Abstract
Rationale: Monocytes are central to the initiation of the inflammatory response in sepsis, with caspase-1 activation playing a key role. Monocyte deactivation during sepsis has been linked to poor outcomes.
Objectives: Given the importance of caspase-1 in the immune response, we investigated whether monocytes from patients early in septic shock demonstrate alterations in mRNAs for caspase-1–related molecules.
Methods: Patients with septic shock (n = 26; age >18 years), critically ill intensive care unit patients (n = 20), and healthy volunteers (n = 22) were enrolled in a prospective cohort study in a university intensive care unit. Demographic, biological, physiologic, and plasma cytokine measurements were obtained. Monocytes were assayed for ex vivo tumor necrosis factor-α production, and fresh monocyte mRNA was analyzed by quantitative reverse-transcription polymerase chain reaction for Toll-like receptors, NOD-LRR proteins, cytokines, and nuclear factor-κB–related genes.
Measurements and Main Results: Relative copy numbers for the inflammasome mRNAs for ASC, caspase-1, NALP1, and Pypaf-7 were significantly lower in patients with septic shock compared with critically ill control subjects. NALP1 mRNA levels were linked to survival in patients with sepsis (P = 0.0068) and correlated with SAPS II scores (r = −0.63).
Conclusions: These data suggest that monocyte deactivation occurs during the earliest stages of the systemic inflammatory response and that changes in inflammasome mRNA expression are part of this process.
Keywords: inflammasome, monocytes, septic shock, messenger RNA, NALP1
AT A GLANCE COMMENTARY
Scientific Knowledge on the Subject
Novel intracellular sensors and regulators of caspase-1 (termed NLRs) modulate innate host responses. These NLRs assemble in response to bacterial and other exogenous challenges and induce major adaptive responses.
What This Study Adds to the Field
This study in critically ill humans shows that major changes in caspase-1 regulatory molecules occur in severe septic shock and correlate with the severity of illness.
Sepsis is a leading cause of death in the United States, accounting for an estimated 215,000 deaths per year (1). Although the precise cause of death in sepsis is unclear, in patients who die there seems to be progression from a generalized inflammatory response, referred to as the systemic inflammatory response syndrome (SIRS) (2), to progressive organ failure, known as multiorgan dysfunction syndrome (3).
Of the initial mediators of sepsis, inflammatory cytokines play a key role (4). There is also evidence for increased antiinflammatory cytokine production including IL-10, IL-1RA, and soluble tumor necrosis factor (TNF) receptors I and II (5, 6). After activation of the innate immune response, monocytes play an important role in initiating the SIRS response. Not only do they release inflammatory cytokines, but de novo replication in the bone marrow increases their numbers (7), and with circulation they can migrate to sites of inflammation in tissue (8).
Recent studies of Toll-like receptors (TLRs) (9) and the activation of IL-1β (10), a key inflammatory cytokine, have resulted in new insights into the sepsis response. TLRs enable cells to identify pathogens such as bacteria, fungi, and viruses (11). Activation of TLRs promotes recruitment and activation of adapters and signaling intermediates collectively termed the “signalosome,” which results in the translocation of nuclear factor (NF)-κB protein dimers to the nucleus, where they promote expression of genes involved in the immune inflammatory response.
Among these acute reactions, IL-1β secretion, through the activation of caspase-1, is a pivotal event in the inflammatory response (12). The post-translational activation of caspase-1 is tightly regulated by a complex of proteins termed the “inflammasome,” the known components of which include caspase-1, apoptosis-associated speck-like protein containing a CARD (ASC), NALP1 (NACHT, leucine-rich repeat and pyrin domain containing 1), and caspase-5 (13). Alternate inflammasome constructions have been suggested to contain pyrin (14, 15), NALP3 (14), and other members of the NOD-LRR (NLR) family (16, 17). ASC facilitates inflammasome assembly, thus triggering caspase-1 activation and IL-1β processing. ASC can also regulate the NF-κB pathway, thus linking the inflammasome to the signalosome (18).
Animal and human studies have highlighted the importance of the inflammasome pathways in the inflammatory response to sepsis (19). For example, caspase-1 knock-out mice are protected from endotoxin and Escherichia coli–induced sepsis (20, 21), while a naturally occurring polymorphism for human caspase-12, a putative regulator of caspase-1, has been linked to sepsis (22, 23). Thus, caspase-1 activation seems to be a prerequisite for a competent immune response (24). Similarly, pyrin—the protein mutated in familial Mediterranean fever—has been shown to regulate production of mature IL-1β by complexing with pro–caspase-1 and ASC (14). When mutated, as in the case of familial Mediterranean fever, excess inflammation results.
Given the relationship of caspase-1 to sepsis, we sought to determine if there were alterations in the caspase-1 inflammasome in human septic shock. We hypothesized that the molecules involved in IL-1β activation (inflammasome) and NF-κB activation (signalosome) would most likely be altered in patients with the most severe form of sepsis (i.e., septic shock) and that these alterations may have prognostic significance. Therefore, we studied patients with septic shock and analyzed whether mRNA expression of components of the inflammasome, the signalosome, and cytokines differed significantly between patients with septic shock, critically ill nonseptic intensive care unit (ICU) patients, and normal control subjects.
METHODS
Study Design
After institutional review board approval and informed consent was obtained, patients admitted to the medical ICU who met the following criteria (modified from the 2001 Society of Critical Care Medicine/European Society of Intensive Care Medicine/American College of Chest Physicians/American Thoracic Society/Surgical Infections Society consensus statement) for the diagnosis of septic shock (25) were prospectively studied. These included only patients who had confirmed or suspected infection (bacterial, viral, fungal) at the time of screening (suspected infection was defined as the presence of SIRS with hypotension in the absence of obvious causes of the same); who had met three of the following four sepsis criteria within a 24-hour period: (1) fever (core temperature >38.3°C) or hypothermia (core temperature <36°C), (2) heart rate greater than 90 beats per minute or more than 2 SD above the normal value for age (except patients with conditions known to increase heart rate or those receiving treatment that would prevent tachycardia), or (3) tachypnea, defined as a respiratory rate ⩾20 breaths per minute or a PaCO2 ⩽32 mm Hg or the use of mechanical ventilation for an acute respiratory process, or (4) leukocytosis (WBC count >12,000/μl) or leukopenia (WBC count <4,000/μl) or normal WBC count with >10% immature forms; and who had cardiovascular dysfunction defined as systolic blood pressure (SBP) ⩽90 mm Hg or mean arterial pressure <60 mm Hg, a decrease of SBP >40 mm Hg from baseline for at least 1 hour despite adequate fluid resuscitation or adequate intravascular volume status, or the use of vasopressors to maintain a SBP ⩾90 mm Hg or a MAP ⩾70 mm Hg. All patients enrolled were required to have had blood sampled within 24 hours of the diagnosis of septic shock. Exclusion criteria primarily excluded patients who were immune compromised and are detailed in the online data supplement (Table E1).
Twenty-two healthy volunteers (age, 46 yr; interquartile range [IQR], 41.5–66.2; male/female ratio, 10/12) and 20 critically ill nonseptic ICU patients (age, 53 yr; IQR, 43.2–61.7; male/female ratio, 8/12) were studied for comparison to the sepsis samples. Patients eligible for inclusion in the critically ill nonseptic arm were patients within 24 hours of ICU admission without known or suspected infection based on review of treating physicians' assessment, lack of antibiotic therapy (except for prophylactic therapy), and negative microbiologic and radiographic signs of infection. Admitting diagnoses included gastrointestinal hemorrhage (n = 6), cerebral vascular accident (n = 2), seizure disorder (n = 2), altered mental status/substance abuse (n = 3), and miscellaneous (n = 7). All sepsis and control blood samples were obtained between 7 a.m. and 2 p.m.
Monocyte Purification
Monocytes from patients with septic shock, critically ill ICU control subjects, and healthy volunteers were isolated from fresh donor blood by Histopaque-1077 (Sigma-Aldrich, St. Louis, MO) density gradient centrifugation at 600 × g for 20 minutes at room temperature. The mononuclear layer was removed and washed twice in RPMI 1640 (BioWhittaker, Walkersville, MD). Monocytes were isolated by positive selection with anti-CD14–coated magnetic beads (Miltenyi Biotec, Auburn, CA) according to the manufacturer's instructions. This method consistently yields ⩾98% pure population of CD14+ cells, confirmed by flow cytometry analysis. Purified monocytes were used in experiments immediately after isolation.
Plasma Cytokine Measurements
Heparinized blood from cases and control subjects was spun at 1,200 × g for 10 minutes at 4°C. The plasma carefully removed and subjected to cytokine analysis using an Immulite automated chemiluminometer (Siemens Medical Solutions Diagnostics, Los Angeles, CA).
RNA Isolation
RNA was extracted by Trizol reagent (Invitrogen, Live Technologies, Carlsbad, CA), and 1 to 2 μg of total RNA was reverse transcribed to cDNA by ThermoScript RNase H- Reverse Transcriptase (Invitrogen Life Technologies) and diluted to 100 μl. The converted cDNA (20–60 ng) was used for quantitative polymerase chain reaction (PCR) with SYBR Green PCR Master Mix in the PRISM 7700 sequence detection system (Applied Biosystems, Foster City, CA). We used relative quantification to evaluate the expression of selected genes linked to endotoxin signaling, cytokines, receptors, and the recently described NLR family. Once selected, primer pairs were validated by PCR and high-resolution gel electrophoresis to have a single band of the desired size dimmers that were free of primer. Relative copy numbers (RCNs) of selected genes were determined by normalization to the expression of two housekeeping genes, GAPDH and CAP-1 (cyclic AMP–accessory protein), and calculated with the following equation:
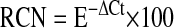 |
where E is the efficiency of PCR, and ΔCt is the Ct target − Ct reference (average of two housekeeping genes). PCR efficiency was calculated by the equation
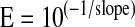 |
as previously reported (26).
Ex Vivo Cytokine Production Assay
Whole blood (50 μl) was added to 500 μl of stimulation solution containing 500 pg per milliliter lipopolysaccharide (Milenia Biotec, Bad Nauheim, Germany). This mixture was incubated at 37°C for 4 hours. After centrifugation at 1000 × g for 5 minutes, the supernatant was assayed for TNF-α using the Immulite automated chemiluminometer (Siemens Medical Solutions Diagnostics).
Statistical Analysis
Comparisons between groups were made on clinical samples collected within 24 hours of the diagnosis of septic shock. Statistical analysis was performed using JMP 6.0.0 (SAS Institute, Cary, NC).
Specific mRNA expression parameters between all patient groups in the inflammasome, signalosome, and inflammatory cytokine groupings were compared with the Kruskal-Wallis nonparametric test using Bonferroni correction for multiple tests. A P value of 0.002 was accepted as statistically significant. Genes meeting significance were analyzed pairwise between septic shock, critically ill ICU, and healthy control groups using the Wilcoxon rank-sum test, and P < 0.05 was accepted as statistically significant. To evaluate the relationship between mRNA levels and survival, comparisons were made between septic shock survivors and nonsurvivors using nominal logistic regression. Spearman's rank correlations were used to describe associations between continuous variables. All reported P values are based on two-tailed tests. Mortality was defined by survival to hospital discharge unless otherwise noted.
RESULTS
Patients
A total of 26 patients with septic shock, 20 critically ill ICU control subjects, and 22 healthy control subjects were enrolled in the study. The majority of the study subjects were male. In the patients with septic shock, the median age was 51.5 years, in-hospital mortality was 57%, and a source of infection was identified in two thirds of patients, similar to previous studies (27). The median times from sepsis onset and septic shock onset to blood sampling were 30 hours (IQR, 15–53) and 10.5 hours (IQR, 8–14), respectively. The median Acute Physiology and Chronic Health Evaluation (APACHE) II score was 34. Critically ill control subjects had a median age of 53 years, an in-hospital mortality of 5%, and a median APACHE II score of 12 (Table 1).
TABLE 1.
CLINICAL PARAMETERS FOR 26 SEPTIC AND 20 CRITICALLY ILL CONTROL PATIENTS
| Characteristics | Septic | Critically Ill Control Subjects | ||
|---|---|---|---|---|
| Age, yr | 51.5 (41.5 to 66.2)* | 53 (43.2 to 61.7) | ||
| Sex (M/F) | 18/8 | 8/12 | ||
| PaO2/FiO2, mm Hg | 154 (101 to 154) | 355 (209 to 513) | ||
| Hospital days | 9.5 (5 to 24) | 6 (4 to 7) | ||
| ICU length of stay, d | 6 (2 to 14) | 2 (2 to 2.8) | ||
| Ventilator days | 5 (1 to 12.5) | 0 (0 to 1) | ||
| 24-h net fluid balance, L | 2.4 (1.2 to 5.7) | 1.2 (−0.4 to 2.9) | ||
| MAP, mm Hg | 59 (52.5 to 62.5) | 59.5 (52.2 to 81.5) | ||
| APACHE score | 34 (27.7 to 45.2) | 12 (8.3 to 18) | ||
| SAPS II score | 52 (38.2 to 67.4) | 30.5 (21 to 37.5) | ||
| SOFA score | 13 (8.7 to 15.5) | 3 (1 to 6.8) | ||
| Hospital mortality | 15/26 (57%) | 1/20 (5%) | ||
| Site of Infection (n = 26) | ||||
| Abdomen | 4 | |||
| Pulmonary | 5 | |||
| Skin/line | 5 | |||
| Urinary tract | 3 | |||
| Multiple sites | 2 | |||
| CSF | 1 | |||
| Unknown | 6 | |||
| Microbiology (n = 26) | ||||
| Gram positive | 10 | |||
| Gram negative | 8 | |||
| Mixed | 2 | |||
| Unknown | 6 | |||
| Positive cultures (n = 26) | ||||
| Blood | 37% | |||
| All sites | 66% | |||
Definition of abbreviations: APACHE = Acute Physiology and Chronic Health Evaluation; CSF = cerebral spinal fluid; MAP = mean arterial pressure; SAPS = Simplified Acute Physiology Score; SOFA = Sepsis-related Organ Failure Assessment.
Median and interquartile range.
Cytokine Measurements
After enrollment, plasma samples taken from patients within 24 hours of onset of septic shock were measured for selected pro- and antiinflammatory cytokines. Plasma cytokine levels were significantly elevated in the patients with septic shock in comparison to critically ill and normal control subjects (Table 2), consistent with the presence of systemic inflammation. When septic survivors were compared with nonsurvivors, plasma IL-6, IL-8, and IL-10 levels were significantly elevated in nonsurvivors (P < 0.0005, P < 0.0012, and P < 0.0096, respectively).
TABLE 2.
CYTOKINE MEASUREMENTS FROM SEPTIC SHOCK CASES AND CONTROLS*
| Septic Shock
|
||||||
|---|---|---|---|---|---|---|
| Cytokine | Control Subjects (n = 10) | Critically Ill Control Subjects (n = 20) | Patients with Sepsis (n = 24) | Survivors (n = 11) | Nonsurvivors (n = 13) | P value (Survivors vs. Nonsurvivors)† |
| IL-1β | All <DL | All <DL | 12.1‡ (19 <DL, 440) | All <DL (5, 5) | 12.1‡ (8 <DL, 440) | 0.041 |
| TNF-α | 5.9 (3 <DL, 10.3) | 10.7 (4 <DL, 37.8) | 28.6 (4.6, 437) | 28.5 (4.6, 437) | 28.7 (6.8, 197) | 0.34 |
| IL-6 | — (9 <DL, 37.1) | 19.9 (2 <DL, 257) | 322 (6.5, 52,668) | 39.9 (6.5, 1,168) | 3,081 (73.4, 52,668) | 0.0005 |
| IL-8 | — (8 <DL, 6.9) | 12 (7 <DL, 52.4) | 76.8 (6.1, 3,205) | 37.6 (6.1, 108) | 76.8 (21.2, 3,205) | 0.0012 |
| IL-10 | All <DL | 9.7 (11 <DL, 33.7) | 39.9 (5 <DL, 934) | 11.5 (4 <DL, 177) | 61.8 (1 <DL, 934) | 0.0096 |
Definition of abbreviations: <DL = less than the detectable limit of 4 pg/ml for TNF-α or 5 pg/ml for rest; TNF-α = tumor necrosis factor-α.
Displayed are the plasma cytokine determinations quantified by chemiluminescence in picograms per milliliter (median ± range) from normal control subjects and critically ill control patients as compared with the initial blood draw from 24 patients with septic shock.
Sepsis survivors were compared with nonsurvivors. P values are shown for IL-1β and IL-10 by Fisher exact and for TNF-α, IL-6, and IL-8 by Wilcoxon.
This value is the median of five detectable values.
Because monocyte deactivation is considered to be a significant contributor to the sepsis response, we measured whole-blood ex vivo lipopolysaccharide-induced TNF-α production capacity in healthy control subjects and in patients with septic shock within our cohort. The median TNF-α release in picograms per milliliter was 649 (IQR, 479–994) for control subjects, 547 (IQR, 204.5–1551) for survivors, and 191 (IQR, 79.3–532.5) for nonsurvivors. Nonsurvivors differed from control subjects (P < 0.008) and trended different from survivors (P = 0.08).
mRNA Expression in Patients with Septic Shock versus Control Groups
The three mRNA groupings (inflammasome, cytokine, and signalosome) are highlighted in Table 3. mRNA expression patterns for these groups were compared using the Kruskal-Wallis nonparametric test with Bonferroni correction for multiple comparisons between control subjects, critically ill subjects, and patients with septic shock. Within the inflammasome group, mRNA levels for caspase-1 and NALP1 were significantly lower in patients with septic shock versus critically ill control or normal subjects, consistent with our hypothesis that sepsis induces substantial alterations in inflammasome components. Septic ASC and Pypaf-7 levels were significantly suppressed compared with critically ill subjects. In the cytokine group, IL-1β and TNF-α mRNA expression were significantly lower in patients with septic shock compared with normal control subjects, whereas IL-18 mRNA levels were significantly elevated compared with critically ill subjects. Within the signalosome grouping, there were significant differences between patients with septic shock and normal subjects in IRAK1 and TLR2 and between patients with septic shock and critically ill control subjects in IRAK1, IRAK2, RIP2, and TLR-2.
TABLE 3.
RELATIVE COPY NUMBERS OF MONOCYTE mRNA FOR GROUPS
| Control Subjects | Critically Ill | Septic Shock | ||||
|---|---|---|---|---|---|---|
| Inflammasome | ||||||
| ASC | 16 (10, 26) | 39.9 (35.6, 45.6) | 19 (13, 29)† | |||
| Caspase-1 | 52 (40, 65) | 33.9 (28.6, 39.9) | 20 (12, 39)*† | |||
| Caspase-5 | 20 (17, 28) | 13.8 (12.2, 16.4) | 18 (11, 25) | |||
| IPAF | 7.8 (6, 10) | 9.4 (8.1, 12.2) | 10 (6.6, 17) | |||
| NALP1 | 11.6 (8, 17) | 8.2 (6.9, 14.8) | 4.0 (2.4, 9.2)*† | |||
| NALP3 | 6.4 (4.7, 8.6) | 5.6 (3.6, 6.5) | 5.2 (2.2, 12) | |||
| Pypaf-7 | 3.6 (2.8, 5.2) | 5.2 (4.8, 6.0) | 2.8 (1.7, 4.3)† | |||
| Pyrin | 10.8 (7.7, 16) | 8.6 (4.4, 11.6) | 5.0 (2.1, 12) | |||
| Cytokine | ||||||
| IL-1β | 7.5 (6, 14) | 4.1 (1.5, 5.6) | 1.9 (1.1, 5.5)* | |||
| IL-6 | 0.008 (0.002, 0.02) | 0.001 (0.0001,0.01) | 0.004 (0.0002, 0.01) | |||
| TNF-α | 2.0 (1.3, 3.8) | 0.6 (0.3, 0.6) | 0.6 (0.2, 0.9)* | |||
| IL-10 | 0.8 (0.3, 1.2) | 0.1 (0.07, 0.2) | 1.4 (0.5, 2.7) | |||
| IL-1ra | 2.8 (1.9, 4.4) | 2.1 (1.7, 3.2) | 1.5 (0.7, 4.2) | |||
| IL-18 | 1.3 (0.8, 1.7) | 0.09 (0.002, 0.15) | 1.0 (0.03, 2.0)† | |||
| Signalosome | ||||||
| Iκ-Bα | 82 (55, 115) | 40 (28, 88) | 82 (42, 135) | |||
| IRAK-1 | 3.8 (1.9, 6.0) | 6.2 (3.9, 7.4) | 1.3 (0.6, 4.3)*† | |||
| IRAK-2 | 0.68 (0.4, 1.2) | 0.09 (0.06, 0.11) | 0.62 (0.1, 1.4)† | |||
| IRAK-M | 8.4 (4.4, 11) | 3.6 (2.1, 5.2) | 10 (2.8, 15.0) | |||
| 5.7 (4, 11) | 8.7 (4.8, 14.8) | 5.9 (4.0, 9.4) | ||||
| NF-κB p50 | 7.3 (3.2, 9.4) | 1.6 (1.3, 2.1) | 3.0 (0.8, 8.0) | |||
| NF-κB p65 | 4.0 (1.3, 6.2) | 1.6 (1.2, 2.2) | 1.6 (0.3, 3.3) | |||
| NOD-1 | 0.4 (0.2, 0.5) | 0.3 (0.2, 0.4) | 0.2 (0.05, 0.8) | |||
| NOD-2 | 4.0 (3.4, 5.7) | 3.8 (1.8, 4.2) | 2.5 (1.0, 5.3) | |||
| RIP-2 | 21 (13, 32) | 3.6 (3.2, 4.2) | 11 (4.8, 26)† | |||
| TLR-2 | 54 (34, 63) | 30 (23, 36) | 70 (59, 91)*† | |||
| TLR-4 | 11 (6.3, 15) | 15 (11, 21) | 9.4 (6.0, 14) | |||
Definition of abbreviations: NF-κB = nuclear factor-κB; TLR = Toll-like receptor; TNF-α = tumor necrosis factor-α.
Shown are monocyte mRNA expression levels, expressed as relative copy numbers median, 25th and 75th quartiles in parentheses, from 26 patients with septic shock within 24 h of onset of diagnosis, 20 critically ill control subjects, and 22 normal individuals. Significance was determined by comparing the three groups by Kruskal-Wallis test after Bonferroni correction for multiple testing within mRNA expression groups. Subsequent subject group pairwise comparisons were made by individual Wilcoxon tests, with P values as listed below. Alternate gene names are listed in Table E2.
P < 0.05 septic shock vs. control subjects.
P < 0.05 septic shock vs. critically ill.
Comparison of Inflammasome mRNA Expression between Septic Shock Survivors and Nonsurvivors
To determine the relationship between survival and selected mRNA levels, comparisons were made between septic shock survivors and nonsurvivors (Table 4). Of the inflammasome components, only NALP1 was significantly different between hospital survivors and nonsurvivors. NALP1 was higher in patients with septic shock who survived to Day 7, Day 30, and to hospital discharge as compared with nonsurvivors. When NALP1 mRNA copies for the normal subjects, critically ill control, septic shock hospital survivor, and nonsurvivor groups were compared, the nonsurvivor group differed from normal control subjects, critically ill control subjects, and the sepsis survivor groups (Figure 1).
TABLE 4.
MONOCYTE mRNA EXPRESSION* IN HOSPITAL SURVIVORS AND NONSURVIVORS
| mRNA Coding for | Survivors | Nonsurvivors | P value |
|---|---|---|---|
| ASC | 17.4 (13.0, 58.8) | 20.2 (9.4, 23.1) | 0.3 |
| Caspase-1 | 18.2 (4.9, 44.1) | 22.1 (15.9, 38.5) | 0.3 |
| Caspase-5 | 19.0 (13.4, 37.3) | 15.5 (9.8, 24.4) | 0.22 |
| IPAF | 11.6 (6.6, 28.6) | 8.8 (6.6, 17.2) | 0.3 |
| NALP1 | 7.8 (4.0, 15.8) | 3.1 (1.6, 4.2) | 0.0064 |
| Pyrin | 3.5 (1.2, 11.2) | 5.6 (3.1, 13.2) | 0.28 |
| NALP3 | 9.1 (4.6, 11.8) | 3.5 (2.0,12.1) | 0.15 |
| Pypaf-7 | 3.1 (1.6, 5.3) | 2.6 (1.9, 3.1) | 0.5 |
mRNA expressed as relative copy numbers showing the median, 25th and 75th quartiles in parentheses, and the P value from Wilcoxon test.
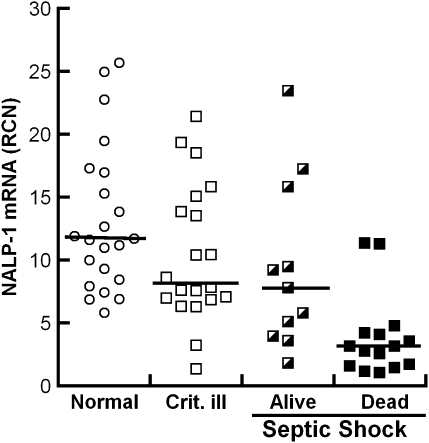
Figure 1.
NALP1 (NACHT, leucine-rich repeat and pyrin domain containing 1) and hospital survival. Plot of NALP1 mRNA levels from monocytes of normal subjects (open circles; n = 22), critically ill control subjects (open squares; n = 20), and patients with septic shock who survived to hospital discharge (half-closed squares; n = 11) or died in hospital (closed squares; n = 15). Monocytes were collected within 24 hours of septic shock and subjected to quantitative polymerase chain reaction. Individual points and medians are shown. Post hoc P values for pairwise comparisons based on Wilcoxon test demonstrated that there was no statistical difference between the normal subjects, the critically ill control subjects, and sepsis survivors; however, the NALP1 levels for sepsis nonsurvivors was less than for sepsis survivors (P = 0.0064).
Logistic regression analysis for the sepsis group revealed that NALP1 was an independent determinant of mortality at 7 days (P = 0.0026), in hospital (P = 0.0068), and at 30 days (P = 0.0312). The sensitivity and specificity of NALP1 mRNA to predict survival, using a cut point of 5.0 RCN, were 69% and 92%, respectively, for 7-day survival and 73% and 87%, respectively, for hospital survival. Stated as an odds ratio, for every 5-RCN-unit increase in NALP1, the odds of hospital survival increases 3.6-fold.
Severity of Illness Scores and Survival
All three severity of illness scoring systems (APACHE II, SAPS II [Simplified Acute Physiology Score II], and SOFA [Sepsis-related Organ Failure Assessment]) had predictive power in identifying 7-day mortality, hospital mortality, and 30-day mortality (logistic regression) in the septic shock cohort of patients. Given the association between NALP1 and survival, it is relevant to note that NALP1 also correlated significantly with severity of illness scores (APACHE II, P = 0.002; SAPS II, P = 0.0005; and SOFA, P = 0.025), providing biological plausibility for the NALP1 association with mortality.
DISCUSSION
As part of the innate host defense, monocytes recognize pathogens with highly conserved sensors, termed pattern recognition receptors. TLRs, which are cell-surface sensors, activate signaling cascades that induce an inflammatory response, whereas intracellular sensors, as exemplified by NLRs, regulate NF-κB and caspase-1 activation (28). Both pattern recognition receptors identify conserved motifs present on many different microorganisms. The TLRs signal the induction of proinflammatory cytokines, including IL-1β, a pivotal cytokine in acute inflammation. Given IL-1β's key role in inflammation, its processing and release is highly regulated. Synthesized in an inactive pro-form, IL-1β requires cleavage by caspase-1 (12). Caspase-1 activity is highly regulated by a complex of proteins termed the inflammasomes (29). The relation of the inflammasome to sepsis may be predicted given the finding that caspase-1–deficient mice are protected when exposed to lethal doses of endotoxin (20) and to lethal peritoneal E. coli infection (21).
The finding that monocyte gene expression for caspase-1, ASC, and NALP1 (major components of the inflammasome) is significantly lower in patients with septic shock than in critically ill control subjects is novel. Indeed, monocyte ex vivo TNF-α release was suppressed in sepsis nonsurvivors as well. This sepsis-induced monocyte deactivation was evident when plasma cytokines were significantly elevated and patients were clinically in septic shock. The source of plasma cytokines was not addressed in this study.
Whether this monocyte inflammasome down-regulation reflects the normal response to infection in general or is specific to septic shock remains to be determined. Further studies of patients with less severe infections may be informative. Also, because it is likely that the earliest sepsis response is immune activation, studying monocyte activation levels even earlier in the course of the response may be informative and relevant to the role of antiinflammatory therapy in sepsis. Analyzing mRNA patterns over time in patients who resolve their sepsis may provide useful information as to how long patient monocytes remain deactivated.
In relation to monocyte cytokine mRNA, the trend was also one of down-regulation, with TNF-α and IL-1β being significantly reduced, whereas IL-10 mRNA levels trended higher in patients with septic shock. Under normal circumstances, the initial proinflammatory monocyte response may be transient, followed by a normal down-regulation of monocyte responsiveness once the inflammatory signal is transferred to other immune cells. This could be due, in part, to the simultaneous release of antiinflammatory signals such as IL-10, which also down-regulates caspase-1 (30). As first suggested over a decade ago, this decreased monocyte inflammatory response in the setting of severe sepsis may be maladaptive, predisposing to opportunistic infections (31, 32). Our data suggest that altered expression of inflammasome components may play an important role in this phenomenon.
In this context, it is important to point out a caveat of our study. We do not have the mechanism to study monocytes that may have been sequestered intravascularly or into sites of infection or organ injury. Therefore, the monocyte deactivation phenomenon that we have described can only be attributed to cells still in circulation. Future studies addressing tissue monocytes in comparison to circulating cells will be important.
Regarding mortality, increased severity of illness scores in our sepsis population were a determinant of hospital mortality, with the SAPS II being most closely linked. Inflammasome components also correlated with disease severity scores, with NALP1 showing the highest correlation, suggesting that regulation of the inflammasome within 24 hours of onset of shock may reflect the severity of illness.
Within the sepsis group, of the inflammasome components that were significantly altered, NALP1 alone was an independent determinant of mortality at 7 days, hospital survival, and 30-day mortality and was more specific than SAPS II in determining 7-day survival. In this context, a recent report demonstrated that NALP1 gene polymorphisms in inbred mouse strains correlate with macrophage lethality to anthrax lethal toxin (33). Furthermore, a human NALP1 polymorphism was recently linked to the immunodeficiency state associated with familial vitiligo (34). Thus, the sepsis-induced suppression of NALP1 may induce a transient immunosuppressive phenotype to monocytes.
In conclusion, deactivation of monocytes appears within 24 hours of onset of human septic shock and is reflected by a decrease in mRNA levels of inflammasome components and proinflammatory cytokine message. Studies regarding role of the inflammasome in the sepsis response may affect future therapeutic interventions.
Supplementary Material
Acknowledgments
The authors gratefully acknowledge the support of the Pulmonary and Critical Care Clinical Trials Office at The Ohio State University.
Supported by National Institutes of Health grants HL69899 (R.J.F.), K12HD43372 (M.W.H.), and HL40871 and HL76278 (M.D.W.) and by American Lung Association grant RG52369N (M.C.E.).
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.200703-418OC on February 8, 2008
Conflict of Interest Statement: None of the authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Anderson RN, Smith BL. Deaths: leading causes for 2002. Natl Vital Stat Rep 2005;53:1–89. [PubMed] [Google Scholar]
- 2.Bone RC. Toward a theory regarding the pathogenesis of the systemic inflammatory response syndrome: what we do and do not know about cytokine regulation. Crit Care Med 1996;24:163–172. [DOI] [PubMed] [Google Scholar]
- 3.Bone RC. Immunologic dissonance: a continuing evolution in our understanding of the systemic inflammatory response syndrome (SIRS) and the multiple organ dysfunction syndrome (MODS). Ann Intern Med 1996;125:680–687. [DOI] [PubMed] [Google Scholar]
- 4.Thijs LG, Hack CE. Time course of cytokine levels in sepsis. Intensive Care Med 1995;21:S258–S263. [DOI] [PubMed] [Google Scholar]
- 5.Damas P, Reuter A, Gysen P, Demonty J, Lamy M, Franchimont P. Tumor necrosis factor and interleukin-1 serum levels during severe sepsis in humans. Crit Care Med 1989;17:975–978. [DOI] [PubMed] [Google Scholar]
- 6.Cavaillon JM, Adib-Conquy M, Fitting C, Adrie C, Payen D. Cytokine cascade in sepsis. Scand J Infect Dis 2003;35:535–544. [DOI] [PubMed] [Google Scholar]
- 7.Cohen J. The immunopathogenesis of sepsis. Nature 2002;420:885–891. [DOI] [PubMed] [Google Scholar]
- 8.van Furth R. Monocyte production during inflammation. Comp Immunol Microbiol Infect Dis 1985;8:205–211. [DOI] [PubMed] [Google Scholar]
- 9.Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell 2006;124:783–801. [DOI] [PubMed] [Google Scholar]
- 10.Burns K, Martinon F, Tschopp J. New insights into the mechanism of IL-1beta maturation. Curr Opin Immunol 2003;15:26–30. [DOI] [PubMed] [Google Scholar]
- 11.Pasare C, Medzhitov R. Toll-like receptors: linking innate and adaptive immunity. Adv Exp Med Biol 2005;560:11–18. [DOI] [PubMed] [Google Scholar]
- 12.Dinarello CA. Interleukin-1β, interleukin-18, and the interleukin-1β converting enzyme. Ann N Y Acad Sci 1998;856:1–11. [DOI] [PubMed] [Google Scholar]
- 13.Martinon F, Burns K, Tschopp J. The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-1β. Mol Cell 2002;10:417–426. [DOI] [PubMed] [Google Scholar]
- 14.Yu JW, Wu J, Zhang Z, Datta P, Ibrahimi I, Taniguchi S, Sagara J, Fernandes-Alnemri T, Alnemri ES. Cryopyrin and pyrin activate caspase-1, but not NF-kappaB, via ASC oligomerization. Cell Death Differ 2005;13:236–249. [DOI] [PubMed] [Google Scholar]
- 15.Seshadri S, Duncan MD, Hart JM, Gavrilin MA, Wewers MD. Pyrin levels in human monocytes and monocyte-derived macrophages regulate IL-1β processing and release. J Immunol 2007;179:1274–1281. [DOI] [PubMed] [Google Scholar]
- 16.Tschopp J, Martinon F, Burns K. NALPs: a novel protein family involved in inflammation. Nat Rev Mol Cell Biol 2003;4:95–104. [DOI] [PubMed] [Google Scholar]
- 17.Ting JP, Davis BK. CATERPILLER: a novel gene family important in immunity, cell death, and diseases. Annu Rev Immunol 2004;23:387–414. [DOI] [PubMed] [Google Scholar]
- 18.Sarkar A, Duncan M, Hart J, Hertlein E, Guttridge DC, Wewers MD. ASC directs NF-κB activation by regulating receptor interacting protein-2 (RIP2) caspase-1 interactions. J Immunol 2006;176:4979–4986. [DOI] [PubMed] [Google Scholar]
- 19.Scott AM, Saleh M. The inflammatory caspases: guardians against infections and sepsis. Cell Death Differ 2007;14:23–31. [DOI] [PubMed] [Google Scholar]
- 20.Li P, Allen H, Banerjee S, Franklin S, Herzog L, Johnston C, McDowell J, Paskind M, Rodman L, Salfeld J, et al. Mice deficient in IL-1beta-converting enzyme are defective in production of mature IL-1beta and resistant to endotoxic shock. Cell 1995;80:401–411. [DOI] [PubMed] [Google Scholar]
- 21.Sarkar A, Hall MW, Exline M, Hart J, Knatz N, Gatson N, Wewers MD. Caspase-1 regulates E. coli sepsis and splenic B cell apoptosis independently of IL-1β and IL-18. Am J Respir Crit Care Med 2006;174:1003–1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Saleh M, Vaillancourt JP, Graham RK, Huyck M, Srinivasula SM, Alnemri ES, Steinberg MH, Nolan V, Baldwin CT, Hotchkiss RS, et al. Differential modulation of endotoxin responsiveness by human caspase-12 polymorphisms. Nature 2004;429:75–79. [DOI] [PubMed] [Google Scholar]
- 23.Saleh M, Mathison JC, Wolinski MK, Bensinger SJ, Fitzgerald P, Droin N, Ulevitch RJ, Green DR, Nicholson DW. Enhanced bacterial clearance and sepsis resistance in caspase-12-deficient mice. Nature 2006;440:1064–1068. [DOI] [PubMed] [Google Scholar]
- 24.Joshi VD, Kalvakolanu DV, Hasday JD, Hebel RJ, Cross AS. IL-18 levels and the outcome of innate immune response to lipopolysaccharide: importance of a positive feedback loop with caspase-1 in IL-18 expression. J Immunol 2002;169:2536–2544. [DOI] [PubMed] [Google Scholar]
- 25.Levy MM, Fink MP, Marshall JC, Abraham E, Angus D, Cook D, Cohen J, Opal SM, Vincent JL, Ramsay G. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Crit Care Med 2003;31:1250–1256. [DOI] [PubMed] [Google Scholar]
- 26.Gavrilin MA, Bouakl IJ, Knatz NL, Duncan MD, Hall MW, Gunn JS, Wewers MD. Internalization and phagosome escape required for Francisella to induce human monocyte IL-1β processing and release. Proc Natl Acad Sci USA 2006;103:141–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Annane D, Aegerter P, Jars-Guincestre MC, Guidet B. Current epidemiology of septic shock: the CUB-Rea Network. Am J Respir Crit Care Med 2003;168:165–172. [DOI] [PubMed] [Google Scholar]
- 28.Martinon F, Tschopp J. NLRs join TLRs as innate sensors of pathogens. Trends Immunol 2005;26:447–454. [DOI] [PubMed] [Google Scholar]
- 29.Martinon F, Tschopp J. Inflammatory caspases: linking an intracellular innate immune system to autoinflammatory diseases. Cell 2004;117:561–574. [DOI] [PubMed] [Google Scholar]
- 30.Kim HJ, Hart J, Knatz N, Hall MW, Wewers MD. Janus kinase 3 down-regulates lipopolysaccharide-induced IL-1 beta-converting enzyme activation by autocrine IL-10. J Immunol 2004;172:4948–4955. [DOI] [PubMed] [Google Scholar]
- 31.Ertel W, Kremer JP, Kenney J, Steckholzer U, Jarrar D, Trentz O, Schildberg FW. Downregulation of proinflammatory cytokine release in whole blood from septic patients. Blood 1995;85:1341–1347. [PubMed] [Google Scholar]
- 32.Docke WD, Randow F, Syrbe U, Krausch D, Asadullah K, Reinke P, Volk HD, Kox W. Monocyte deactivation in septic patients: restoration by IFN-gamma treatment. Nat Med 1997;3:678–681. [DOI] [PubMed] [Google Scholar]
- 33.Boyden ED, Dietrich WF. Nalp1b controls mouse macrophage susceptibility to anthrax lethal toxin. Nat Genet 2006;38:240–244. [DOI] [PubMed] [Google Scholar]
- 34.Jin Y, Mailloux CM, Gowan K, Riccardi SL, LaBerge G, Bennett DC, Fain PR, Spritz RA. NALP1 in vitiligo-associated multiple autoimmune disease. N Engl J Med 2007;356:1216–1225. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.