Summary
Neutrophil Gelatinase-Associated Lipocalin (NGAL) is a lipocalin that is well known for its functions as a shuttle for iron and siderophores, which comprises a critical component of innate immunity to exogenous bacterial infections. However, several lines of recent evidence have added new dimensions of functionality that have attracted the interest of cancer biologists and oncologists. This review will highlight the exciting new paths and roles that are emerging for NGAL in human cancers, in the tissue response to anticancer therapy, and in the acute kidney injuries that commonly complicate the care of patients with cancer. The evidence for NGAL induction and its role in various human cancers will be explored. The current status of NGAL as a predictive non-invasive biomarker of acute kidney injuries will also be examined.
Keywords: Lipocalin, biomarker, cancer, acute renal failure, acute kidney injury, cisplatin, nephrotoxicity
I. Introduction
Lipocalins constitute a family of over 20 small secreted proteins defined on the basis of their three- dimensional structure, consisting of a single eight-stranded anti-parallel b-barrel which forms an enclosing calyx capable of binding to a wide variety of molecules (Flower, 1996; Flower et al, 2000). This unique structure renders lipocalins as efficient shuttles and transporters for diverse substances such as retinoids, arachidonic acid, prostaglandins, fatty acids, pheromones, steroids, and iron (Goetz et al, 2000). Neutrophil gelatinase-associated lipocalin (NGAL, also known as lipocalin 2, siderocalin, uterocalin, and neu-related lipocalin) is a prominent member of the lipocalin family that has captured the interest of structural biologists for several years now. It was first isolated as a 25 kDa glycoprotein covalently bound with matrix metalloproteinase-9 (MMP-9) in human neutrophils (Triebel et al, 1992; Kjeldsen et al, 1993). Despite significant lack of conservation in the residues in other parts of the protein, the amino acids constituting the central calyx that characterize NGAL as a lipocalin are highly preserved (Flower et al, 2000). Besides MMP-9, a prominent ligand for NGAL was identified as enterochelin (Goetz et al, 2002), a siderophore that binds iron with extremely high affinity (Raymond et al, 2003). Bacteria produce siderophores to shuttle iron from the extracellular space, thereby providing an efficient means of iron acquisition (Schmidt-Ott et al, 2006; Schmidt-Ott et al, 2007). The siderophore-chelating property of NGAL renders it as a bacteriostatic agent that prevents growth of those bacterial strains by depleting their intracellular iron stores (Goetz et al, 2002). The biological significance of this finding has recently been highlighted in NGAL deficient mice, which develop a markedly increased sensitivity to Gram-negative bacterial infections and an increased susceptibility to death from sepsis (Flo et al, 2004; Berger et al, 2006). Thus, NGAL comprises a critical component of innate immunity to exogenous bacterial infections, consistent with its normal expression in a number of human tissues that are typically exposed to the external environment, including the respiratory, gastro-intestinal, and urinary tracts (Xu and Venge, 2000). Elevated tissue NGAL expression has been documented under diverse infectious and inflammatory conditions such as inflammatory bowel disease, appendicitis, diverticulitis, and urinary tract infections (Xu and Venge, 2000).
However, several lines of recent evidence in humans have challenged the prevailing notion of NGAL as an iron- transporting shuttle, and have added new dimensions of functionality to this enigmatic molecule that have sparked the interest of cancer biologists and oncologists. This review will highlight the new paths and roles that are emerging for NGAL in human cancers, in the tissue response to anticancer therapy, and in the acute kidney injuries that commonly complicate the care of patients with cancer.
II. NGAL in human cancers
The gene encoding NGAL (also known as 24p3 in mice and as LCN2 for lipocalin 2) was first identified almost twenty years ago as a transcript that was rapidly overexpressed by SV40 tumor virus in quiescent mouse primary kidney cells (Hraba-Reveney et al, 1989). NGAL is also induced by a number of other tumor-promoting agents in vitro, including polyoma virus (Hanai et al, 2005), the phorbol ester PMA (Davis et al, 1991), the transforming factor neu (Stoesz and Gould, 1995), hepatocyte growth factor (Gwira et al, 2005), retinoic acid (Garay-Rojas et al, 1996), and glucocorticoids (Bratt, 2000). A number of related lipocalins are overexpressed in a variety of human cancers, and lipocalin ligands have been shown to regulate proliferation, differentiation, and protease activities (Bratt, 2000). In this section, the expression and potential role of NGAL in cancer is reviewed.
Mounting evidence indicates that NGAL is induced in a number of human cancers (Table 1). A heterogeneous expression of NGAL was first documented in a subset of subjects with primary breast carcinoma at both the mRNA and protein levels (Stoesz et al, 1998). The NGAL protein was found within breast carcinoma cells but not in normal ductal epithelium. The largest quantity of the NGAL protein was observed in the lumen of normal ducts in the vicinity of NGAL-expressing tumors. These findings have been recently confirmed in a large study of 207 invasive breast cancer samples, which documented the diffuse labeling of NGAL in tumor cells. Approximately one-third of breast cancer cases were interpreted as strongly positive for NGAL staining, with the overwhelming majority representing ductal carcinomas (Bauer et al, 2007). While the significance of these findings remains to be fully elucidated, several lines of preliminary evidence suggest that NGAL expression may represent a predictor of poor prognosis in primary human breast cancer. Stoesz and colleagues (1998) showed a significant correlation between NGAL expression and other markers of poor prognosis such as estrogen and progesterone receptor-negative status and high proliferation (S-phase) fraction. The correlation between NGAL expression and negative ER status has now been confirmed by several gene expression profiling studies (Gruvberger et al, 2001; Van de Vijyer et al, 2002; Van’t Veer et al, 2002; Wang et al, 2005). Bauer et al have recently extended these findings to show that NGAL expression correlated with negative steroid receptor status, poor histologic grade, the presence of lymph node metastasis, and high proliferation (Ki-67) index (Bauer et al, 2007). In univariate analysis, NGAL expression was associated with decreased disease-specific survival and decreased disease-free survival. Thus, tissue NGAL expression may provide prognostic information for risk assessment and potential identification of a subset of breast cancer patients who may benefit from more aggressive adjuvant therapy.
Table 1.
Expression of NGAL in human cancers.
| Reference | Cancer Type | Comments |
|---|---|---|
| Stoesz et al, 1998 | Breast Adenocarcinoma | Correlated with steroid negative status and with high proliferation |
| Bauer et al, 2007 | Breast Adenocarcinoma | Correlated with steroid negative status, high proliferation, poor histology grade and metastasis |
| Kubben et al, 2007 | Gastric Adenocarcinoma | Tissue NGAL/MMP-9 complexes correlated with severity and worse survival |
| Zhang et al, 2007 | Esophageal Squamous Cell Ca | Tissue NGAL/MMP-9 complexes correlated with tumor invasion |
| Nielsen et al 1996 | Colonic Adenocarcinoma | Ectopic expression of NGAL in colon cancer cells suppressed invasion (Lee et al, 2005b) |
| Furutani et al 1998 | Pancreatic Adenocarcinoma | Upregulation of NGAL confirmed by transcriptome profiling analysis (Argani et al, 2001) |
| Friede et al 1999 | Lung Adenocarcinoma | Heterogeneous expression, largely confined to the tumor cells |
| Lim et al, 2007 | Ovarian Tumors | Highest expression in grade 1 tumors, reduced expression in grade 2 and 3 tumors |
Some lines of in vitro evidence support NGAL’s putative role in breast tumor progression. For example, over-expression of NGAL in MCF-7 human breast cancer cells resulted in increased growth rates, proliferation, angiogenesis, and increased levels of MMP-9 (Fernandez et al, 2005). NGAL is well known to complex with MMP-9, thereby preventing MMP-9 degradation and increasing MMP-9 enzyme activity in vitro (Yan et al, 2001) and in patients with breast cancer (Fernandez et al, 2005). In turn, MMP activity has been shown to promote cancer progression by degrading the basement membranes and extracellular matrix, liberating vascular endothelial growth factor (VEGF), and thus enabling angiogenesis, invasion and metastasis (Lochner et al, 1998; Lee et al, 2005).
However, in striking contrast, a recent study by Hanai et al showed that introduction of NGAL into Ras-transformed 4T1 mouse mammary tumor cells enhanced the epithelial phenotype, reduced tumor growth, and suppressed metastasis (Hanai et al, 2005). These protective actions of NGAL were enhanced by iron-siderophore, and are consistent with the well-known epithelial induction actions of NGAL on embryonic kidney mesenchyme (Yang et al, 2002). Thus, even within breast cancer cells, the actions of NGAL may be heterogeneous and specific to the cancer cell type.
A number of investigators have documented the induction of NGAL in cancers of other organs (Friedl et al, 1999). In a study of 81 patients who underwent resection of primary gastric adenocarcinoma, NGAL was found to colocalize with MMP-9 in epithelial cells by immunofluorescence, and complexes of NGAL and MMP-9 were abundantly detected in tissue homogenates (Kubben et al, 2007). The complexes were significantly correlated with pathological classifications of severity, and with worse survival, suggesting a role for NGAL in tumor progression via suppression of MMP-9 auto-degradation (Kubben et al, 2007). Along the same lines, a recent examination of 30 cases of esophageal squamous cell carcinomas revealed robust NGAL staining in the cancer cells, and tissue extracts demonstrated an increase in NGAL/MMP-9 enzymatic activity that correlated strongly with tumor invasion (Zhang et al, 2007). On the other hand, while several studies have demonstrated the enhanced and ectopic expression of NGAL in the epithelial cells of colonic adenocarcinoma cases (Nielsen et al, 1996; Madoz-Gurpide et al, 2006), the ectopic expression of NGAL in colon cancer cells suppressed invasion in vitro and inhibited liver metastasis in an animal model, suggesting that NGAL may be a suppressor of metastasis in this case (Lee et al, 2005b). Finally, while NGAL upregulation has been demonstrated at both the protein and mRNA level in human pancreatic cancers (Furutani et al, 1998; Argani et al, 2001; Terris et al, 2002; Laurell et al, 2006), the potential clinical significance of these findings remains unclear at the present time.
In light of the findings that (a) MMP-9 forms a stable and enzymatically active complex with NGAL in the urine (Yan et al, 2001), (b) MMP-9 can be detected in the urine of cancer patients (Moses et al, 1998), and (c) the induced NGAL in some cancer types could mediate tumor progression via suppression of MMP-9 auto-degradation, the potential role of urinary NGAL/MMP-9 has been investigated. In urine samples from 22 patients with breast cancer and 27 age-matched controls, the NGAL/MMP-9 complex was detected in 86% of the cases but in none of the healthy controls (Fernandez et al, 2005). These findings have led to the suggestion that urinary detection of the NGAL/MMP-9 complex may represent a novel non-invasive method for predicting disease status in breast cancer patients, with potential for generalized application in cancer diagnostics and prognostics. The source of the NGAL/MMP-9 complex in the urine remains somewhat puzzling. The relationship between free NGAL and the NGAL-MMP-9 complex also requires further clarification. Any NGAL systemically released from malignantly transformed epithelia would be freely filtered by the kidney glomerulus, but would be expected to be largely reabsorbed by efficient endocytic mechanisms in the proximal tubules (Schmidt-Ott et al, 2006; Schmidt-Ott et al, 2007). Urinary excretion of NGAL is more likely to be present when there is concomitant renal tubular injury that increases de novo NGAL synthesis and/or precludes NGAL reabsorption (Mori et al, 2005). The clinical utility of urinary measurements of NGAL, MMP-9 and their complex as predictive biomarkers in human cancers will hopefully be clarified in future validation studies.
III. NGAL in cisplatin nephrotoxicity
Cisplatin is one of the most widely used and effective chemotherapeutic agents, with clinical applications in more than 50% of human cancers (Boulikas, 2007). While it is clearly a potent antitumor drug, the efficacy is dose dependent but the significant risk of nephrotoxicity frequently precludes the use of higher doses to maximize its antineoplastic effects (Hanigan and Devarajan, 2003). Approximately one-third of patients who receive cisplatin develop evidence for acute kidney injury (AKI), previously referred to as acute renal failure (Santoso et al, 2003). This situation can have dire consequences for morbidity and mortality, especially in combination with other multi-organ involvements typical in patients with underlying malignancies. While several therapeutic maneuvers have been efficacious in animal models, successful translation to humans has remained anecdotal at best (Sheikh-Hamad et al, 1997). One reason for this state of affairs may be the lack of early biomarkers for nephrotoxic AKI, and hence a potential delay in initiating therapy. Current clinical practice relies on serial measurements of serum creatinine for the diagnosis of AKI, which is an unreliable and delayed measure of nephrotoxic injury.
Transcriptome profiling studies have identified NGAL as one of the most highly induced genes in animal models of cisplatin nephrotoxicity (Hung et al, 2007). In an established animal model of cisplatin nephrotoxicity, NGAL protein was rapidly induced in the kidney, and detected in a punctuate cytoplasmic distribution within tubule cells, reminiscent of a secreted protein (Mishra et al, 2004a; Devarajan, 2005). NGAL protein was also easily detected and quantified in the urine within 3 hours of cisplatin administration in a dose- and duration- dependent manner, whereas an increase in serum creatinine was not evident until 96 hours after cisplatin. These pre-clinical studies suggest that NGAL may represent a novel, non-invasive, early urinary biomarker for cisplatin-induced AKI. The results of translational studies examining NGAL in urines of human subjects with varying degrees of cisplatin nephrotoxicity are awaited with anticipation.
IV. NGAL in other forms of acute kidney injury
AKI is a common problem in hospitalized patients with cancer (Devarajan, 2005), and a major cause of morbidity and mortality in this population (Lamiere et al, Devarajan: Neutrophil gelatinase-associated lipocalin: new paths for an old shuttle 466 2005). AKI in patients with malignancies limits our ability to deliver the most effective care, and is one of the most important risk factors for the development of non-renal complications. While several factors can contribute to the development of AKI in subjects with cancer, the major causes of severe AKI include sepsis, ischemic injury, and nephrotoxic injury (Devarajan, 2005). While recent advances have suggested novel therapeutic approaches in animal models, translational efforts in humans have yielded disappointing results. The reasons for this include an incomplete understanding of the underlying pathophysiology, and the lack of early biomarkers for AKI (Devarajan 2006; Devarajan 2007a). In current clinical practice, AKI is typically diagnosed by measuring serum creatinine. Unfortunately, creatinine is an unreliable indicator during acute changes in kidney function (Parikh et al, 2007a). First, serum creatinine levels can vary widely with age, gender, muscle mass, muscle metabolism, medications, and hydration status. Second, during acute changes in glomerular filtration, serum creatinine does not accurately depict kidney function until steady state equilibrium has been reached, which may require several days. However, animal studies have shown that while AKI can be prevented and/or treated by several maneuvers, these must be instituted very early after the initiating insult, well before the serum creatinine even begins to rise. The quest to improve early diagnosis of AKI is an area of intense research (Zhou et al, 2006). Conventional urinary biomarkers such as casts and fractional excretion of sodium have been insensitive and non-specific for the early recognition of AKI. Other traditional urinary biomarkers such as filtered high molecular weight proteins and tubular proteins or enzymes have also suffered from lack of specificity and dearth of standardized assays.
Fortunately, technologies such as functional genomics and proteomics have uncovered novel candidates that are emerging as biomarkers (Devarajan 2007b). In animal models, NGAL was identified by microarray analysis as one of the earliest induced genes and proteins in the kidney after AKI, and NGAL protein was easily detected in the blood and urine soon after AKI (Mishra et al, 2003; Supavekin et al, 2003; Devarajan et al, 2003; Mishra et al, 2004b). In a cross-sectional study, human adults with established AKI (defined as a doubling of the serum creatinine in less than 5 days) displayed a greater than 10-fold increase in plasma NGAL and more than a 100-fold increase in urine NGAL when compared to controls (Mori et al, 2005). Kidney biopsies in these patients showed intense accumulation of immuno-reactive NGAL in 50% of the cortical tubules.
In a prospective study of children undergoing cardiopulmonary bypass, the diagnosis of AKI (defined as a 50% increase in serum creatinine) was only possible 1–3 days after surgery (Mishra et al, 2005). In marked contrast, NGAL measurements revealed a 10-fold increase in the urine and plasma, within 2–6 hours of the surgery in patients who subsequently developed AKI. Both urine and plasma NGAL were powerful independent predictors of AKI, with an area under the curve (AUC) of 0.998 for the 2 hour urine NGAL and 0.91 for the 2 hour plasma NGAL measurement. These findings have been confirmed in a prospective study of adults who developed AKI after cardiac surgery, in whom urinary NGAL was significantly elevated by 1–3 hours after the operation (Wagener et al, 2006).
NGAL has also been evaluated as a biomarker of AKI in kidney transplantation. Biopsies of kidneys obtained 1 hour after vascular anastomosis revealed a significant correlation between NGAL staining intensity and the development of delayed graft function (Mishra et al, 2006). In a prospective multicenter study, urine NGAL levels in samples collected on the day of transplant identified cadaveric kidney recipients who subsequently developed delayed graft function and dialysis requirement (which typically occurred 2–4 days later). The receiver- operating characteristic (ROC) curve for prediction of delayed graft function based on urine NGAL at day 0 showed an AUC of 0.9, indicative of an excellent predictive biomarker (Parikh et al, 2006). Urine NGAL has also been shown to predict the severity of AKI and dialysis requirement in a multicenter study of diarrhea-associated hemolytic uremic syndrome (Trachtman et al, 2006). Accumulating evidence also suggests that plasma and urine NGAL measurements represent predictive biomarkers of AKI following contrast administration in prospective studies (Hirsch et al, 2007). Finally, urine NGAL predicted development and severity of AKI in a heterogeneous prospective cohort of critically ill patients with unknown timing of kidney injury (Zapitelli et al, 2007).
In summary, NGAL has emerged as a sensitive and specific biomarker of AKI from diverse etiologies, with significant potential for early diagnosis. Standardized point-of-care clinical assays for NGAL are currently undergoing large-scale validation (Dent et al, 2007), and hold tremendous promise for the early diagnosis of AKI in patients with cancer.
The biological role of NGAL induction by the injured kidney has recently attracted a great deal of attention. In a mouse model of renal ischemia-reperfusion injury, administration of recombinant NGAL either before or shortly after the insult resulted in marked preservation of kidney function and structure, attenuation of tubule cell apoptosis, and an enhanced tubule cell proliferative response (Mishra et al, 2004b). This protective effect is dependent on the concomitant delivery of siderophore and iron to the proximal tubule, and is accompanied by upregulation of heme oxygenase-1 (a protective anti-oxidant) and preservation of proximal tubule N-cadherin expression (to enhance the epithelial phenotype) (Mori et al, 2005). These protective properties in the mature kidney are consistent with the epithelial induction actions of NGAL on embryonic kidney mesenchyme during kidney development (Yang et al, 2002). Translation of these findings to the treatment of human AKI is anticipated in the next few years.
V. A unifying hypothesis
How does one reconcile the multifunctional and seemingly contradictory roles of NGAL in human biology? A potentially unifying hypothesis is offered in Figure 1. Efficient mechanisms have been identified for the intracellular uptake of NGAL via receptors such as 24p3R and megalin, and for intracellular trafficking via endosomes (Schmidt-Ott, 2007). The subsequent molecular path taken by this shuttle may be largely dependent on the type of molecule being shuttled. NGAL that is devoid of siderophore and iron (holo-NGAL) rapidly scavenges intracellular iron and exits the cell via endosomal recycling pathways. The resultant intracellular iron depletion is the basis of NGAL’s primordial function as a bacteriostatic agent, but the same path results in a decrease in the mammalian cell’s proliferative ability and in induction of apoptosis. On the other hand, NGAL that is bound to siderophore and iron is endocytosed, there is a rapid release of iron with regulation of iron-dependent molecular pathways and downstream induction of proliferation and epithelial transformation. When NGAL is shuttling MMP-9 instead, there is enhancement of the active MMP-9 pool with resultant upregulation of MMP- 9’s well known pro-angiogenic and pro-invasive properties. Future studies aimed at testing these hypotheses hold promise for advancing our understanding of tumor biology as well as for validating diagnostic and prognostic biomarkers for cancer.
Figure 1.
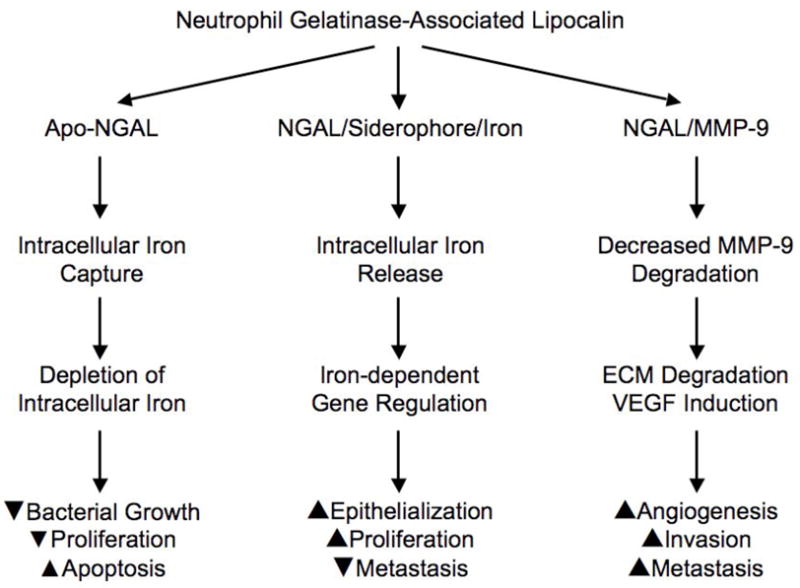
New paths for the NGAL shuttle. The molecular path taken by NGAL may be largely dependent on the type of molecule being shuttled. Apo-NGAL denotes NGAL that is devoid of siderophore or iron. ECM denotes extra-cellular matrix, and VEGF denotes vascular endothelial growth factor.
Acknowledgments
Studies cited in this review that were performed in the author’s laboratory were supported by grants from the NIH/NIDDK (R01 DK53289 and R21 DK070163).
Abbreviations
- AKI
acute kidney injury
- AUC
area under the curve
- NGAL
Neutrophil Gelatinase-Associated Lipocalin
References
- Argani P, Rosty C, Reiter RE, Wilentz RE, Murugesan SR, Leach SD, Ryu B, Skinner HG, Goggins M, Jaffee EM, Yeo CJ, Cameron JL, Kern SE, Hruban RH. Discovery of new markers of cancer through serial analysis of gene expression: prostate stem cell antigen is overexpressed in pancreatic adenocarcinoma. Cancer Res. 2001;61:4320–4324. [PubMed] [Google Scholar]
- Bauer M, Eickhoff JC, Gould MN, Mundhenke C, Maass N, Friedl A. Neutrophil gelatinase-associated lipocalin (NGAL) is a predictor of poor prognosis in human primary breast cancer. Breast Cancer Res Treat. 2007 Jun 7; doi: 10.1007/s10549-007-9619-3. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Berger T, Togawa A, Duncan GS, Elia AJ, You-Ten A, Wakeham A, Fong HE, Cheung CC, Mak TW. Lipocalin 2-deficient mice exhibit increased sensitivity to E. coli infection but not to ischemia-reperfusion injury. Proc Natl Acad Sci USA. 2006;103:1834–1839. doi: 10.1073/pnas.0510847103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulikas T. Molecular mechanisms of cisplatin and its liposomally encapsulated form, Lipoplatin™. Lipoplatin™ as a chemotherapy and antiangiogenesis drug. Cancer Ther. 2007;5:349–376. [Google Scholar]
- Bratt T. Lipocalins and cancer. Biochim Biophys Acta. 2000;1482:318–326. doi: 10.1016/s0167-4838(00)00154-0. [DOI] [PubMed] [Google Scholar]
- Davis TR, Tabatabai L, Bruns K, Hamilton RT, Nilsen-Hamilton M. Basic fibroblast growth factor induces 3T3 fibroblasts to synthesize and secrete a cyclophilin-like protein and b2-microglobulin. Biochim Biophys Acta. 1991;1095:145–152. doi: 10.1016/0167-4889(91)90077-b. [DOI] [PubMed] [Google Scholar]
- Dent CL, Ma Q, Dastrala S, Bennett M, Mitsnefes MM, Barasch J, Devarajan P. Plasma NGAL predicts acute kidney injury, morbidity and mortality after pediatric cardiac surgery: a prospective uncontrolled cohort study. Crit Care. 2007;11:R127. doi: 10.1186/cc6192. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devarajan P. Novel biomarkers for the early prediction of acute kidney injury. Cancer Therapy. 2005;3:477–488. [Google Scholar]
- Devarajan P. Update on mechanisms of ischemic acute kidney injury. J Am Soc Nephrol. 2006;17:1503–1520. doi: 10.1681/ASN.2006010017. [DOI] [PubMed] [Google Scholar]
- Devarajan P. Emerging biomarkers of acute kidney injury. Contrib Nephrol. 2007a;156:203–212. doi: 10.1159/000102085. [DOI] [PubMed] [Google Scholar]
- Devarajan P. Proteomics for Biomarker Discovery in Acute Kidney Injury. Semin Nephrol. 2007b;27:637–651. doi: 10.1016/j.semnephrol.2007.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devarajan P, Mishra J, Supavekin S, Patterson LT, Steven Potter S. Gene expression in early ischemic renal injury: clues towards pathogenesis, biomarker discovery, and novel therapeutics. Mol Genet Metab. 2003;80:365–376. doi: 10.1016/j.ymgme.2003.09.012. [DOI] [PubMed] [Google Scholar]
- Flo TH, Smith KD, Sato S, Rodriguez DJ, Holmes MA, Strong RK, Akira S, Aderem A. Lipocalin 2 mediates an innate immune response to bacterial infection by sequestering iron. Nature. 2004;432:917–921. doi: 10.1038/nature03104. [DOI] [PubMed] [Google Scholar]
- Flower D, North A, Sansom C. The lipocalin protein family: structural and sequence overview. Biochim Biophys Acta. 2000;1482:9–24. doi: 10.1016/s0167-4838(00)00148-5. [DOI] [PubMed] [Google Scholar]
- Flower DR. The lipocalin family: structure and function. Biochem J. 1996;318:1–14. doi: 10.1042/bj3180001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedl A, Stoesz SP, Buckley P, Gould MN. Neutrophil gelatinase-associated lipocalin in normal and neoplastic human tissues. Cell type-specific pattern of expression. Histochem J. 1999;31:433–441. doi: 10.1023/a:1003708808934. [DOI] [PubMed] [Google Scholar]
- Furutani M, Arii S, Mizumoto M, Kato M, Inamura M. Identification of a neutrophil gelatinase-associated lipocalin mRNA in human pancreatic cancers using a modified signal sequence trap method. Cancer Lett. 1998;122:209–214. doi: 10.1016/s0304-3835(97)00391-1. [DOI] [PubMed] [Google Scholar]
- Garay-Rojas E, Harper M, Hraba-Renevey S, Kress M. An apparent autocrine mechanism amplifies the dexamethasone- and retinoic acid-induced expression of mouse lipocalin- encoding gene 24p3. Gene. 1996;170:173–180. doi: 10.1016/0378-1119(95)00896-9. [DOI] [PubMed] [Google Scholar]
- Goetz DH, Holmes MA, Borregaard N, Bluhm ME, Raymond KN, Strong RK. The neutrophil lipocalin NGAL is a bacteriostatic agent that interferes with siderophore-mediated iron acquisition. Mol Cell. 2002;10:1033–1043. doi: 10.1016/s1097-2765(02)00708-6. [DOI] [PubMed] [Google Scholar]
- Goetz DH, Willie ST, Armen RS, Bratt T, Borregaard N, Strong RK. Ligand preference inferred from the structure of neutrophil gelatinase associated lipocalin. Biochem J. 2000;39:1935–1941. doi: 10.1021/bi992215v. [DOI] [PubMed] [Google Scholar]
- Gruvberger S, Ringnér M, Chen Y, Panavally S, Saal LH, Borg A, Fernö M, Peterson C, Meltzer PS. Estrogen receptor ststus in breast cancer is associated with remarkably distinct gene expression patterns. Cancer Res. 2001;61:5979– 5984. [PubMed] [Google Scholar]
- Gwira JA, Wei F, Ishibe S, Ueland JM, Borasch J, Cantley LG. Expression of Ngal regulates epithelial morphogenesis in vitro. J Biol Chem. 2005;280:7875–7882. doi: 10.1074/jbc.M413192200. [DOI] [PubMed] [Google Scholar]
- Hanai J, Mammoto T, Seth P, Mori K, Karumanchi A, Barasch J, Sukhatme VP. Lipocalin 2 diminishes invasiveness and metastasis of ras-transformed cells. J Biol Chem. 2005;280:13641–13647. doi: 10.1074/jbc.M413047200. [DOI] [PubMed] [Google Scholar]
- Hanigan MH, Devarajan P. Cisplatin nephrotoxicity: molecular mechanisms. Cancer Therapy. 2003;1:1–15. [PMC free article] [PubMed] [Google Scholar]
- Hirsch R, Dent C, Pfriem H, Allen J, Beekman RH, 3rd, Ma Q, Dastrala S, Bennett M, Mitsnefes M, Devarajan P. NGAL is an early predictive biomarker of contrast-induced nephropathy in children. Pediatr Nephrol. 2007;22:2089–2095. doi: 10.1007/s00467-007-0601-4. [DOI] [PubMed] [Google Scholar]
- Hraba-Reveney S, Turler H, Kress M, Salomon C, Weil R. SV40-induced expression of mouse gene 24p3 involves a post-transcriptional mechanism. Oncogene. 1989;4:601–608. [PubMed] [Google Scholar]
- Hung Y-C, Huang GS, Lin L-W, Hong M-Y, Se P-S. Thea sinensis melanin prevents cisplatin-induced nephrotoxicity in mice. Food Chem Toxicol. 2007;45:1123–1130. doi: 10.1016/j.fct.2006.12.017. [DOI] [PubMed] [Google Scholar]
- Kjeldsen L, Johnsen AH, Sengelov H, Borregaard N. Isolation and primary structure of NGAL, a novel protein associated with human neutrophil gelatinase. J Biol Chem. 1993;268:10425–10432. [PubMed] [Google Scholar]
- Kubben FJGM, Sier CFM, Hawinkels LJAC, Tschesche H, Duijn WV, Zuidwijk K, van der Reijden JJ, Hanemaaijer R, Griffioen G, Lamers CBHW, Varspaget HW. Clinical evidence for a protective role of lipocalin-2 against MMP-9 autodegradation and the impact for gastric cancer. European J Cancer. 2007;43:1869–1876. doi: 10.1016/j.ejca.2007.05.013. [DOI] [PubMed] [Google Scholar]
- Lamiere NH, Flombaum CD, Moreau D, Ronco C. Acute renal failure in cancer patients. Ann Med. 2005;37:13–25. doi: 10.1080/07853890510007205. [DOI] [PubMed] [Google Scholar]
- Laurell H, Bouisson M, Berthelemy P, Rochaix P, Dejean S, Besse P, Susini C, Pradayrol L, Vaysse N, Buscail L. Identification of biomarkers of human pancreatic adenocarcinomas by expression profiling and validation with gene expression analysis in endoscopic ultrasound-guided fine needle aspiration samples. World J Gastroenterol. 2006;12:3344–3351. doi: 10.3748/wjg.v12.i21.3344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H-J, Lee E-K, Lee K-J, Hong S-W, Yoon Y, Kim J-S. Ectopic expression of neutrophil gelatinase- associated lipocalin suppresses the invasion and liver metastasis of colon cancer cells. Int J Cancer. 2005b;118:2490–2497. doi: 10.1002/ijc.21657. [DOI] [PubMed] [Google Scholar]
- Lee S, Jilani SM, Nikolova GV, Carpizo D, Iruela-Arispe ML. Processing of VEGF-A by matrix metalloproteinases regulates bioavailability and vascular patterning in tumors. J Cell Biol. 2005;169:681–691. doi: 10.1083/jcb.200409115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lochner A, Sternlicht MD, Werb Z, Bissell MJ. The significance of matrix metalloproteinases during early stages of tumor progression. Ann NY Acad Sci. 1998;857:180–193. doi: 10.1111/j.1749-6632.1998.tb10116.x. [DOI] [PubMed] [Google Scholar]
- Madoz-Gurpide J, Lopez-Serra P, Martinez-Torrecuadrada JL, Sanchez L, Lombardia L, Casal JI. Proteomics-based validation of genomic data. Applications in colorectal cancer diagnosis. Molecular & Cellular Proteomics. 2006;5:1471–1483. doi: 10.1074/mcp.M600048-MCP200. [DOI] [PubMed] [Google Scholar]
- Mishra J, Dent C, Tarabishi R, Mitsnefes MM, Ma Q, Kelly C, Ruff SM, Zahedi K, Shao M, Bean J, Mori K, Barasch J, Devarajan P. Neutrophil gelatinase-associated lipocalin (NGAL) as a biomarker for acute renal injury after cardiac surgery. Lancet. 2005;365:1231–1238. doi: 10.1016/S0140-6736(05)74811-X. [DOI] [PubMed] [Google Scholar]
- Mishra J, Ma Q, Kelly C, Mitsnefes M, Mori K, Barasch J, Devarajan P. Kidney NGAL is a novel early marker of acute injury following transplantation. Pediatr Nephrol. 2006;21:856–863. doi: 10.1007/s00467-006-0055-0. [DOI] [PubMed] [Google Scholar]
- Mishra J, Ma Q, Prada A, Mitsnefes M, Zahedi K, Yang J, Barasch J, Devarajan P. Identification of neutrophil gelatinase-associated lipocalin as a novel early urinary biomarker for ischemic renal injury. J Am Soc Nephrol. 2003;14:2534–2543. doi: 10.1097/01.asn.0000088027.54400.c6. [DOI] [PubMed] [Google Scholar]
- Mishra J, Mori K, Ma Q, Kelly C, Barasch J, Devarajan P. Neutrophil Gelatinase-Associated Lipocalin: A novel early urinary biomarker for cisplatin nephrotoxicity. Am J Nephrol. 2004a;24:307–315. doi: 10.1159/000078452. [DOI] [PubMed] [Google Scholar]
- Mishra J, Mori K, Ma Q, Kelly C, Yang J, Mitsnefes M, Barasch J, Devarajan P. Amelioration of ischemic acute renal injury by neutrophil gelatinase-associated lipocalin. J Am Soc Nephrol. 2004b;15:3073–3082. doi: 10.1097/01.ASN.0000145013.44578.45. [DOI] [PubMed] [Google Scholar]
- Mori K, Lee HT, Rapoport D, Drexler IR, Foster K, Yang J, Schmidt-Ott KM, Chen X, Li JY, Weiss S, Mishra J, Cheema FH, Markowitz G, Suganami T, Sawai K, Mukoyama M, Kunis C, D’Agati V, Devarajan P, Barasch J. Endocytic delivery of lipocalin-siderophore-iron complex rescues the kidney from ischemia-reperfusion injury. J Clin Invest. 2005;115:610–621. doi: 10.1172/JCI23056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moses MA, Wiederschain D, Loughlin KR, Zurakowski D, Lamb CC, Freeman MR. Increased incidence of matrix metalloproteinases in urine of cancer patients. Cancer Res. 1998;58:1395–1399. [PubMed] [Google Scholar]
- Nielsen BS, Borregaard N, Bundgaard JR, Timshel S, Sehested M, Kjeldsen L. Induction of NGAL synthesis in epithelial cells of human colorectal neoplasia and inflammatory bowel disease. Gut. 1996;38:414–420. doi: 10.1136/gut.38.3.414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parikh CR, Edelstein CL, Devarajan P, Cantley L. Biomarkers of acute kidney injury: early diagnosis, pathogenesis, and recovery. J Investig Med. 2007a;55:333–340. doi: 10.2310/6650.2007.00015. [DOI] [PubMed] [Google Scholar]
- Parikh CR, Jani A, Mishra J, Ma Q, Kelly C, Barasch J, Edelstein CL, Devarajan P. Urine NGAL and IL-18 are predictive biomarkers for delayed graft function following kidney transplantation. Am J Transplant. 2006;6:1639–1645. doi: 10.1111/j.1600-6143.2006.01352.x. [DOI] [PubMed] [Google Scholar]
- Raymond KN, Dertz EA, Kim SS. Enterobactin: an archetype for microbial iron transport. Proc Natl Acad Sci USA. 2003;100:3584–3588. doi: 10.1073/pnas.0630018100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santoso JT, Lucci JA, Coleman RL, Schafer I, Hannigan EV. Saline, mannitol, and furosemide hydration in acute cisplatin nephrotoxicity: a randomized trial. Cancer Chemother Pharmacol. 2003;52:13–18. doi: 10.1007/s00280-003-0620-1. [DOI] [PubMed] [Google Scholar]
- Schmidt-Ott KM, Mori K, Kalandadze A, Li JY, Paragas N, Nicholas T, Devarajan P, Barasch J. Neutrophil gelatinase-associated lipocalin-mediated iron traffic in kidney epithelia. Curr Opin Nephrol Hypertens. 2006;15:442– 449. doi: 10.1097/01.mnh.0000232886.81142.58. [DOI] [PubMed] [Google Scholar]
- Schmidt-Ott KM, Mori K, Li JY, Kalandadze A, Cohen DJ, Devarajan P, Barasch J. Dual action of neutrophil gelatinase-associated lipocalin. J Am Soc Nephrol. 2007;18:407–413. doi: 10.1681/ASN.2006080882. [DOI] [PubMed] [Google Scholar]
- Sheikh-Hamad D, Timmins K, Jalali Z. Cisplatin-induced renal toxicity: Possible reversal by N-acetylcysteine. J Am Soc Nephrol. 1997;8:1640–1645. doi: 10.1681/ASN.V8101640. [DOI] [PubMed] [Google Scholar]
- Stoesz SP, Gould MN. Overexpression of neu-related lipocalin (NRL) in neu-initiated but not ras or chemically initiated rat mammary carcinomas. Oncogene. 1995;11:2233–2241. [PubMed] [Google Scholar]
- Stoesz SP, Friedl A, Haag JD, Lindstrom MJ, Clark GM, Gould MN. Heterogeneous expression of the lipocalin NGAL in primary breast cancers. Int J Cancer. 1998;79:565–572. doi: 10.1002/(sici)1097-0215(19981218)79:6<565::aid-ijc3>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- Supavekin S, Zhang W, Kucherlapati R, Kaskel FJ, Moore LC, Devarajan P. Differential gene expression following early renal ischemia/reperfusion. Kidney Int. 2003;63:1714–1724. doi: 10.1046/j.1523-1755.2003.00928.x. [DOI] [PubMed] [Google Scholar]
- Terris B, Blaveri E, Crnogorac-Jurcevic T, Jones M, Missiaglia E, Ruszniewski P, Sauvanet A, Lemoine NR. Characterization of gene expression profiles in intraductal papillary-mucinous tumors of the pancreas. Am J Pathol. 2002;160:1745–1754. doi: 10.1016/S0002-9440(10)61121-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trachtman H, Christen E, Cnaan A, Patrick J, Mai V, Mishra J, Jain A, Bullington N, Devarajan P Investigators of the HUS- SYNSORB Pk Multicenter Clinical Trial. Urinary neutrophil gelatinase-associated lipocalcin in D+HUS: a novel marker of renal injury. Pediatr Nephrol. 2006;21:989–994. doi: 10.1007/s00467-006-0146-y. [DOI] [PubMed] [Google Scholar]
- Triebel S, Blaser J, Reinke H, Tschesche H. A 25 kDa α2- microglobulin-related protein is a component of the 125 kDa form of human gelatinase. FEBS Lett. 1992;314:386–388. doi: 10.1016/0014-5793(92)81511-j. [DOI] [PubMed] [Google Scholar]
- van de Vijver MJ, He YD, van’t Veer LJ, Dai H, Hart AA, Voskuil DW, Schreiber GJ, Peterse JL, Roberts C, Marton MJ, Parrish M, Atsma D, Witteveen A, Glas A, Delahaye L, van der Velde T, Bartelink H, Rodenhuis S, Rutgers ET, Friend SH, Bernards R. A gene-expression signature as a predictor of survival in breast cancer. N Engl J Med. 2002;347:1999–2009. doi: 10.1056/NEJMoa021967. [DOI] [PubMed] [Google Scholar]
- Van’t Veer LJ, Dai H, Van de Vijyer MJ, He YDD, Hart AAM, Mao M, Peterse HL, van der Kooy K, Marton MJ, Witteveen AT, Schreiber GJ, Kerkhoven RM, Roberts C, Linsley PS, Bernards R, Friend SH. Gene expression profiling predicits clinical outcome of breast cancer. Nature. 2002;415:530–536. doi: 10.1038/415530a. [DOI] [PubMed] [Google Scholar]
- Wagener G, Jan M, Kim M Mori K, Barasch JM, Sladen RN, Lee HT. Association between increases in urinary neutrophil gelatinase-associated lipocalin and acute renal dysfunction after adult cardiac surgery. Anesthesiology. 2006;105:485–491. doi: 10.1097/00000542-200609000-00011. [DOI] [PubMed] [Google Scholar]
- Wang Y, Klijn JG, Zhang Y, Sieuwerts AM, Look MP, Yang F, Talantov D, Timmermans M, Meijer-van Gelder ME, Yu J, Jatkoe T, Berns EM, Atkins D, Foekens JA. Gene- expression profiles to predict distant metastasis of lymph- node-negative primary breast cancer. Lancet. 2005;365:671–679. doi: 10.1016/S0140-6736(05)17947-1. [DOI] [PubMed] [Google Scholar]
- Xu S, Venge P. Lipocalins as biochemical markers of disease. Biochim Biophys Acta. 2000;1482:298–307. doi: 10.1016/s0167-4838(00)00163-1. [DOI] [PubMed] [Google Scholar]
- Yan L, Borregaard N, Kjeldsen L, Moses MA. The high molecular weight urinary matrix metalloproteinase (MMP) activity is a complex of gelatinase B/MMP-9 and neutrophil gelatinase-associated lipocalin (NGAL) J Biol Chem. 2001;276:37258–37265. doi: 10.1074/jbc.M106089200. [DOI] [PubMed] [Google Scholar]
- Yang J, Goetz D, Li JY, Wang W, Mori K, Setlik D, Du T, Erdjument-Bromage H, Tempst P, Strong R, Barasch J. An iron delivery pathway mediated by a lipocalin. Mol Cell. 2002;10:1045–1056. doi: 10.1016/s1097-2765(02)00710-4. [DOI] [PubMed] [Google Scholar]
- Zappitelli M, Washburn KK, Arikan AA, Loftis L, Ma Q, Devarajan P, Parikh CR, Goldstein SL. Urine neutrophil gelatinase-associated lipocalin is an early marker of acute kidney injury in critically ill children: a prospective cohort study. Crit Care. 2007;2(11):4–R84. doi: 10.1186/cc6089. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Xu L, Xiao D, Xie J, Zeng H, Wang Z, Zhang X, Niu Y, Shen Z, Shen J, Wu X, Li E. Upregulation of neutrophil gelatinase-associated lipocalin in oesophageal squamous cell carcinoma: significant correlation with cell differentiation and tumour invasion. J Clin Pathol. 2007;60:555–561. doi: 10.1136/jcp.2006.039297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou H, Hewitt SM, Yuen PST, Star R. Acute kidney injury biomarkers – needs, present status, and future promise. NephSAP. 2006;5:63–71. [PMC free article] [PubMed] [Google Scholar]


