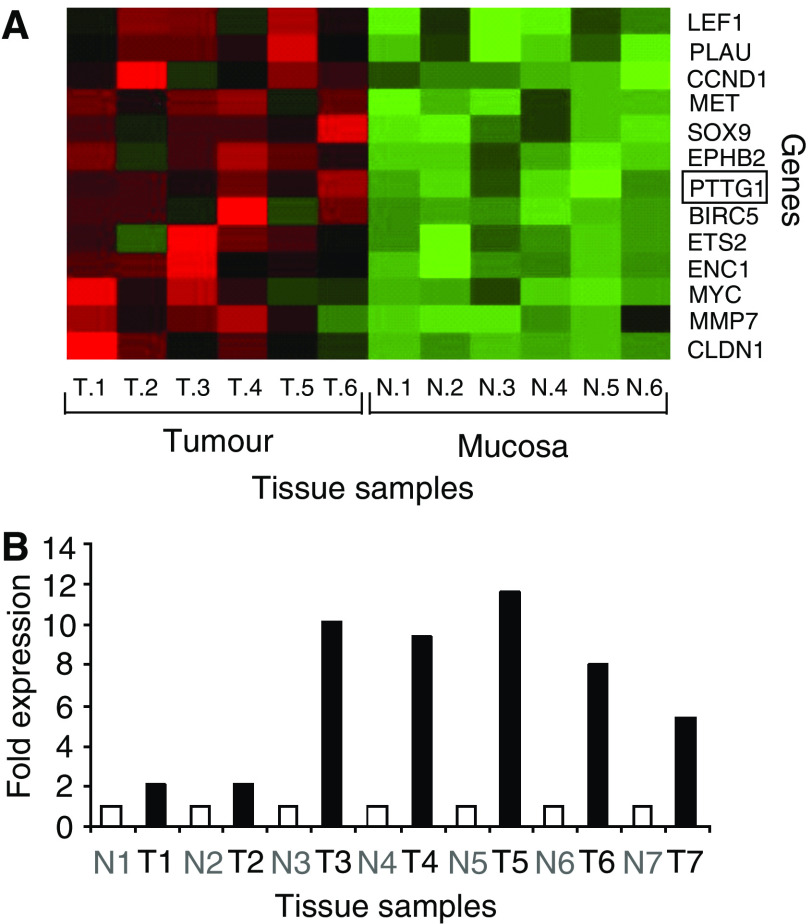
DNA microarray analysis revealed significant overexpression of securin (threefold, P-value <0.00001) in six human colorectal carcinomas compared to the corresponding normal mucosa (Figure 1A; detailed results will be published elsewhere (Hlubek et al, in preparation). Similarly, securin overexpression was found by real-time PCR analysis of seven different colorectal tumours compared to the corresponding mucosa (Figure 1B). Thus, securin shows a very similar differential expression pattern between tumour and mucosa tissue like 12 other well-characterised β-catenin/TCF target genes (fold changes between 3.3 and 1.8-fold, P-value <0.005; Figure 1A). This observation gave rise to the hypothesis, that securin is a target gene of β-catenin/TCF signalling, which is constitutively active in these tumours. Therefore, we analysed the expression pattern of β-catenin and securin protein in 30 colorectal adenocarcinomas by immunohistochemistry. We found a perfect correlation of aberrant nuclear β-catenin expression and securin overexpression in colorectal carcinoma and adenoma tissue, in contrast to very low and restricted expression of securin at the colon crypt base in normal mucosa. In line with securin function in mitosis, the proliferation marker Ki-67 displayed the same expression pattern as securin and nuclear β-catenin, indicating high proliferative activity of these tumour cells (Figure 2 and Supplementary Figure 1). These results support the hypothesis that accumulated β-catenin is an activator of securin expression in human colorectal carcinoma.
Figure 1.
Similar expression pattern of securin (hPTTG1) and 12 established β-catenin/TCF target genes in tumour vs mucosa tissue revealed by hierarchical clustering of microarray data (A). All 13 genes are significantly upregulated in colorectal tumour tissue (T.1–T.6) compared to corresponding normal mucosa (N.1–N.6). Gene expression data are displayed as a heatplot with high expression encoded in red and low gene expression shown in green relative to the mean expression (black). Columns represent tissue samples with the corresponding expression level of the individual genes located in rows. Overexpression of securin in colorectal carcinoma (T) compared to mucosa (N) of seven different cases analysed by real-time RT–PCR (B). Securin-specific mRNA expression in seven tumour tissues relative to corresponding normal mucosa was determined following normalisation to the matching expression values of the housekeeping gene β-actin.
Figure 2.
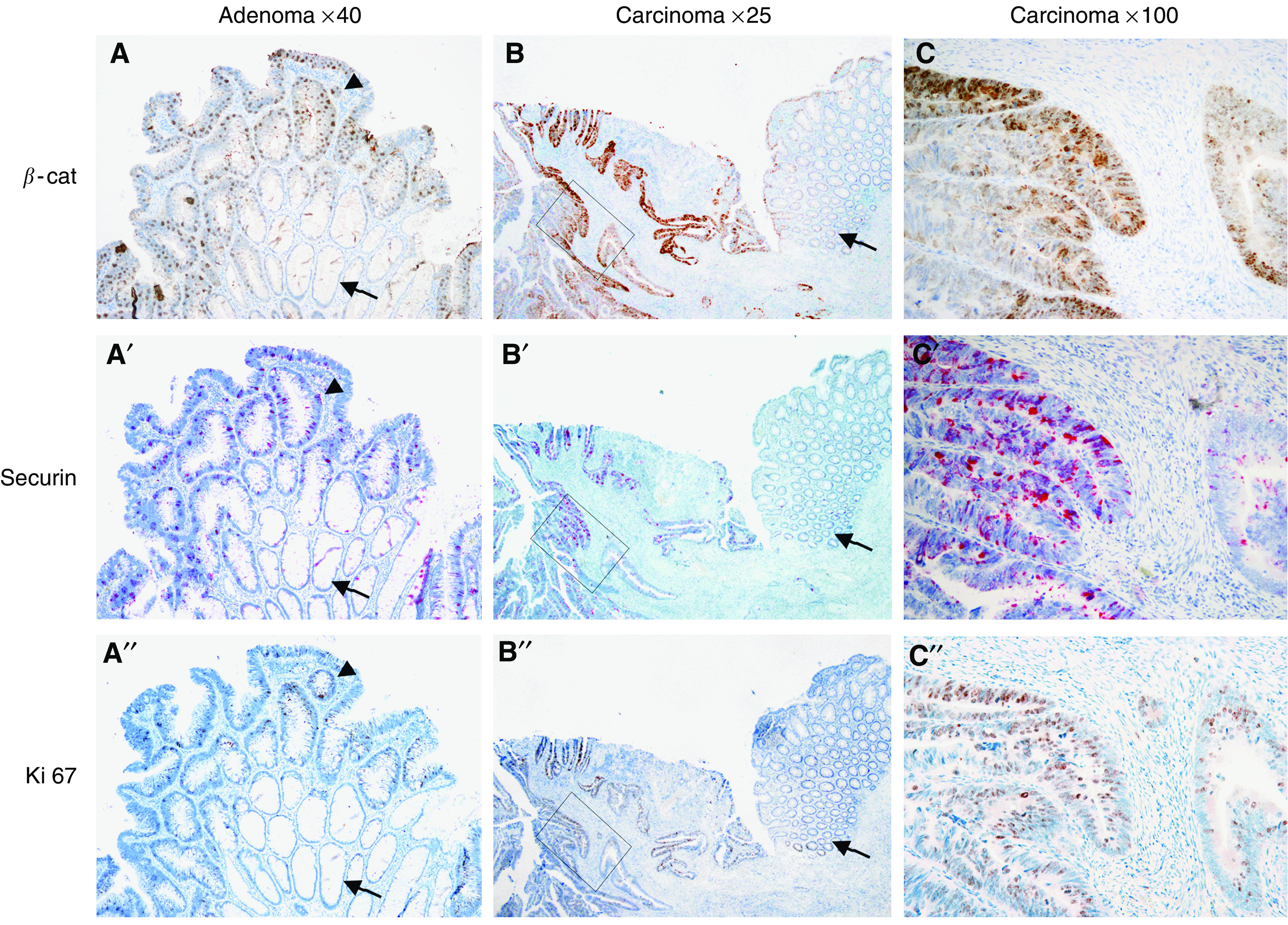
Correlated overexpression of nuclear β-catenin, securin and Ki-67 in colorectal adenoma and carcinoma. Immunohistochemical detection of β-catenin (first row), securin (second row) and Ki-67 (third row) using serial sections. Tissue sections show adenoma (a, a′, a′′), carcinoma (c, c′, c′′) and the normal mucosa (arrows in a, a′, a″ and b, b′, b′′) of the same case. Note that securin is already overexpressed in the adenoma tissue (arrowhead in a′) and only weakly expressed in the underlying normal colon mucosa (arrow in a′). A substantial overexpression of securin, Ki-67 and nuclear β-catenin can be seen in the carcinoma part (b, b′, b′′ and c, c′, c′′; the rectangle in b indicates the magnified area shown in c). Concordant with securin function in mitosis, the proliferation marker Ki-67 displayed the same expression pattern as securin. Nuclear accumulation of β-catenin in many of these tumour cells may suggest a function of this transcriptional activator protein for securin expression. Magnifications are indicated at the top of the figure.
β-Catenin binds to a TCF-binding site of the human securin promoter
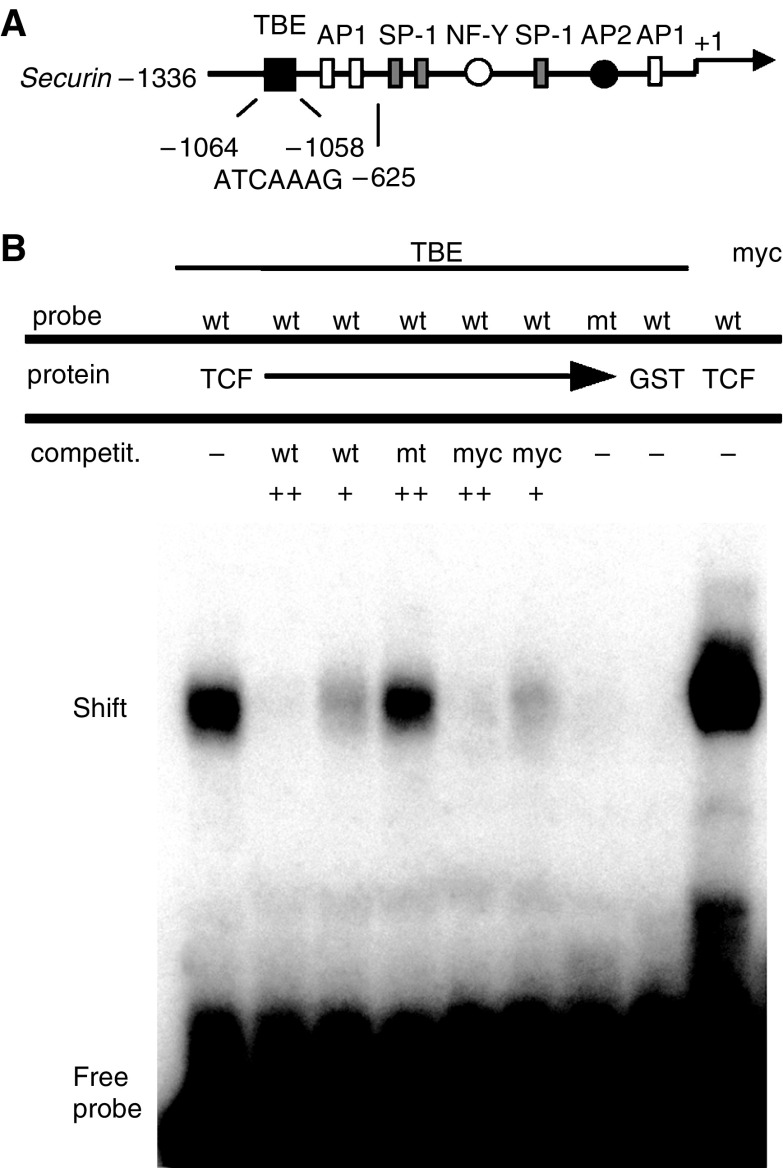
We searched for potential TCF-binding elements (TBE) in the securin promoter/enhancer sequence (Kakar, 1999; Clem et al, 2003) as β-catenin/TCF might directly regulate securin expression. Two sites comprising the TBE motif (5′-WWCAAAG-3′) were identified (nt −1064 to −1058, nt −796 to −790) and tested for interaction with TCF-4 in electromobility shift assays (EMSA). One TBE (nt −1064 to −1058; Figure 3A) was found to bind recombinant TCF-4 (Figure 3B), which is consistent with the very recent report by Zhou et al (2005). The TCF–DNA interaction was abrogated by specific competitor DNA containing the wild-type TBE site (wt) whereas the mutant TBE motif competitor (mt) had no effect (Figure 3B). Competitor DNA containing the TBE of the c-myc promoter (myc) inhibited TCF binding to TBE to the same extent. Mutant TBE-DNA showed no TCF binding and GST protein alone did not bind to wild-type TBE (Figure 3B), indicating specific binding of TCF-4 to TBE of the securin promoter in vitro.
Figure 3.
Identification of a TCF-binding element in the human securin promoter. Structure of the human securin promoter (A). The TBE motif is shown as a closed box and the corresponding nucleotide sequence as well as the location relative to the transcription initiation site is indicated below (not drawn to scale). Known transcription factor binding sites are shown (Kakar, 1999; Clem et al, 2003). Electromobility shift assay shows specific interaction of recombinant TCF-4 DNA-binding domain (TCF) with oligonucleotides containing the wild-type TBE motif (TBE, wt) of the securin promoter by complex formation (B, shift). Oligonucleotides were used as labelled probes and as unlabelled competitors (competit.) in two different amounts (wt, wild type; mt, mutant; myc, TBEs of the c-myc promoter as positive control). Competition of wild-type securin TBE and c-myc TBE, but not mutant TBE, indicates specific TCF-4 protein binding. The wild-type DNA probe does not bind to GST protein alone nor does TCF bind to mutant TBE (B).
The human securin promoter is activated by β-catenin/TCF
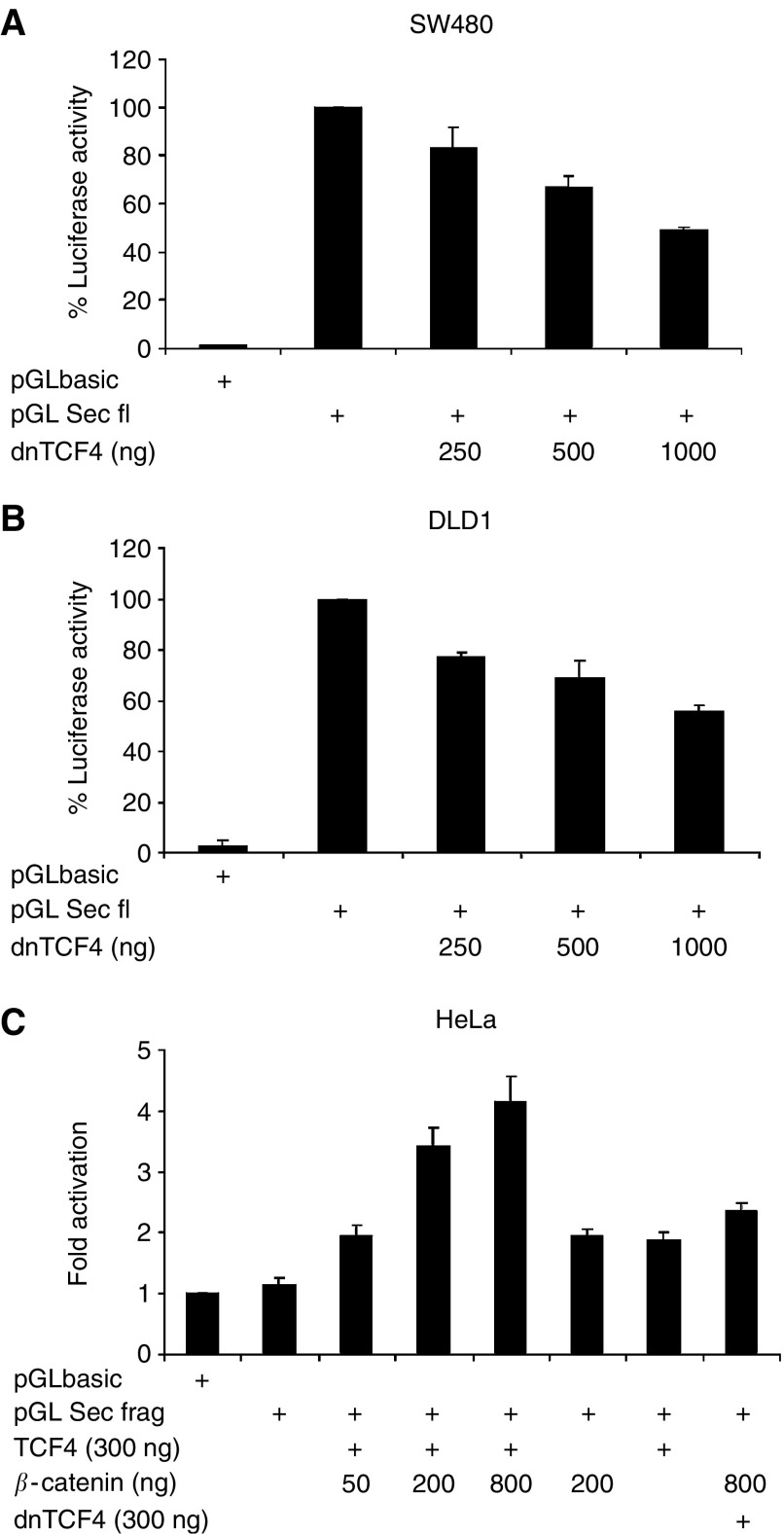
The functional relevance of TCF-4 binding to the TBE motive was investigated in luciferase reporter assays using a full-length securin promoter (−1336 to +34). In β-catenin-overexpressing colorectal carcinoma cell lines SW480 and DLD1, the securin promoter (pGL Sec fl.) exhibits strong transcriptional activity, 74 and 35-fold over background (pGLbasic). Addition of dominant-negative TCF-4 (dnTCF), which lacks the N-terminal β-catenin interaction domain, reduced the securin promoter activity in a dose-dependent manner by up to 51 and 44 % (Figure 4A and B). As it is known that complete abrogation of β-catenin/TCF transcriptional activity by dnTCF can induce a G1-phase arrest of the cell cycle (van de Wetering et al, 2002) possibly influencing the cell cycle-dependent expression of securin, we determined cell cycle progression by fluorescence-activated cell sorting (FACS) analysis (Supplementary Figure 2). Unsynchronised DLD1 and SW480 cells showed no significant change in cell cycle phases after transient transfection with dnTCF compared to cells transfected with control vector.
Figure 4.
The β-catenin/TCF complex activates the human securin promoter activity. Inhibition of securin promoter activity by dnTCF in colorectal carcinoma cells with constitutively active β-catenin. The results of the transient transfection reporter assays are shown as percentage of full-length securin promoter activity (pGL Sec fl.; pGLbasic, vector control; A, B). Securin promoter fragment (pGL Sec frag.) activity is enhanced by β-catenin and TCF-4 in a dose-dependent manner. In contrast, transfection of either β-catenin or TCF-4 alone does not augment securin promoter activity (C). Results are represented as fold activation of promoter activity by transfection of increasing amounts of β-catenin related to the vector control transfected (pGLbasic). Cotransfection of dnTCF inhibits the augmentation of securin promoter fragment activity induced by β-catenin. The experiments were carried out in triplicate and repeated three times with similar results.
Furthermore, in HeLa cells, which do not have an activated β-catenin/TCF-signalling pathway, the activity of the securin promoter fragment (−1336 to −625) was enhanced by overexpression of TCF4 and β-catenin in a dose-dependent manner (Figure 4C). Neither β-catenin nor TCF alone was able to activate the securin promoter fragment. In addition, dnTCF abolished promoter activation by β-catenin (Figure 4C).
Expression of endogenous human securin is regulated by β-catenin
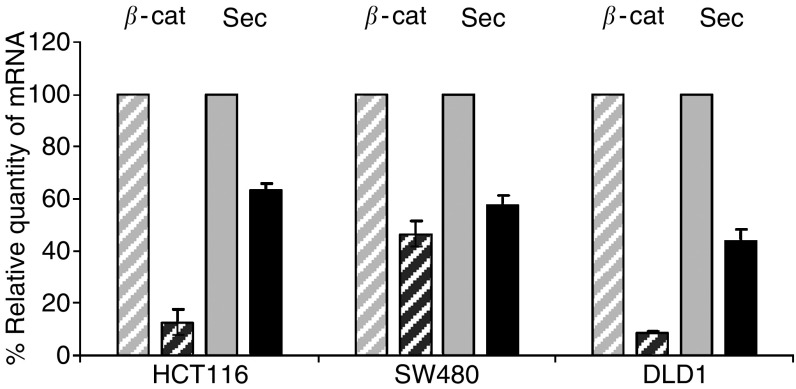
To test the regulatory function of β-catenin on the expression of the endogenous securin gene in colorectal carcinoma cells, we utilised siRNA knockdown technology. The expression of endogenous β-catenin was inhibited by transfection of HCT116, SW480 and DLD1 colon cancer cells with β-catenin-specific siRNA. β-catenin expression, measured by quantitative RT–PCR, was clearly reduced compared to the control cells (transfected with green fluorescent protein (GFP)-siRNA; Figure 5). Specific reduction of β-catenin expression by β-catenin-specific siRNA transfection was confirmed by Western blot in control experiments (Supplementary Figure 3; see also Hlubek et al, 2004). Importantly, transfection of β-catenin-specific siRNA decreased endogenous securin expression by 37, 43 and 56% compared to control cells, but not β-actin expression (Figure 5, and Supplementary Figure 3). To exclude the possibility that reduction of securin expression was caused by changes in cell cycle progression, we performed control experiments using FACS analysis. Transient transfection of HCT116 cells with β-catenin-specific siRNA did not result in significant changes in the cell cycle phase distribution (Supplementary Figure 4).
Figure 5.
Endogenous securin expression is activated by β-catenin. HCT116, SW480 and DLD1 colorectal cancer cells were transfected with β-catenin-specific siRNA and control siRNA (GFP-siRNA). The effect of β-catenin knockdown on securin expression was measured by quantitative real-time RT–PCR and β-catenin expression was confirmed by Western blotting (Supplemental Figure 3). Inhibition of β-catenin expression by siRNA in colorectal cancer cells clearly reduces the endogenous securin expression. Black bars: β-catenin siRNA-transfected cells; grey bars: control cells (GFP-siRNA transfected); hatched bars: quantity of β-catenin mRNA; closed bars: quantity of securin mRNA. Quantification of securin and β-catenin-specific mRNA was done in triplicates, the amounts were normalised to the amount of β-actin mRNA, and expressed in percent relative to the control cells. The experiments were repeated twice with similar results.
DISCUSSION
The results presented above demonstrate a correlated expression of β-catenin, securin and Ki-67 in human colorectal adenomas and carcinomas, indicating that securin is a potential β-catenin/TCF target gene. In support of this hypothesis, we show that securin, like 12 well-known β-catenin/TCF target genes (e.g. c-myc, c-Met, survivin and MMP7), is significantly overexpressed in colorectal cancer compared to normal mucosa using DNA microarray analysis, real-time PCR and immunohistochemistry. Moreover, we found a functional TCF-binding element in the securin promoter sequence identical to the TBE found to be functional in esophageal squamous cell carcinoma, published during the preparation of this manuscript (Zhou et al, 2005). In EMSA experiments, we show specific TCF–DNA interaction. The functional relevance of this interaction is shown by reporter-gene experiments in colorectal cancer cell lines demonstrating that dnTCF inhibits securin promoter activity in a dose-dependent manner. As securin expression is cell cycle dependent, peaking in mitosis (Ramos-Morales et al, 2000), and β-catenin can influence cell cycle progression by regulating c-myc expression (van de Wetering et al, 2002), we performed FACS anaylsis. However, we did not observe significant differences in cell cycle progression beween dnTCF-transfected and control vector-tranfected cells (Supplementary Figure 2). The transient transfection of dnTCF or β-catenin siRNA (see below) reduces β-catenin transcriptional activity, but does not completely abolish it, which would be necessary for inducing a G1-phase arrest of the cell cycle (van de Wetering et al, 2002, 2003).
Additionally, we show that securin promoter fragment containing the TBE is activated in HeLa cells by overexpression of TCF-4 and β-catenin, but not by either protein alone. However, we were unable to activate the full-length promoter construct significantly by overexpression of TCF-4 and β-catenin, because the promoter region (659 nucleotides) proximal to the transcriptional start site contains additional regulatory elements (Kakar, 1999; Clem et al, 2003) presumably obscuring this effect. We tested the function of β-catenin/TCF in the regulation of the endogenous securin gene by transfection of colorectal carcinoma cells with β-catenin-specific siRNA. Owing to the transient transfection procedure used, no complete abrogation of β-catenin expression was observed and cell cycle progression was not changed. However, a potent inhibition of β-catenin expression was induced, resulting in a significant reduction of the endogenous securin gene expression. Even though we do not exclude the possibility of an indirect effect of β-catenin on securin expression by affecting cell cycle progression, our results show the relevance of β-catenin for the direct regulation of securin gene expression in colorectal tumour cells.
The oncogene securin is deregulated in many human tumours and is able to contribute to several main aspects of tumorigenesis. In several tumours, overexpression of securin is associated with tumour metastasis and poor clinical outcome and has been identified as a significant prognostic marker in esophageal carcinoma (Zhang et al, 1999; Heaney et al, 2000; Shibata et al, 2002; Ramaswamy et al, 2003). The identification of securin as a Wnt target gene activated early in the adenoma–carcinoma sequence further supports a decisive role of aberrant overexpression of β-catenin in all phases of colorectal carcinogenesis.
Acknowledgments
We thank C Knoll and U Suchy for expert technical assistance. We especially like to thank Ludger Klein-Hitpass for performing the microarray hybridisations and Bert Vogelstein and Hans Clevers for providing expression plasmids. This work was supported by Johannes und Frieda Marohn-Stiftung to FH and TB, and DFG Grant no. BR 1399/4-1 to TK and TB.
Footnotes
Supplementary Information accompanies the paper on British Journal of Cancer website (http://www.nature.com/bjc)
Supplementary Material
References
- Benjamini Y, Hochberg Y (1995) Controlling the false discovery rate: a practical and powerful approach to multiple testing. J Royal Stat Soc Ser B 57: 289–300 [Google Scholar]
- Bernal JA, Luna R, Espina A, Lazaro I, Ramos-Morales F, Romero F, Arias C, Silva A, Tortolero M, Pintor-Toro JA (2002) Human securin interacts with p53 and modulates p53-mediated transcriptional activity and apoptosis. Nat Genet 32: 306–311 [DOI] [PubMed] [Google Scholar]
- Brabletz T, Hlubek F, Spaderna S, Schmalhofer O, Hiendlmeyer E, Jung A, Kirchner T (2005) Invasion and metastasis in colorectal cancer: epithelial–mesenchymal transition, mesenchymal–epithelial transition, stem cells and b-catenin. Cells Tissues Organs 179: 56–65 [DOI] [PubMed] [Google Scholar]
- Brabletz T, Jung A, Kirchner T (2002) Catenin and the morphogenesis of colorectal cancer. Virchows Arch 441: 1–11 [DOI] [PubMed] [Google Scholar]
- Clem AL, Hamid T, Kakar SS (2003) Characterization of the role of Sp1 and NF-Y in differential regulation of PTTG/securin expression in tumor cells. Gene 322: 113–121 [DOI] [PubMed] [Google Scholar]
- Cleveland W (1977) Robust locally weighted regression and smoothing scatterplots. J Am Stat Assoc 74: 829–836 [Google Scholar]
- Dominguez A, Ramos-Morales F, Romero F, Rios RM, Dreyfus F, Tortolero M, Pintor-Toro JA (1998) hpttg, a human homologue of rat pttg, is overexpressed in hematopoietic neoplasms. Evidence for a transcriptional activation function of hPTTG. Oncogene 17: 2187–2193 [DOI] [PubMed] [Google Scholar]
- Hamid T, Kakar SS (2004) PTTG/securin activates expression of p53 and modulates its function. Mol Cancer 3: 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanahan D, Weinberg RA (2000) The hallmarks of cancer. Cell 100: 57–70 [DOI] [PubMed] [Google Scholar]
- Heaney AP, Singson R, McCabe CJ, Nelson V, Nakashima M, Melmed S (2000) Expression of pituitary-tumour transforming gene in colorectal tumours. Lancet 355: 716–719 [DOI] [PubMed] [Google Scholar]
- Hlubek F, Spaderna S, Jung A, Kirchner T, Brabletz T (2004) Beta-Catenin activates a coordinated expression of the proinvasive factors laminin-5 gamma2 chain and MT1-MMP in colorectal carcinomas. Int J Cancer 108: 321–326 [DOI] [PubMed] [Google Scholar]
- Ishikawa H, Heaney AP, Yu R, Horwitz GA, Melmed S (2001) Human pituitary tumor-transforming gene induces angiogenesis. J Clin Endocrinol Metab 86: 867–874 [DOI] [PubMed] [Google Scholar]
- Jallepalli PV, Waizenegger IC, Bunz F, Langer S, Speicher MR, Peters JM, Kinzler KW, Vogelstein B, Lengauer C (2001) Securin is required for chromosomal stability in human cells. Cell 105: 445–457 [DOI] [PubMed] [Google Scholar]
- Kakar SS (1998) Assignment of the human tumor transforming gene TUTR1 to chromosome band 5q35.1 by fluorescence in situ hybridization. Cytogenet Cell Genet 83: 93–95 [DOI] [PubMed] [Google Scholar]
- Kakar SS (1999) Molecular cloning, genomic organization, and identification of the promoter for the human pituitary tumor transforming gene (PTTG). Gene 240: 317–324 [DOI] [PubMed] [Google Scholar]
- Korinek V, Barker N, Morin PJ, van Wichen D, de Weger R, Kinzler KW, Vogelstein B, Clevers H (1997) Constitutive transcriptional activation by a beta-catenin-Tcf complex in APC−/− colon carcinoma [see comments]. Science 275: 1784–1787 [DOI] [PubMed] [Google Scholar]
- Ramaswamy S, Ross KN, Lander ES, Golub TR (2003) A molecular signature of metastasis in primary solid tumors. Nat Genet 33: 49–54 [DOI] [PubMed] [Google Scholar]
- Ramos-Morales F, Dominguez A, Romero F, Luna R, Multon MC, Pintor-Toro JA, Tortolero M (2000) Cell cycle regulated expression and phosphorylation of hpttg proto-oncogene product. Oncogene 19: 403–409 [DOI] [PubMed] [Google Scholar]
- Shibata Y, Haruki N, Kuwabara Y, Nishiwaki T, Kato J, Shinoda N, Sato A, Kimura M, Koyama H, Toyama T, Ishiguro H, Kudo J, Terashita Y, Konishi S, Fujii Y (2002) Expression of PTTG (pituitary tumor transforming gene) in esophageal cancer. Jpn J Clin Oncol 32: 233–237 [DOI] [PubMed] [Google Scholar]
- van de Wetering M, Oving I, Muncan V, Pon Fong MT, Brantjes H, van Leenen D, Holstege FC, Brummelkamp TR, Agami R, Clevers H (2003) Specific inhibition of gene expression using a stably integrated, inducible small-interfering-RNA vector. EMBO Rep 4: 609–615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Wetering M, Sancho E, Verweij C, de Lau W, Oving I, Hurlstone A, van der Horn K, Batlle E, Coudreuse D, Haramis AP, Tjon-Pon-Fong M, Moerer P, van den Born M, Soete G, Pals S, Eilers M, Medema R, Clevers H (2002) The beta-catenin/TCF-4 complex imposes a crypt progenitor phenotype on colorectal cancer cells. Cell 111: 241–250 [DOI] [PubMed] [Google Scholar]
- Wang Z, Yu R, Melmed S (2001) Mice lacking pituitary tumor transforming gene show testicular and splenic hypoplasia, thymic hyperplasia, thrombocytopenia, aberrant cell cycle progression, and premature centromere division. Mol Endocrinol 15: 1870–1879 [DOI] [PubMed] [Google Scholar]
- Yu R, Heaney AP, Lu W, Chen J, Melmed S (2000) Pituitary tumor transforming gene causes aneuploidy and p53-dependent and p53-independent apoptosis. J Biol Chem 275: 36502–36505 [DOI] [PubMed] [Google Scholar]
- Zhang X, Horwitz GA, Prezant TR, Valentini A, Nakashima M, Bronstein MD, Melmed S (1999) Structure, expression, and function of human pituitary tumor-transforming gene (PTTG). Mol Endocrinol 13: 156–166 [DOI] [PubMed] [Google Scholar]
- Zhou C, Liu S, Zhou X, Xue L, Quan L, Lu N, Zhang G, Bai J, Wang Y, Liu Z, Zhan Q, Zhu H, Xu N (2005) Overexpression of human pituitary tumor transforming gene (hPTTG), is regulated by beta-catenin/TCF pathway in human esophageal squamous cell carcinoma. Int J Cancer 113: 891–898 [DOI] [PubMed] [Google Scholar]
- Zou H, McGarry TJ, Bernal T, Kirschner MW (1999) Identification of a vertebrate sister-chromatid separation inhibitor involved in transformation and tumorigenesis. Science 285: 418–422 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.