Abstract
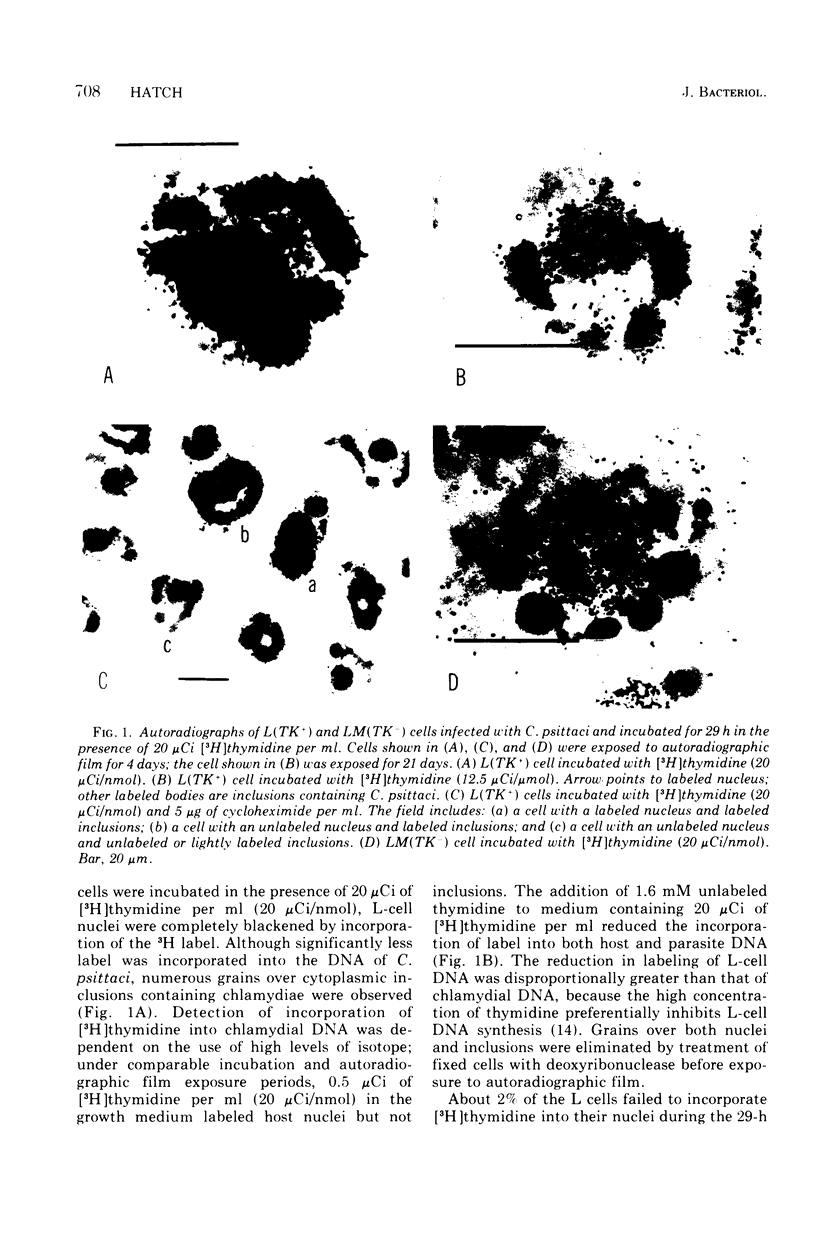
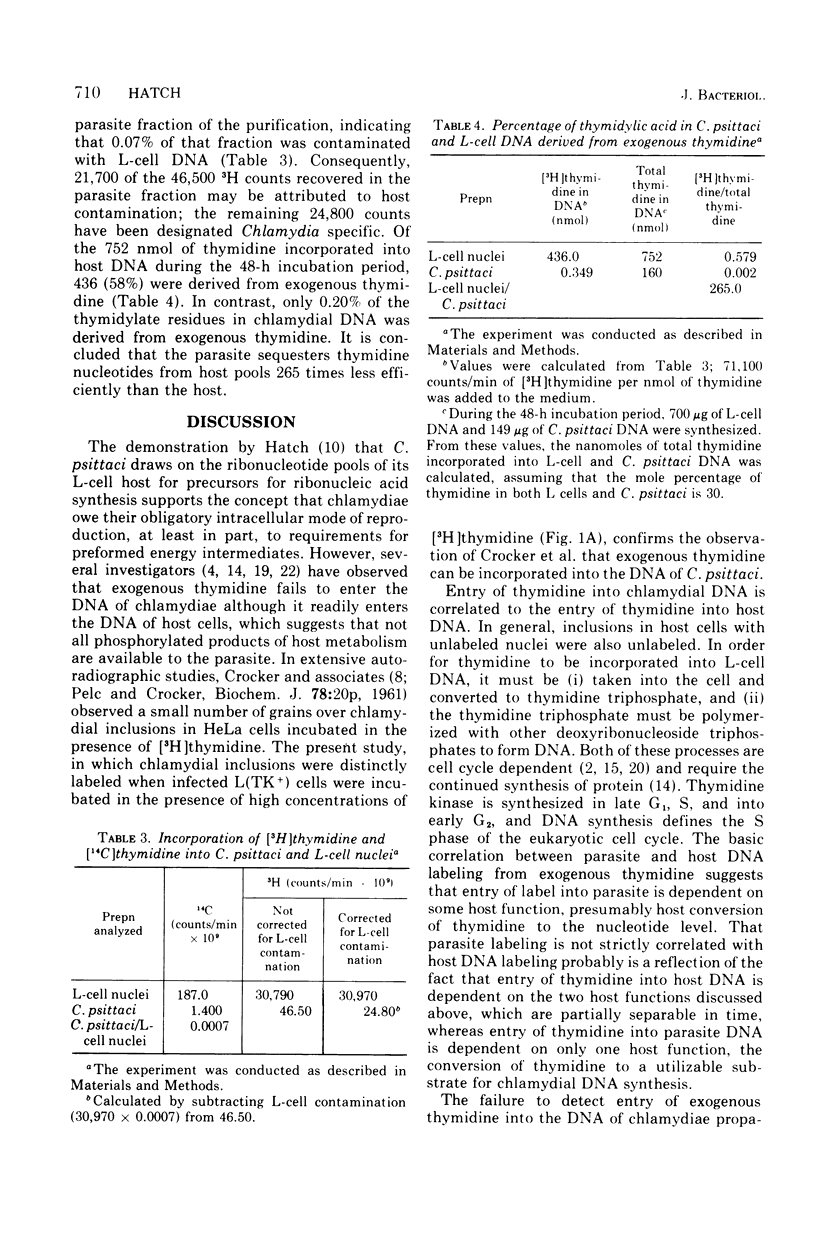
The incorporation of [3H]thymidine into the deoxyribonucleic acid (DNA) of Chlamydia psittaci (strain 6BC) growing in thymidine kinase (adenosine 5'-triphosphate-thymidine 5'-phosphotransferase, EC 1.7.1.21)-containing L cells, L(TK+), and thymidine kinase-deficient L cells, LM(TK-), was examined by autoradiography. Label was detected over C. psittaci inclusions in L(TK+) but not LM(TK-) cells. No evidence for a chlamydia-specific thymidine kinase activity in either L(TK+) or LM(TK-) cells was obtained. Entry of [3H]thymidine into the DNA of C. psittaci growing in L(TK+) cells was quantitated by measuring label in purified C. psittaci. It was 265 times less efficient than entry into infected host cell DNA. It is concluded that low levels of exogenous thymidine are incorporated into the DNA of C. psittaci and that this incorporation is dependent on a fully competent host thymidine kinase activity. Evidence also is presented that L cells possess at least two thymidine kinase activities, both of which are capable of supplying thymidylate precursors for nuclear DNA synthesis.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexander J. J. Effect of infection with the meningopneumonitis agent on deoxyribonucleic acid and protein synthesis by its L-cell host. J Bacteriol. 1969 Feb;97(2):653–657. doi: 10.1128/jb.97.2.653-657.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BERNKOPF H., MASHIAH P., BECKER Y. Correlation between morphological and biochemical changes and the appearance of infectivity in FL cell cultures infected with trachoma agent. Ann N Y Acad Sci. 1962 Mar 5;98:62–81. doi: 10.1111/j.1749-6632.1962.tb30532.x. [DOI] [PubMed] [Google Scholar]
- BURTON K. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J. 1956 Feb;62(2):315–323. doi: 10.1042/bj0620315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bello L. J. Regulation of thymidine kinase synthesis in human cells. Exp Cell Res. 1974 Dec;89(2):263–274. doi: 10.1016/0014-4827(74)90790-3. [DOI] [PubMed] [Google Scholar]
- Berk A. J., Clayton D. A. A genetically distinct thymidine kinase in mammalian mitochondria. Exclusive labeling of mitochondrial deoxyribonucleic acid. J Biol Chem. 1973 Apr 25;248(8):2722–2729. [PubMed] [Google Scholar]
- Brown R. L., Stubblefield E. Use of mutant cells to localize thymidine kinase in a mammalian cell-free system. Exp Cell Res. 1974 Aug;87(2):400–402. doi: 10.1016/0014-4827(74)90502-3. [DOI] [PubMed] [Google Scholar]
- CROCKER T. T., PELC S. R., NIELSEN B. I., EASTWOOD J. M., BANKS J. POPULATION DYNAMICS AND DEOXYRIBONUCLEIC ACID SYNTHESIS IN HELA CELLS INFECTED WITH AN ORNITHOSIS AGENT. J Infect Dis. 1965 Apr;115:105–122. doi: 10.1093/infdis/115.2.105. [DOI] [PubMed] [Google Scholar]
- Colby C., Edlin G. Nucleotide pool levels in growing, inhibited, and transformed chick fibroblast cells. Biochemistry. 1970 Feb 17;9(4):917–920. doi: 10.1021/bi00806a029. [DOI] [PubMed] [Google Scholar]
- Hatch T. P. Competition between Chlamydia psittaci and L cells for host isoleucine pools: a limiting factor in chlamydial multiplication. Infect Immun. 1975 Jul;12(1):211–220. doi: 10.1128/iai.12.1.211-220.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatch T. P. Utilization of L-cell nucleoside triphosphates by Chlamydia psittaci for ribonucleic acid synthesis. J Bacteriol. 1975 May;122(2):393–400. doi: 10.1128/jb.122.2.393-400.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KIT S., DUBBS D. R., PIEKARSKI L. J., HSU T. C. DELETION OF THYMIDINE KINASE ACTIVITY FROM L CELLS RESISTANT TO BROMODEOXYURIDINE. Exp Cell Res. 1963 Aug;31:297–312. doi: 10.1016/0014-4827(63)90007-7. [DOI] [PubMed] [Google Scholar]
- Kit S., Leung W. C., Kaplan L. A. Distinctive molecular forms of thymidine kinase in mitochondria of normal and bromodeoxyuridine-resistant HeLa cells. Eur J Biochem. 1973 Nov 1;39(1):43–48. doi: 10.1111/j.1432-1033.1973.tb03101.x. [DOI] [PubMed] [Google Scholar]
- Klemperer H. G., Haynes G. R., Shedden W. I., Watson D. H. A virus-specific thymidine kinase in BHK-21 cells infected with herpes simplex virus. Virology. 1967 Jan;31(1):120–128. doi: 10.1016/0042-6822(67)90015-3. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lin H. S. Inhibition of thymidine kinase activity and deoxyribonucleic acid synthesis in L cells infected with the meningopneumonitis agent. J Bacteriol. 1968 Dec;96(6):2054–2065. doi: 10.1128/jb.96.6.2054-2065.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Littlefield J. W. The periodic synthesis of thymidine kinase in mouse fibroblasts. Biochim Biophys Acta. 1966 Feb 21;114(2):398–403. doi: 10.1016/0005-2787(66)90319-4. [DOI] [PubMed] [Google Scholar]
- STARR T. J., SHARON N. AUTORADIOGRAPHY WITH THE AGENTS OF PSITTACOSIS AND TRACHOMA. Proc Soc Exp Biol Med. 1963 Aug-Sep;113:912–914. doi: 10.3181/00379727-113-28529. [DOI] [PubMed] [Google Scholar]
- Skoog L., Bjursell G. Nuclear and cytoplasmic pools of deoxyribonucleoside triphosphates in Chinese hamster ovary cells. J Biol Chem. 1974 Oct 25;249(20):6434–6438. [PubMed] [Google Scholar]
- Tribby I. I. Cell Wall Synthesis by Chlamydia psittaci Growing in L Cells. J Bacteriol. 1970 Dec;104(3):1176–1188. doi: 10.1128/jb.104.3.1176-1188.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tribby I. I., Moulder J. W. Availability of bases and nucleosides as precursors of nucleic acids in L cells and in the agent of meningopneumonitis. J Bacteriol. 1966 Jun;91(6):2362–2367. doi: 10.1128/jb.91.6.2362-2367.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss E. Adenosine Triphosphate and Other Requirements for the Utilization of Glucose by Agents of the Psittacosis-Trachoma Group. J Bacteriol. 1965 Jul;90(1):243–253. doi: 10.1128/jb.90.1.243-253.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss E., Schramek S., Wilson N. N., Newman L. W. Deoxyribonucleic Acid Heterogeneity Between Human and Murine Strains of Chlamydia trachomatis. Infect Immun. 1970 Jul;2(1):24–28. doi: 10.1128/iai.2.1.24-28.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss E., Wilson N. N. Role of exogenous adenosine triphosphate in catabolic and synthetic activities of Chlamydia psittaci. J Bacteriol. 1969 Feb;97(2):719–724. doi: 10.1128/jb.97.2.719-724.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]



