Abstract
Deficient levels of serotonin are associated with suicide and depression. Paradoxically, in the dorsal raphe nucleus (DRN) there are more serotonin neurons and more neuronal tryptophan hydroxylase-2 (TPH2) expression postmortem in depressed suicides. In this study, we sought to determine whether greater TPH2 expression in depressed suicides was the result of more TPH2 expression per neuron. In situ hybridization and computer-assisted image analysis were performed on tissue sections throughout the extent of the raphe nuclei at the level of silver grains per neuron to systematically quantify TPH2 neuronal expression. Depressed suicides have 26.5% more TPH2 grain density per neuron in the DRN compared with matched controls (P= 0.04). The difference in grain density is greater at mid- and caudal anatomical levels across the rostrocaudal axis of the DRN. Densitometric analysis of TPH2 expression in the DRN subnuclei showed that higher expression levels were observed at posterior anatomical levels of depressed suicides (121% of control in the caudal subnucleus). Higher TPH2 expression in depressed suicides may explain more TPH2 protein and reflect a homeostatic response to deficient serotonin levels in the brains of depressed suicides. Localized changes in TPH2 expression in specific subnuclei of the DRN suggest that the serotonergic compensatory mechanism in depression and suicide is specifically regulated within the DRN and has implications for regions innervated by this subnucleus.
Keywords: serotonin, postmortem, in situ hybridization
Introduction
An impaired brain serotonergic system is hypothesized to contribute to the etiology of major depressive disorder (MDD) and suicide. The findings of low serotonin and/or its major metabolite 5-hydroxyindoleacetic acid in suicides and depressed suicide attempters1 suggest deficient serotonergic neurotransmission. Furthermore, in suicide attempters, serotonin activity measured by the release of prolactin in response to administration of fenfluramine was found to be blunted.2–7 Brain serotonin (5-HT) is produced by neurons in the midline of the brainstem, the raphe nuclei. Of the several raphe nuclei, the dorsal (DRN) and median raphe nuclei (MRN) provide the innervation to the forebrain,8 including the prefrontal cortex, a region that modulates mood and major cognitive processes. The DRN is anatomically subdivided into five subnuclei, that is, the dorsal (DRd), ventrolateral (DRvl), ventral (DRv), interfascicular (DRif), and caudal (DRc). Each of these subnuclei is believed to project to specific parts of the cortex and subcortical regions and consequently may have differential regulation of 5-HT synthesis.
In postmortem studies of suicides, some reported findings in the serotonergic system are not consistent with reduced function and may represent an upregulation of the serotonergic system as part of a homeostatic response to deficient 5-HT neurotransmission. In the brainstem, there are more serotonin neurons, fewer presynaptic serotonin transporter (SERT) binding sites and altered 5-HT1A autoreceptor binding and some, but not all, studies report higher 5-HT1A and 5-HT2A postsynaptic receptor binding in the prefrontal cortex (reviewed by Mann9).
Tryptophan hydroxylase (TPH) is the rate-limiting enzyme in the biosynthesis of 5-HT, converting tryptophan to 5-hydroxytryptophan (5-HTP) on route to decarboxylation into 5-hydroxytryptamine (see Mockus and Vrana10 for review). Alterations in TPH expression or catalytic activity can potentially cause changes in the levels of 5-HT in the brain. At the transcript level, brain 5-HT in rodents is regulated by a neuron-specific isoform of TPH, known as TPH2.11,12 We found specific TPH2 transcript expression in the human DRN and MRN and further discovered that TPH2 mRNA expression is increased in suicides.13 At the protein level, we found higher density and number of TPH immunoreactive (IR) neurons in the DRN of depressed suicides by immunocytochemistry,14 a finding replicated in a second cohort using immunoautoradiography.15 Another group reported higher TPH protein expression restricted to the dorsal subnucleus of the DRN in alcohol-dependent depressed suicides16 but found no change in non-alcoholic depressed suicides,17 demonstrating the anatomically restricted contributions of individual DRN subnuclei in regulating serotonin.
In the present study we sought to further analyze the increase in TPH2 gene expression in suicides to determine whether the increase in TPH2 expression was due to more neurons or whether there was an increase in expression at the neuron level. We, therefore, analyzed emulsion-dipped slides of the cases studied in our previous report13 and quantified expression at a per neuron level in suicides and matched controls. We also examined expression in subnuclei of the DRN.
Materials and methods
Subjects
Study procedures were approved by the applicable Institutional Review Boards. Consent was given by next of kin for tissue collection, review of relevant records and psychological autopsy (see Mann et al.18 for description of psychological autopsy procedures). All cases died suddenly. Controls died of either cardiac arrest (n = 8), vehicular injury (n=1) or industrial accident.1 Suicides occurred by hanging (n = 5), fall from height (n = 3), gunshot (n=1) or drowning (n = 1). Control cases had no psychiatric diagnoses based on a structured clinical interview SCID I and II.19–22 A total of 9 of the 10 suicides had a diagnosis of Axis I major depression. For one case, the diagnosis of major depression was part of a schizoaffective disorder. Comorbidities included one case with an eating disorder and one with obsessive-compulsive disorder; 1 of the 10 suicide cases met criteria for alcohol abuse in the past and 3 were moderate-to-heavy cigarette smokers.
All procedures involving brain collection, neuropathology, toxicology and tissue sectioning protocols are discussed by Bach-Mizrachi et al.,13 and are briefly summarized here. For all subjects, postmortem interval (PMI) averaged 15.6 h. Brain pH was 6.44±0.12 in controls and 6.63±0.08 in suicides (P > 0.05). In situ hybridization experiments were performed in 10 matched case–control pairs. Cases are matched first according to sex (eight males, two females), then age within 5 years (controls, 53.9±5 vs suicides, 54.5±5 years), PMI (controls, 13.1±1.7 vs suicides, 20±1.6) and whenever possible race (controls comprise six Caucasian, one Hispanic and three African-American, whereas suicides comprise six Caucasian and four Hispanic). Cause of death is determined by the medical examiner’s office. All cases had clear peripheral and brain toxicological screens at the time of death. See Table 1 for summary of subject demographics.
Table 1.
Case demographics
| Pair no. |
Age (years) |
Sex | Race | PMI | pH | Brain toxicology |
Axis I | Cause of death |
Antemortem prescriptions |
Mean grain counts per neuron |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 66 | Male | White | 19 | 6.1 | None | None | Myocardial infarction | None | 220 |
| 1aa | 63 | Male | Hispanic | 17 | 6.74 | None | None | Hanging (S) | None | 308 |
| 2 | 30 | Female | African-American | 8 | 6.73 | None | None | Heart attack | None | 466 |
| 2aa,b | 26 | Female | Hispanic | 18 | 6.88 | None | Schizoaffective, depressed type | Hanging (S) | Mood stabilizers | 651 |
| 3 | 79 | Male | White | 9.75 | 5.96 | Lidocaine/antiarrhythmic | None | Heart attack | None | 341 |
| 3a | 77 | Male | White | 18 | 6.40 | None | MDD | Hanging (S) | None | 396 |
| 4 | 27 | Female | White | 15 | 6.72 | None | None | MVA | None | 291 |
| 4a | 28 | Female | White | 19 | 6.32 | None | Eating disorder, MDD | Fall from height (S) | TCA | 456 |
| 5 | 51 | Male | Hispanic | 7.5 | 6.43 | None | None | Myocardial infarction | None | 941 |
| 5a | 66 | Male | White | 11 | 6.27 | Caffeine | None | Gun shot (S) | None | 664 |
| 6 | 53 | Male | White | 18.5 | NA | None | None | Myocardial infarction | None | 900 |
| 6a | 50 | Male | Hispanic | 30 | 6.82 | None | MDD | Fall from height (S) | None | 1010 |
| 7 | 37 | Male | African-American | 15 | 6.75 | None | None | Myocardial infarction | None | 438 |
| 7a | 40 | Male | White | 20 | 6.77 | Analgesics | MDD | Hanging (S) | SSRI | 382 |
| 8 | 85 | Male | White | 7 | 6.22 | None | None | Myocardial infarction | None | 555 |
| 8a | 74 | Male | White | 21 | 5.93 | Opiates, analgesics, caffeine | MDD | Fall from height (S) | None | 1011 |
| 9b | 53 | Male | African-American | 9 | NA | None | None | Industrial accident | None | 87 |
| 9a | 59 | Male | Hispanic | 24.5 | 6.77 | None | MDD | Hanging (S) | None | 148 |
| 10 | 58 | Male | White | 22 | 6.44 | Lidocaine | None | Myocardial infarction | None | 211 |
| 10a | 62 | Male | White | 21 | 6.37 | None | MDD | Drowning (S) | SSRI | 192 |
|
| ||||||||||
| Mean | 54 | 15.9 | 6.48 | |||||||
| s.e.m. | 6 | 1.9 | 0.07 | |||||||
Abbreviations: MDD, major depression; MVA, motor vehicle accident; NA, not available; PMI, postmortem interval, in hours; S, suicide; SSRI, serotonin-specific reuptake inhibitor; TCA, tricyclic antidepressants.
Alcohol abuse.
Cigarette smoker.
All demographic information was made available by the medical examiner and psychological autopsy provided by next of kin.
Brainstems
Brains were collected at autopsy (see Arango et al.23). The brainstem tissue block used for sectioning was approximately 3cm in length and included the midbrain and rostral pons, which contained the DRN and MRN. The block, standing on a glass slide on its rostral cut surface, was flash-frozen in freon (−20 °C) and stored at −80 °C. The brainstem was sectioned exhaustively at 20 μm. Sections for in situ hybridization experiments were taken every 1200 μm throughout the rostrocaudal extent of the DRN, corresponding to 16–20 sections per case. Sections were mounted and stored at −80 °C until assayed. Brain pH was measured to obtain an indication of the integrity of the RNA.24
Grain counting of in situ hybridization autoradiograms
Production of riboprobes specific for TPH2 and in situ hybridization assays were described by Bach-Mizrachi et al.13 After the completion of the in situ hybridization procedure, slides were dried and exposed to autoradiography film (Kodak BioMax MR) with 14C calibration standard slides (ARC-146, 146A, American Radiolabeled Chemicals Inc.) for 3 days. After the films were developed, the slides were dipped in photographic emulsion (Kodak NTB-2) and lightly stained with hematoxylin and eosin, allowing for the visualization of cellular structures without obstructing the silver grains produced by the reduction of silver following emulsion dipping, exposure and development.
Bright-field microscope images of the DRN/MRN at ×400 magnification were digitized by a charge-coupled device (Dage CCD-72). The images were analyzed for silver grain counts (number of grains per neuron) and grain density (grains per unit area) using computer-assisted image analysis (microcomputer imaging device (MCID); Elite Software, version 7.0). A systematic randomized method of selecting and sampling neurons was employed to measure representative cells for each subject in sufficient numbers (Q- = 150 neurons per case) so as to reduce measurement error bias and allow for the estimation of the total number of labeled cells in the DRN/MRN per subject. The boundaries of the DRN and MRN were outlined on the slide by matching to the corresponding in situ hybridization autoradiogram. These drawn boundaries were used as guidelines and were also used to create graphical coordinates for each neuron sampled in the DRN and MRN to obtain a permanent record of the locations and measurements taken on each slide.
For each slide, the most ventral point of the fourth ventricle was used as the origin. The total DRN or MRN area of each section was sampled by moving the microscope stage at 500 μm intervals and measuring grains in all neurons in a spatially quantified upper right quadrant (75 × 50 μm area) of the computer monitor. Exclusion and inclusion lines were drawn as boundaries of the sampling frame. Overlapping cells were not sampled. For each neuron, grain number and grain density was measured.
Grains were measured using a fixed area target circle (3317 μm2) placed over the cell soma. Group differences were quantified by determining the mean number of grains per neuron in the DRN and MRN using this fixed area circle. Using the image analysis software, the number of grains within the circle was estimated by dividing the total grain area by the predetermined average grain size of 0.3 μm2. The values for grain densities for all the neurons sampled (including labeled and unlabeled neurons) were divided into fixed ranges. The number of labeled and unlabeled neurons in each range was counted and plotted in a histogram. The plotted densities displayed a bimodal distribution. Neurons were considered positively labeled when their density reading was three times over background, which was determined by extrapolating a normal distribution from the histogram.25,26 Background grain counts were taken from five regions: adjacent pons, unlabeled neurons in the raphe, a region devoid of neurons, a section hybridized with the sense probe, and slide background without tissue. The mean measurement of the background grain density was calculated and the value was subtracted from neuron measurements of grain density to gain greater specificity and reduce variability. TPH2 silver grain density did not correlate with age, postmortem interval, pH or freezer storage time (P > 0.05 for all variables).
Statistical analyses
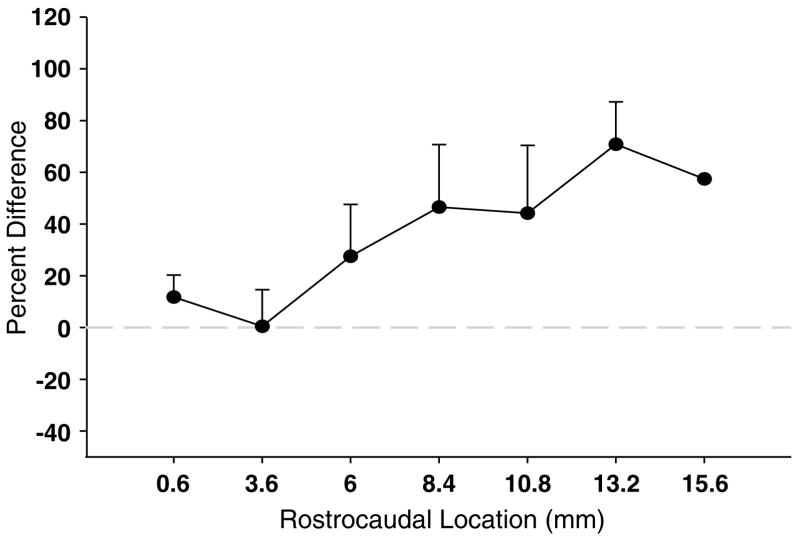
Expression along the rostrocaudal axis was determined by grouping and averaging the densities across 2.4mm intervals for each case. Mean grain counts per neuron at each anatomical level across the rostrocaudal axis were plotted for the control group and the suicide group (see Figure 3). These data were analyzed using a linear mixed effect model. The data were log transformed. The fixed effect was the subject type, and the random effect was the pair assignment since the subjects were matched by key demographics and postmortem characteristics. In the primary analysis, the anatomical level was included as a predictor. A similar analysis was carried out with a binary predictor (‘rostral DRN’ and ‘caudal DRN’) in place of anatomical level (‘rostral DRN’ = the most rostral 8mm of the DRN). The percent change at each anatomical level was also calculated using mean percent of control within each interval and plotted (see Figure 4).
Figure 3.
Mean grain count at 2.4mm intervals along the rostrocaudal axis of the dorsal raphe nucleus (DRN). Suicides have more tryptophan hydroxylase-2 (TPH2) grains per neuron than matched controls (P = 0.03). The largest difference is seen at more caudal levels of the DRN.
Figure 4.
Mean difference in tryptophan hydroxylase-2 (TPH2) grain density between suicides and controls across the rostrocaudal axis in the dorsal raphe nucleus (DRN). Difference in expression is determined as the mean percent of control at 2.4mm intervals across the rostrocaudal axis. The mean change in grain density per neuron is 48%higher in suicides as compared to controls, and is present throughout the extent of the DRN and median raphe nucleus (MRN), but peaks at caudal levels (t = 3.90, P = 0.008, d.f. = 6).
Analysis of covariance and another mixed model analysis were performed on the overall mean grain density to examine the effects of age, sex and PMI on grain density. Again, pair was used as a random effect. Since the overall mean grain counts were used, there was no need to include anatomical level.
Results
Microscopic analysis of TPH2 mRNA in situ hybridization autoradiograms
Silver grains representing TPH2 mRNA expression were exclusively clustered over neurons in the DRN and MRN (Figure 1). Smaller neurons and glia tended to have fewer or no grains and approximated background levels. Sections assayed with the sense probe resulted in background levels of silver grains throughout the entire tissue section, demonstrating the specificity of the antisense probe. The mean number of neurons counted per anatomical level was not significantly different between the groups (Figure 2). The coefficient of error was the same for both the control and depressed suicide group (0.22). Our previous analysis of TPH2 films from these subjects demonstrated no difference in total area or volume of the DRN/MRN between depressed suicides and controls.13
Figure 1.

(a) In situ hybridization film autoradiogram of tryptophan hydroxylase-2 (TPH2) in the human dorsal (DRN) and median raphe nuclei (MRN), Bar = 1mm. (b) High magnification (× 400) photomicrograph of an emulsion- dipped section of a TPH2 in situ hybridization slide demonstrating silver grains specific to the neurons of the dorsal (DRN) and median raphe nuclei (MRN). An unlabeled neuron, where very few silver grains are found, is also visible above the encircled neuron, Bar = 25 μm.
Figure 2.
Mean number of neurons counted at each anatomical interval for suicides and controls. No significant difference in the number of neurons counted between suicides and controls was found (P > 0.05).
Since the grains were quantified using a fixed area target sampling circle placed over the soma of individual neurons (see Figure 1b), density measures per unit area are equivalent to grain density per neuron. Mean TPH2 grain density per neuron was higher in the DRN and MRN of depressed suicides as compared to matched controls (d.f. = 5.19, P = 0.03). The average grain density of DRN/MRN neurons throughout the DRN/MRN in depressed suicides was 26.5% greater than controls (see Table 1).
Mean TPH2 grain density did not correlate with age, or PMI as determined by regression analysis. Neither mixed model analysis nor ANCOVA, with age, sex or PMI as covariates, showed an effect of demographic variables on TPH2 expression (P > 0.05 for all variables).
Background density in the fixed area circle sampled inside the DRN, but not including a neuron, was higher in depressed suicides than controls (controls, 0.005±0.001 vs suicides, 0.012±0.003 grains per μm2, t =−2.34, d.f. = 7, P = 0.05) This represents the elevation in TPH2 expression in neuronal processes within the DRN. The other four background counts taken outside the raphe nuclei did not differ between suicides and controls.
When the grain density per neuron was examined at 2.4mm intervals across the rostrocaudal axis of the DRN/MRN (Figures 3 and 4), the elevation in grain density per neuron in suicides appeared to have the largest difference in caudal levels of the DRN/MRN. Therefore, we divided the DRN into rostral (0–8.4mm) and caudal (8.4–18.0mm) portions recognizing and respecting the anatomical organization of the nucleus. Mixed model analysis of grain density at these two anatomical levels indicated that the difference between depressed suicides and controls was present in the caudal (d.f. = 6.06, P = 0.02), but not in the rostral DRN (P = 0.46). The greatest difference was located at 13.2mm caudal from the most rostral section of the DRN (168±13% of control).
We analyzed the individual DRN subnuclei in autoradiograms and further defined the anatomy of the difference in expression of TPH2 mRNA density in the DRN subnuclei. TPH2 expression in suicides was greater in magnitude than in controls in all five subnuclei ranging from 107 to 145% of control, but failed to reach significance (P > 0.05). In the caudal subnucleus.
Discussion
We have expanded on our original report of elevated TPH2 gene expression in the DRN and MRN of depressed suicides,13 greater density of serotonergic neurons in depressed suicides14 and greater amount of TPH protein in depressed suicides.15 We now report that elevated TPH2 gene product in suicides is the result of transcriptional upregulation at the level of individual serotonergic neurons. It appears that depressed suicides may have more serotonergic neurons, as previously shown by immunocytochemistry14 with higher TPH2 transcriptional capacity. We further determined that in suicides, transcriptional upregulation of TPH2-expressing neurons is more pronounced at caudal levels of the DRN and in the MRN. Specifically, the only subnucleus with increased TPH2 expression in the DRN of suicides was the caudal subnucleus. The pattern of distribution of change in grain density per neuron expression of TPH2 in suicides across the rostrocaudal axis correlates13 with our previous densitometric analysis of TPH2 in situ hybridization autoradiograms (r = 0.96, P = 0.002).
Elevated TPH2 expression in suicides does not correlate with age, sex, pH or PMI, signifying that the statistically significant differences in TPH2 grain counts between controls and suicides are due to the biology of the disorder rather than an effect of demographic variables. Although we cannot discount the affects of drug therapies prescribed before death, or the consequence of alcohol or nicotine use on TPH2 expression, we can, at least, conclude that at the time of death, peripheral and brain toxicological screens were negative, and therefore, the elevation in TPH2 expression may be a result of MDD and/or suicide.
Upregulation of TPH2 gene expression may account for higher levels of DRN TPH immunoreactivity in depressed suicides.14,15 The original finding of greater TPH protein immunoreactivity in suicides reflected an increase in the number TPH immunoreactive neurons and also a darker reaction product in individual neurons in suicides as compared to controls. Combined, an increase in TPH2 transcription and a larger number of immunoreactive neurons in suicides both contribute to more expression and more protein. Our previous study indicates that there are more neurons expressing TPH2 transcript in depressed suicides compared with non-psychiatric controls. However, since an estimate of number of neurons was not the goal of this study, we did not employ an unbiased counting approach, and thus estimation of the total number of neurons in the DRN was not possible in this study.
Since TPH is the rate-limiting 5-HT biosynthetic enzyme and is increased in depressed suicides, it remains to be determined why brain serotonin and cerebrospinal fluid levels of 5-hydroxyindole acetic acid levels are low in suicides and serious suicide attempters.1,27 One possible explanation is that the TPH isoenzyme in depressed suicides is mutated and has lower catalytic activity. In fact, one functional missense variant ARG441HIS has been identified in the coding region of the TPH2 gene and found to be present in approximately 10% of a population of elder major depressives, and has not been detected in a cohort affected with bipolar disorder.28 When expressed in PC12 cells, this genetic variant was found to decrease serotonin levels by 80% compared to wild type, demonstrating that this single nucleotide polymorphism (SNP) is a loss of function mutation. This SNP represents a rare functional polymorphism.29–33 However, in vitro, it has been shown to have decreased catalytic activity, stability and solubility as compared to wild type.34 Future studies in a large postmortem sample can assess whether elevated TPH2 expression correlates with genotype.
We found that the largest change in TPH2 expression was in caudal levels of the DRN. In mice, it has been shown that in response to swim stress, 5-HT levels decrease in the lateral septum, increase in the striatum and do not change in the cortex.35 A retrograde labeling study determined that the DRN innervation of these serotonergic targets is regionally restricted. Interestingly, the lateral septum, in which 5-HT levels were found to decrease in response to stress, is innervated by the caudal subnucleus, at least in the rodent.36 Elevated TPH2 expression was found throughout all DRN subnuclei in suicides, and did not appear regionally restricted. However, the larger increase at caudal levels compared to more rostral levels suggests that the caudal subnucleus, which predominates at these levels, may be critically involved. Perhaps, the caudal subnucleus is most sensitive to low 5-HT and therefore has the largest increase in TPH2 expression in response to low 5-HT. In fact, corticotrophin-releasing factor type 2 receptor agonist treatment in rats results in increased c-FOS expression in TPH immunoreactive neurons in mid- and caudal levels of the DRN.37
Other evidence has been found for the functional selectivity of DRN subnuclei. In ovariectomized rats, estrogen increased TPH2 expression only in mid- and caudal regions of the DRN and was correlated with decreased anxiety behaviors.38 In stress-sensitive female monkeys, SERT mRNA was found to be decreased specifically in the caudal subnucleus.39 Although 5-HT levels were not measured in these studies, both of these findings are consistent with a compensatory response to deficient serotonergic neurotransmission.
In rodents, acute stress increases TPH2 transcript and protein expression,40,41 suggesting that depression and suicide-related stress may account for elevated TPH gene expression.42 Hyperactivity of the corticotrophin-releasing hormone (CRH) and diminished functioning of the hypothalamic–pituitary–adrenal axis have been implicated in depression and suicidal behavior.43 Future studies are necessary to explore the relationship between CRH and TPH2 elevation in depression and suicide.
Acknowledgments
We thank Mihran J Bakalian, Suham A Kassir and Jennifer Lau for technical assistance. The work was supported by PHS grants MH40210, MH62185 and MH64168, the American Foundation for Suicide Prevention and the Diane Goldberg Foundation.
References
- 1.Mann JJ, Malone KM. Cerebrospinal fluid amines and higher-lethality suicide attempts in depressed inpatients. Biol Psychiatry. 1997;41:162–171. doi: 10.1016/s0006-3223(96)00217-x. [DOI] [PubMed] [Google Scholar]
- 2.Mann JJ, McBride PA, Malone KM, DeMeo MD, Keilp JG. Blunted serotonergic responsivity in depressed patients. Neuropsychopharmacology. 1995;13:53–64. doi: 10.1016/0893-133X(95)00016-7. [DOI] [PubMed] [Google Scholar]
- 3.Pandey GN. Altered serotonin function in suicide. Evidence from platelet and neuroendocrine studies. Ann NYAcad Sci. 1997;836:182–200. doi: 10.1111/j.1749-6632.1997.tb52360.x. [DOI] [PubMed] [Google Scholar]
- 4.Corrêa H, Duval F, Mokrani M-C, Bailey P, Trémeau F, Staner L, et al. Prolactin response to D-fenfluramine and suicidal behavior in depressed patients. Psychiatry Res. 2000;93:189–199. doi: 10.1016/s0165-1781(00)00114-1. [DOI] [PubMed] [Google Scholar]
- 5.Malone KM, Corbitt EM, Li S, Mann JJ. Prolactin response to fenfluramine and suicide attempt lethality in major depression. Br J Psychiatry. 1996;168:324–329. doi: 10.1192/bjp.168.3.324. [DOI] [PubMed] [Google Scholar]
- 6.Weiss D, Coccaro EF. Neuroendocrine challenge studies of suicidal behavior. Psychiatr Clin North Am. 1997;20:563–579. doi: 10.1016/s0193-953x(05)70330-0. [DOI] [PubMed] [Google Scholar]
- 7.Dulchin MC, Oquendo MA, Malone KM, Ellis SP, Li S, Mann JJ. Prolactin response to dl-fenfluramine challenge before and after treatment with paroxetine. Neuropsychopharmacology. 2001;25:395–401. doi: 10.1016/S0893-133X(01)00239-1. [DOI] [PubMed] [Google Scholar]
- 8.Törk I, Hornung J-P. Raphe nuclei and the serotonergic system. In: Paxinos G, editor. The Human Nervous System. Academic Press; San Diego: 1990. pp. 1001–1022. [Google Scholar]
- 9.Mann JJ. Neurobiology of suicidal behaviour. Nat Rev Neurosci. 2003;4:819–828. doi: 10.1038/nrn1220. [DOI] [PubMed] [Google Scholar]
- 10.Mockus SM, Vrana KE. Advances in the molecular characterization of tryptophan hydroxylase (Review) J Mol Neurosci. 1998;10:163–179. doi: 10.1007/BF02761772. [DOI] [PubMed] [Google Scholar]
- 11.Patel PD, Pontrello C, Burke S. Robust and tissue-specific expression of TPH2 versus TPH1 in rat raphe and pineal gland. Biol Psychiatry. 2004;55:428–433. doi: 10.1016/j.biopsych.2003.09.002. [DOI] [PubMed] [Google Scholar]
- 12.Zhang X, Beaulieu JM, Sotnikova TD, Gainetdinov RR, Caron MG. Tryptophan hydroxylase-2 controls brain serotonin synthesis. Science. 2004;305:217. doi: 10.1126/science.1097540. [DOI] [PubMed] [Google Scholar]
- 13.Bach-Mizrachi H, Underwood MD, Kassir SA, Bakalian MJ, Sibille E, Tamir H, et al. Neuronal tryptophan hydroxylase mRNA expression in the human dorsal and median raphe nuclei: major depression and suicide. Neuropsychopharmacology. 2006;31:814–824. doi: 10.1038/sj.npp.1300897. [DOI] [PubMed] [Google Scholar]
- 14.Underwood MD, Khaibulina AA, Ellis SP, Moran A, Rice PM, Mann JJ, et al. Morphometry of the dorsal raphe nucleus serotonergic neurons in suicide victims. Biol Psychiatry. 1999;46:473–483. doi: 10.1016/s0006-3223(99)00043-8. [DOI] [PubMed] [Google Scholar]
- 15.Boldrini M, Underwood MD, Mann JJ, Arango V. More tryptophan hydroxylase in the brainstem dorsal raphe nucleus in depressed suicides. Brain Res. 2005;1041:19–28. doi: 10.1016/j.brainres.2005.01.083. [DOI] [PubMed] [Google Scholar]
- 16.Bonkale WL, Turecki G, Austin MC. Increased tryptophan hydroxylase immunoreactivity in the dorsal raphe nucleus of alcohol-dependent, depressed suicide subjects is restricted to the dorsal subnucleus. Synapse. 2006;60:81–85. doi: 10.1002/syn.20278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bonkale WL, Murdock S, Janosky JE, Austin MC. Normal levels of tryptophan hydroxylase immunoreactivity in the dorsal raphe of depressed suicide victims. J Neurochem. 2004;88:958–964. doi: 10.1046/j.1471-4159.2003.02225.x. [DOI] [PubMed] [Google Scholar]
- 18.Mann JJ, Huang YY, Underwood MD, Kassir SA, Oppenheim S, Kelly TM, et al. A serotonin transporter gene promoter polymorphism (5-HTTLPR) and prefrontal cortical binding in major depression and suicide. Arch Gen Psychiatry. 2000;57:729–738. doi: 10.1001/archpsyc.57.8.729. [DOI] [PubMed] [Google Scholar]
- 19.First MB, Spitzer RL, Gibbon M, Williams JMG, Benjamin L. Structured Clinical Interview for DSM-IV Axis II Personality Disorders (SCID-II) (Version 2.0) Biometrics Research Department, New York State Psychiatric Institute; New York: 1996. [Google Scholar]
- 20.Spitzer RL, Williams JBW, Gibbon M, First MB. The structured clinical interview for DSM-III-R (SCID). I: history, rationale, and description. Arch Gen Psychiatry. 1992;49:624–629. doi: 10.1001/archpsyc.1992.01820080032005. [DOI] [PubMed] [Google Scholar]
- 21.Williams JBW, Gibbon M, First MB, Spitzer RL, Davies M, Borus J, et al. The structured clinical interview for DSM-III-R (SCID). II. Multisite test-retest reliability. Arch Gen Psychiatry. 1992;49:630– 636. doi: 10.1001/archpsyc.1992.01820080038006. [DOI] [PubMed] [Google Scholar]
- 22.Kelly TM, Mann JJ. Validity of DSM-III-R diagnosis by psychological autopsy: a comparison with clinician ante-mortem diagnosis. Acta Psychiatr Scand. 1996;94:337–343. doi: 10.1111/j.1600-0447.1996.tb09869.x. [DOI] [PubMed] [Google Scholar]
- 23.Arango V, Underwood MD, Boldrini M, Tamir H, Kassir SA, Hsiung S, et al. Serotonin 1A receptors, serotonin transporter binding and serotonin transporter mRNA expression in the brainstem of depressed suicide victims. Neuropsychopharmacology. 2001;25:892–903. doi: 10.1016/S0893-133X(01)00310-4. [DOI] [PubMed] [Google Scholar]
- 24.Harrison PJ, Heath PR, Eastwood SL, Burnet PWJ, McDonald B, Pearson RCA. The relative importance of premortem acidosis and postmortem interval for human brain gene expression studies: selective mRNA vulnerability and comparison with their encoded proteins. Neurosci Lett. 1995;200:151–154. doi: 10.1016/0304-3940(95)12102-a. [DOI] [PubMed] [Google Scholar]
- 25.Volk DW, Austin MC, Pierri JN, Sampson AR, Lewis DA. Decreased glutamic acid decarboxylase 67 messenger RNA expression in a subset of prefrontal cortical gamma-aminobutyric acid neurons in subjects with schizophrenia. Arch Gen Psychiatry. 2000;57:237–245. doi: 10.1001/archpsyc.57.3.237. [DOI] [PubMed] [Google Scholar]
- 26.Gerfen CR, McGinty JF, Young WS., III Dopamine differentially regulates dynorphin, substance P, and enkephalin expression in striatal neurons: in situ hybridization histochemical analysis. J Neurosci. 1991;11:1016–1031. doi: 10.1523/JNEUROSCI.11-04-01016.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Placidi GP, Oquendo MA, Malone KM, Huang YY, Ellis SP, Mann JJ. Aggressivity, suicide attempts, and depression: relationship to cerebrospinal fluid monoamine metabolite levels. Biol Psychiatry. 2001;50:783–791. doi: 10.1016/s0006-3223(01)01170-2. [DOI] [PubMed] [Google Scholar]
- 28.Zhang X, Gainetdinov RR, Beaulieu JM, Sotnikova TD, Burch LH, Williams RB, et al. Loss-of-function mutation in tryptophan hydroxylase-2 identified in unipolar major depression. Neuron. 2005;45:11–16. doi: 10.1016/j.neuron.2004.12.014. [DOI] [PubMed] [Google Scholar]
- 29.Blakely RD. Overview: a rare opportunity or just one less reason to be depressed. Neuron. 2005;48:701–702. doi: 10.1016/j.neuron.2005.11.029. [DOI] [PubMed] [Google Scholar]
- 30.Glatt CE, Carlson E, Taylor TR, Risch N, Reus VI, Schaefer CA. Response to Zhang et al. (2005): loss-of-function mutation in tryptophan hydroxylase-2 identified in unipolar major depression. Neuron. 45:11–16. doi: 10.1016/j.neuron.2004.12.014. [DOI] [PubMed] [Google Scholar]; Neuron. 2005;48:704–705. doi: 10.1016/j.neuron.2005.11.019. [DOI] [PubMed] [Google Scholar]
- 31.Zhou Z, Peters EJ, Hamilton SP, McMahon F, Thomas C, McGrath PJ, et al. Response to Zhang et al. (2005): loss-of-function mutation in tryptophan hydroxylase-2 identified in unipolar major depression. Neuron. 45:11–16. doi: 10.1016/j.neuron.2004.12.014. [DOI] [PubMed] [Google Scholar]; Neuron. 2005;48:702–703. doi: 10.1016/j.neuron.2005.11.018. [DOI] [PubMed] [Google Scholar]
- 32.Van Den BA, De ZS, Heyrman L, Mendlewicz J, Adolfsson R, Van BC, et al. Response to Zhang et al. (2005): loss-of-function mutation in tryptophan hydroxylase-2 identified in unipolar major depression. Neuron. 45:11–16. doi: 10.1016/j.neuron.2004.12.014. [DOI] [PubMed] [Google Scholar]; Neuron. 2005;48:704–706. doi: 10.1016/j.neuron.2005.11.019. [DOI] [PubMed] [Google Scholar]
- 33.Delorme R, Durand CM, Betancur C, Wagner M, Ruhrmann S, Grabe HJ, et al. No human tryptophan hydroxylase-2 gene R441H mutation in a large cohort of psychiatric patients and control subjects. Biol Psychiatry. 2006;60:202–203. doi: 10.1016/j.biopsych.2005.12.014. [DOI] [PubMed] [Google Scholar]
- 34.Winge I, McKinney JA, Knappskog PM, Haavik J. Characterization of wild-type and mutant forms of human tryptophan hydroxylase 2. J Neurochem. 2007;100:1648–1657. doi: 10.1111/j.1471-4159.2006.04290.x. [DOI] [PubMed] [Google Scholar]
- 35.Kirby LG, Allen AR, Lucki I. Regional differences in the effects of forced swimming on extracellular levels of 5-hydroxytryptamine and 5-hydroxyindoleacetic acid. Brain Res. 1995;682:189–196. doi: 10.1016/0006-8993(95)00349-u. [DOI] [PubMed] [Google Scholar]
- 36.Waselus M, Galvez JP, Valentino RJ, Van Bockstaele EJ. Differential projections of dorsal raphe nucleus neurons to the lateral septum and striatum. J Chem Neuroanat. 2006;31:233–242. doi: 10.1016/j.jchemneu.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 37.Staub DR, Evans AK, Lowry CA. Evidence supporting a role for corticotropin-releasing factor type 2 (CRF2) receptors in the regulation of subpopulations of serotonergic neurons. Brain Res. 2006;1070:77–89. doi: 10.1016/j.brainres.2005.10.096. [DOI] [PubMed] [Google Scholar]
- 38.Hiroi R, McDevitt RA, Neumaier JF. Estrogen selectively increases tryptophan hydroxylase-2 mRNA expression in distinct subregions of rat midbrain raphe nucleus: association between gene expression and anxiety behavior in the open field. Biol Psychiatry. 2006;60:288–295. doi: 10.1016/j.biopsych.2005.10.019. [DOI] [PubMed] [Google Scholar]
- 39.Bethea CL, Streicher JM, Mirkes SJ, Sanchez RL, Reddy AP, Cameron JL. Serotonin-related gene expression in female monkeys with individual sensitivity to stress. Neuroscience. 2005;132:151– 166. doi: 10.1016/j.neuroscience.2004.11.022. [DOI] [PubMed] [Google Scholar]
- 40.Chamas F, Serova L, Sabban EL. Tryptophan hydroxylase mRNA levels are elevated by repeated immobilization stress in rat raphe nuclei but not in pineal gland. Neurosci Lett. 1999;267:157–160. doi: 10.1016/s0304-3940(99)00340-7. [DOI] [PubMed] [Google Scholar]
- 41.Chamas FM, Underwood MD, Arango V, Serova L, Kassir SA, Mann JJ, et al. Immobilization stress elevates tryptophan hydroxylase mRNA and protein in the rat raphe nuclei. Biol Psychiatry. 2004;55:278–283. doi: 10.1016/s0006-3223(03)00788-1. [DOI] [PubMed] [Google Scholar]
- 42.McEwen BS, Brinton RE, Sapolsky RM. Glucocorticoid receptors and behavior: implications for the stress response. Adv Exp Med Biol. 1988;245:35–45. doi: 10.1007/978-1-4899-2064-5_4. [DOI] [PubMed] [Google Scholar]
- 43.Swaab DF, Bao AM, Lucassen PJ. The stress system in the human brain in depression and neurodegeneration. Ageing Res Rev. 2005;4:141–194. doi: 10.1016/j.arr.2005.03.003. [DOI] [PubMed] [Google Scholar]