Abstract
This study examined the control of elbow force in nine patients with Parkinson’s disease when visual feedback was available and when visual feedback was removed to determine how medication (Meds) and unilateral deep brain stimulation (DBS) of the subthalamic nucleus (STN) affect memory guided force control. Patients were examined in each of four treatment conditions: 1) off treatment; 2) Meds; 3) STN DBS; and 4) Meds plus STN DBS. With visual feedback available, there was no difference in force output across treatment conditions. When visual feedback was removed force output drifted under the target in both the off-treatment and the Meds conditions. However, when on STN DBS or Meds plus STN DBS force output drifted above the target. As such, only STN DBS had a significant effect on force output in the vision removed condition. Increased force output when on STN DBS may have occurred due to disruptions in the basal ganglia-thalamo-cortical circuitry. We suggest that modulation of output of the internal segment of the globus pallidus by STN DBS may drive the effect of STN DBS on memory guided force control.
Index Terms: Deep brain stimulation (DBS), force, globus pallidus, motor memory, vision
I. Introduction
For several decades levodopa and other forms of antiparkinsonian medication have been effective treatments for reducing the symptoms of Parkinson’s disease (PD) [1]–[3]. A more recent treatment of PD is deep brain stimulation (DBS) of the subthalamic nucleus (STN) [4]. However, STN DBS has been shown to have adverse affects on some aspects of cognition including executive functioning, attention, and working memory [5]–[8]. Additionally, application of STN lesions in the rat has been shown to lead to slower reaction times [9]. Based on the changes in reaction time, it has been suggested that STN lesions may impair the operation of a “motor” working memory which holds a selected response in readiness for performance until needed [9]. In humans, STN DBS has been shown to improve performance on a simple reaction time task to a greater extent than on a choice reaction time task [10]. Since choice reaction time tasks involve more complex information processing than simple reaction time tasks, less of an effect of STN DBS on choice reaction time suggests that the benefit of STN DBS may be attenuated in tasks requiring greater cognitive processing.
One example of a cognitively driven motor task is memory guided force control. Previously, it has been shown that when healthy subjects generate isometric force at a set level with visual feedback and attempt to maintain this level when visual feedback is removed, the normal response to removal of visual feedback is a slight decline in force output across time which begins within a narrow time window [11]. This decline in force following the removal of visual feedback has been attributed to decay in short-term motor memory. Patients with PD, when on their medication, have also been studied using this paradigm and show similar patterns of force control to healthy controls when visual feedback is available. However, they show a decline in force output when visual feedback is removed that is of a greater extent and occurs at a faster rate to that of healthy controls [12]. This increased decline in PD has been attributed to faster memory decay, related to deficits in higher order sensory-motor processes. The motor memory process in healthy individuals during continuous force production has been shown to be confined to the prefrontal network. The specific regions involved are the dorsolateral prefrontal cortex, the ventral pre-frontal cortex, and the anterior cingulate [13]. Since the pre-frontal cortex has been shown to be anatomically connected to the basal ganglia [14], altering STN output via DBS may directly affect the motor memory process.
While the majority of studies have found that medication and STN DBS have similar effects on motor control [15]–[18], medication and STN DBS have been shown to have different effects on selected aspects of motor control. For example, medication has been shown to lead to increased postural sway in patients with PD, while STN DBS has been shown to lead to reduced postural sway [19], [20]. In another study, medication has not been shown to significantly improve simple and choice reaction time in patients with PD, while STN DBS has been shown to lead to significantly shorter reaction times [10]. Finally, medication has been shown to be more effective than STN DBS on shortening the timing of the early phase of a grip-lift synergy in a reach and grasp task [21]. Taken together with the adverse effects of STN DBS on cognition that have previously been described [22], we hypothesize that STN DBS will have an effect on memory guided force control whereas medication may not [10], [23]. As such, the purpose of this study was to examine the control of elbow force in patients with PD to determine how medication and STN DBS affect memory guided force control.
II. Methods
A. Subjects and Stimulation Parameters
Nine patients with PD who underwent unilateral STN DBS participated in the study (Table I). One of the patients was tested twice: first, following unilateral STN DBS surgery, and then following implantation of a second STN DBS stimulator on the opposite side (Table I subject P1). Data from this patient following the second DBS surgery are included for a comparison of unilateral versus staged bilateral DBS effects. One of the patients studied had previously had a pallidotomy on the same side of the basal ganglia as STN DBS surgery (Table I subject P9). Data from this patient were analyzed separately from the other eight patients. All clinical and motor control evaluations for all of the patients were performed an average of 7.2 months post surgery and after optimization of DBS parameters had occurred. A priori, inclusion criteria were established for the study, and the first nine PD patients who received STN DBS and met the criteria were chosen for the study. We did not exclude any patients based on this inclusion criterion because the first nine patients met the requirement. Patients were examined by a movement disorders neurologist and included in the study if they: 1) had idiopathic PD as outlined by the Parkinson’s Disease Society Brain Bank diagnostic criteria [24], [25] and 2) had a positive clinical improvement [greater than a 15% reduction in scores from the entire motor section of the Unified Parkinson’s Disease Rating Scale (UPDRS)] from the Off treatment condition compared to the on STN DBS condition. Note: a 15% improvement in UPDRS reflects bilateral changes while we are investigating a unilateral intervention. We chose the UPDRS because it is the gold standard in PD research for assessing treatment efficacy, and we chose to study patients with at least a 15% reduction in score to make sure we were studying patients who responded efficaciously to STN DBS. All subjects gave informed consent to all experimental procedures, which were approved by the local Institutional Review Boards at the University of Illinois at Chicago and Rush University Medical Center.
TABLE I.
Profile of each subject
| Postsurgery UPDRS (motor section)
|
||||||||
|---|---|---|---|---|---|---|---|---|
| Subject | Age (years) | Gender | Stim Level (volts) | Pre-operative Single Dose Anti-Parkinsonian Medications | Off treatment | Meds | STN DBS | Meds plus STN DBS |
| Patients without prior surgery | ||||||||
| P1 | 60 | M | 1.5 | Carbidopa/Levodopa CR 25/250 2 tabs
Ropinerole 2 mg 2 tabs |
31 | 19 | 21.5 | 14.5 |
| P2 | 43 | F | 2.8 | Carbidopa/Levodopa 25/100 .5 tabs | 54 | 22 | 15 | 7 |
| P3 | 65 | F | 1.8 | Carbidopa/Levodopa CR 25/1 00 1 tab
Pramipexole l.Smg 1 tab |
25 | 6.5 | 21 | 6.5 |
| P4 | 46 | M | 2.1 | Carbidopa/Levodopa 25/100 3 tabs
Pramipexole Img 3 tabs |
37.5 | 8 | 9.5 | 2.5 |
| P5 | 47 | M | 2.0 | Carbidopa/Levodopa 25/1 00 1.5 tabs | 36.5 | 10.5 | 21 | 7.5 |
| P6 | 53 | M | 2.0 | Carbidopa/Levodopa 25/100 2 tabs Ropinerole 5mg 2 tabs
Amantadine 100mg 1 tab Entacapone 200mg 2 tabs |
19.5 | 3 | 14.5 | 2.5 |
| P7 | 62 | M | 3.1 | Carbidopa/Levodopa CR 50/200 1.5 tabs
Carbidopa/Levodopa 25/1 00 1 tab Entacapone 200mg 1 tab Pergolide 1 mg 1 tab |
33 | 3 | 27 | 2 |
| P8 | 34 | M | 5.3 | Carbidopa/Levodopa 257 100 2 tabs
Pramipexole 1 mg 1 tab |
6.5 | 4.5 | 4.5 | 3 |
|
| ||||||||
| Patient with a prior pallidotomy | ||||||||
|
| ||||||||
| P9 | 59 | M | 2.5 | Carbidopa/Levodopa CR 50/200 1 tab
Carbidopa/Levodopa 25/1 00 1 tab Pramipexole 0.5 mg 2 tabs |
45 | 33.5 | 35 | 25 |
M = male; F = female; tab = tablet
In all cases, a quadripolar stimulation electrode was placed in the STN opposite to the most severely affected body side. Surgery was performed as part of clinical care according to standard procedures [26], [27]. Briefly, the anatomical target was based on standard stereotactic coordinates while the patient was in the MRI scanner with a Leksell stereotactic head frame in place [28]. MRI images were subsequently reformatted and coordinates refined using a Stealth system. Intra-operatively, microelectrode penetrations were made through a burr hole to verify the neurophysiological target [27], [29]. Responses to sensory stimuli (passive movements of upper and lower limbs) were sought to identify the sensorimotor portion of the STN, and the DBS zero electrode was placed at the ventral border of STN. Microelectrode recordings and postoperative MRI demonstrated appropriate placement of the DBS lead.
The same movement disorders neurologist administered the entire motor section of the UPDRS to each patient post surgery, and these scores are reported in Table I. Following surgery, stimulation parameters were optimized in order to reduce presurgery scores on the motor section of the UPDRS, and these parameters are shown in Table II. Optimization took place during several visits over the ensuing months, and at the time of the experiments we tested the individuals on the stimulator settings determined by the patient’s neurologist.
TABLE II.
Deep brain stimulation parameters
| Contact | Pulse Width | Frequency | Voltage | |
|---|---|---|---|---|
| P1 | Monopolar 0− C+ | 60 μs | 185 Hz | 1.5 |
| P2 | Monopolar 0− C+ | 60 μs | 185 Hz | 2.8 |
| P3 | Monopolar 2− C+ | 60 μs | 185 Hz | 1.8 |
| P4 | Monopolar 1− C+ | 60 μs | 185 Hz | 2.1 |
| P5 | Monopolar 1− C+ | 60 μs | 185 Hz | 2.0 |
| P6 | Monopolar 1− C+ | 60 μs | 185 Hz | 2.0 |
| P7 | Monopolar 0− C+ | 60 μs | 185 Hz | 3.1 |
| P8 | Monopolar 1− C+ | 60 μs | 185 Hz | 5.3 |
| P9 | Monopolar 0− C+ | 60 μs | 185 Hz | 2.5 |
B. Experimental Setup
The experiments were performed on two consecutive days in each of four treatment conditions: 1) off treatment; 2) Meds; 3) STN DBS; and 4) Meds plus STN DBS. Examination of Figs. 1 and 2 do not show any trend in the data that can be attributed to testing order. On day 1, testing of condition 1 took place between 9–11 am and condition 2 occurred between 1–3 pm. On day 2, the same testing schedule as Day 1 was set for conditions 3 and 4, respectively. To ensure that each PD patient was in the operationally defined Off state, patients were always tested in the Off condition following 12 hours without the specific treatment [30], [31]. For instance, the night prior to Day 1 both treatments were withheld, and the night prior to Day 2 only STN DBS was withheld. Each patient stayed in a hotel close to the laboratory and was examined using a Medtronic Console Programmer (Model 7432) on each night prior to Day 1 and 2 to make certain that the stimulator was off.
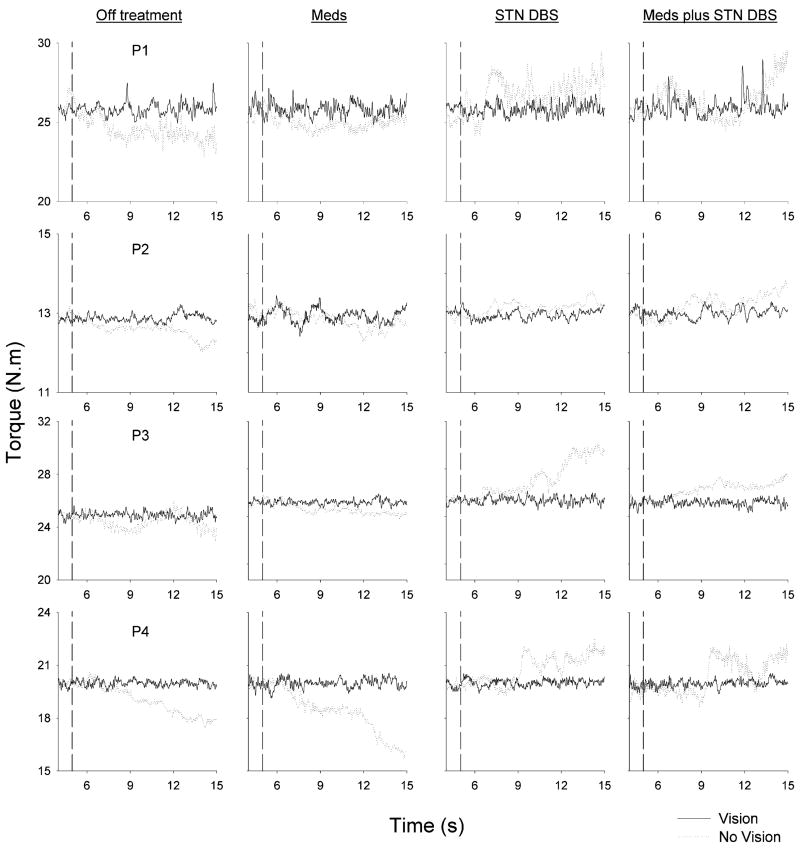
Fig. 1.
Individual force trial records from four PD patients (P1–P4) under each of four treatment conditions (Off treatment, Meds, STN DBS, and Meds plus STN DBS) under full visual feedback (solid lines) and visual feedback removed (dotted lines). The vertical dashed line in each plot represents the point at which visual feedback was removed in the visual feedback removed condition.
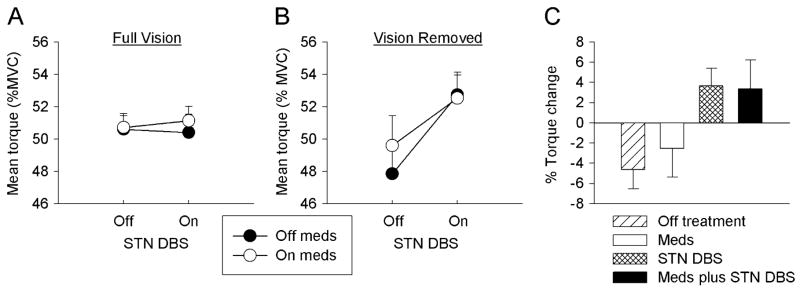
Fig. 2.
Mean torque as a percentage of MVC over the final 3 s of the trials from 8 PD patients under each of the four treatment conditions in the full visual feedback condition (A) and visual feedback removed condition (B). (C) is the percent of torque change (change between the mean torque of the final 3 s and the mean torque 3 s prior to the removal of visual feedback under each of the four treatment conditions). Data are the means and standard errors.
To ensure that each patient was ON STN DBS, the motor section of the UPDRS was administered 90 min after activation of the stimulator. All patients had reduced UPDRS values 90 min following reactivation of the stimulator. Patients’ normal medication dose schedule was restarted on Day 1 after morning testing was completed and continued through Day 2 of testing. On Day 2, all patients took medication between 6:00–7:00 am and then again at 9:00 am. 45 min after the 9:00 am dosage, the motor section of the UPDRS was administered to ensure that medication efficacy was optimized. All patients responded to levodopa therapy, so testing proceeded 50 min after administration of the 9:00 am levodopa dose. For the afternoon session on Day 2, patients took medication at 12:30 pm, and the same protocol for the morning session was followed. For all patients in this study, the afternoon testing commenced 50 min later. At each dose, prior to testing, patients took the medication listed in Table I. It should be noted that during a typical day patients would routinely take several of these doses throughout the day. Patients took the optimal levodopa treatment, which is the clinically determined preoperative medication dosage [32]. We used presurgery dosage levels instead of postsurgery dosage levels because most patients had a reduction in their medication post-surgery, and we wanted to make certain that each patient received a therapeutic benefit from their medication. The data in Table I indicates that this was the case.
C. Force Control Task
The subject was seated with the arm abducted between 75°–90° depending on the comfort of each subject. The arm examined in this study was always contralateral to the placement of the DBS electrode. The chair height was set during the first testing session and along with the arm abduction angle remained constant across all experimental testing sessions. The forearm rested on a rigid, lightweight manipulandum that was fixed at 90° flexion in the horizontal plane. The axis of rotation was aligned with the elbow joint. Full extension of the elbow was defined as 0°. Joint torque was measured by a capacitive transducer mounted on the shaft at the axis of rotation.
The subject viewed a computer monitor that displayed a vertical cursor and a vertical target that were positioned along the horizontal axis. The cursor represented the amount of torque generated at the elbow joint. The starting position was shown as a stationary bar on the monitor and the target force level was always placed in the center of the screen. The monitor distance was set to 110 cm from the subject and this distance remained constant across the experimental sessions. The height of the monitor was set level to the eyes of each individual subject and also remained constant across testing sessions.
During the initial portion of the study, the subject’s maximum voluntary contraction (MVC) was estimated. Subjects flexed “as hard as possible” on the manipulandum for four consecutive 6-s trials. Proper rest was given between each trial. Because patient’s with PD experience bradykinesia, maximal force contractions may take a long time period before reaching a plateau [33]. Upon inspection of each trial, the 6-s time period used in this study allowed each participant to achieve a plateau of maximal force. Maximal force within each trial was averaged across the four trials.
The force vision task examined the PD patients’ ability to sustain force output with visual feedback and after the removal of visual feedback. The task required subjects to adjust their level of torque to a target level represented by a cursor on the video monitor. The minimum and maximum display range of the video monitor was set to ±3 N/m from the target force. This resulted in a highly sensitive control-to-display gain [11]. Subjects viewed online feedback of their performance in the form of a red cursor that moved from left to right across the video monitor. The subjects were instructed to match the cursor to the target throughout each trial. The force target used in the study was 50% of the maximum voluntary contraction because this force level consistently shows a reduction in force output following visual feedback removal in healthy individuals and patients with PD [11]. In the visual feedback condition, the subject received continuous, online visual feedback for the entire 15-s trial. In the visual feedback removal condition, visual feedback was displayed for the initial 5 s, and then the force cursor and target line disappeared from the video monitor. During this time, subjects were instructed to maintain their force output at the level previously specified by the target. They alternated between one trial of full visual feedback and one trial of visual feedback removal for a total of 12 consecutive trials. Proper rest of at least 60 s was given between each trial.
During testing of patients on medication, two of the patients (P3 and P6) in Table I had dyskinesias that were severe enough to affect the data collection. When this occurred, we stopped the experiment and asked the patient to repeat the trials once the dyskinesias were no longer present. We compared the data from these two patients to the other patients in the study and a similar effect for memory guided force control was present in these patients.
D. Data Processing and Analysis
Before all data analyses, the force trajectories were filtered with a fourth-order (in both forward and reverse directions) Butterworth filter using a 20-Hz lowpass cutoff. The dependent measures calculated included the mean torque for the final 3s of the trial as a percentage of MVC, the percent of torque change, and the drift time after vision removal. The percent torque change was calculated by subtracting the mean torque of the final 3 s (T2) from the mean torque 3 s (T1) prior to the removal of visual feedback (2–5 s) and dividing this by the mean 3 s T1 torque. This value was then multiplied by 100. Reduced and increased torque following visual feedback removal would have a negative and positive percent torque change, respectively. To calculate drift time, we examined the time period between visual feedback removal and the onset of change in the force signal with a method used in previous work [11]. We fit a two-parameter linear function to the force signal from 2 s before vision was removed until the time vision was removed. Then, we fit an exponential decay function (y = α × e(−β × χ)to the remaining portion of the force trial. We calculated the mean squared error from each fitted function. This sequence was repeated for 3 s by incrementally moving the point where the linear function stopped and the exponential fit began one data sample at a time. The total mean squared error was calculated for each fitted function over the 3 s time period, and the function with the minimum mean square error value provided the time (i.e., data sample) when change in force output (increase or decrease) began.
The dependent variables were examined using repeated measures analysis of variance models. All statistics in the analysis of variance were evaluated as significant when there was less than a 5% chance of making a Type I error (p < .05). All statistical analyses were calculated using Statistica statistical package (StatSoft Inc., Tulsa, OK).
III. Results
A. Medication and Unilateral STN DBS
Fig. 1 shows an elbow torque trial with vision (solid line) and without vision (dotted line) from four PD patients across each of the four treatment conditions: 1) off treatment; 2) Meds; 3) STN DBS; and 4) Meds plus STN DBS. When visual feedback was available, each subject maintained their mean torque output to similar levels under each treatment condition. When visual feedback was removed (vertical dashed line in Fig. 1), the torque output drifted over or under the force target depending on the specific treatment condition. Fig. 1 demonstrates that in the Off treatment condition the subjects decreased their torque output following visual feedback removal. In the Meds condition, torque output decreased in a similar manner to the Off treatment condition following visual feedback removal. However, in the STN DBS condition, each of the four subjects in Fig. 1 increased their torque output following visual feedback removal. These findings were robust across the group of eight patients including patient P8. We recognize that P8 has a much lower UPDRS score and a higher voltage on his stimulator postoperatively than the other patients, but it should be noted that the effects of DBS on the UPDRS and force drift patterns in this patient were consistent with the other patients. On a few isolated trials for some subjects without visual feedback, the change in torque output in the STN DBS condition was not above the target force, but the measured torque always reached levels that were much greater than the negative drift in the Off treatment condition and the medication only condition. Finally, Meds plus STN DBS resulted in an increased torque output following removal of visual feedback, and this increased torque was consistent with the increased torque which occurred in the STN DBS condition.
Group data supported the individual patient data shown in Fig. 1. Fig. 2(a) depicts mean torque output from the final 3 s of the trial expressed as a percentage of MVC across all eight subjects in each of the four treatment conditions when visual feedback was present. When visual feedback was available, there was no difference in mean torque across treatment conditions. Fig. 2(b) depicts mean torque when visual feedback was removed and shows that mean torque output differed as a function of treatment condition. A three-factor analysis of variance (Meds × STN DBS X Vision) supported the findings from Fig. 2(a) and (b). There was a nonsignificant main effect for Meds but a significant main effect for STN DBS. The main effect for STN DBS was qualified by a significant STN DBS × Vision interaction. Post hoc analyses indicated that this interaction occurred because STN DBS only had an effect when vision was removed. In the vision removed condition, mean torque was higher when on STN DBS than when off STN DBS. None of the other main effects or interactions were significant [Table III, Fig. 2(b)].
TABLE III.
Statistical results
| Three-way ANOVA
|
Two-way ANOVA
|
|||||
|---|---|---|---|---|---|---|
| Mean torque | Percent torque change | Drift time | ||||
| F
|
p
|
F
|
p
|
F
|
p
|
|
| Meds | 0.796 | .402 | 0.17 | 0.692 | 3.68 | 0.100 |
| STNDBS | 17.41 | .004 | 15.24 | .006 | 0.001 | 0.973 |
| Meds × STN DBS | 0.28 | .615 | 0.30 | .601 | 2.72 | 0.143 |
| Vision | 0.003 | .957 | ||||
| Meds × Vision | 0.08 | .791 | ||||
| STN DBS × Vision | 12.44 | .010 | ||||
| Meds × STN DBS × Vision | 1.20 | .310 | ||||
(values in bold type identify results significant at p<.05)
Fig. 2(c) shows the percent torque change from 3 s prior to visual feedback removal compared to the final 3 s of the trial. The percent torque change closely followed the mean torque change with a negative percent torque change in the OFF treatment and Meds conditions. In previous work that investigated flexion of the index finger, the percent change in isometric force decreased on average by 12% in healthy individuals [11]. This is greater than the 4% torque change found in the patients off treatment [Fig. 2(c)] in this study, although a direct comparison is premature because different effectors were used. In contrast, the STN DBS condition produced a positive percent torque change. Turning STN DBS on when the subjects were medicated reversed the percent torque from the negative values seen in the Meds condition to a positive change in the Meds plus STN DBS condition. The findings from Fig. 2(c) were supported by the two-way analysis of variance (Table III) where there was a significant main effect for STN DBS but not for Meds. The interaction between Meds and STN DBS was not significant.
To examine the temporal capacity of short-term motor memory across treatment conditions, we calculated the drift time. Across treatment condition, the two-way analysis of variance showed that there was no significant change in drift time after vision removal (Table II). Off treatment, mean drift time was 0.99 ± 0.10 SEM, on Meds it was 1.51 ± 0.13, on STN DBS it was 1.20 ± 0.17, and on Meds plus STN DBS it was 1.29 ± 0.17.
B. Bilateral STN DBS
A patient (P1 from Fig. 1) who participated following right, unilateral STN DBS surgery returned to the laboratory after left, unilateral STN DBS surgery. The patient could now be considered to have staged bilateral STN DBS. The stimulator settings set by the neurologist had changed from the first testing session to the second testing session when we compared unilateral versus bilateral stimulation. The left-side stimulator was previously at monopolar (0- C+, 60 μs, 185 Hz, 1.5 V) and the same left-side stimulator during this new testing session was also monopolar (1- C+, 60 μs, 185, 1.5 V). The effects of the unilateral stimulator on memory-guided force were similar despite the change in contact. The patient’s new right-side stimulator was monopolar (0- C+, 60 μs, 185 Hz, 1.5 V).
In a new testing session, we compared the torque output from the right elbow following visual feedback and visual feedback removal when the patient was Off treatment, on unilateral STN DBS (U-STN DBS), and on bilateral DBS (B-STN DBS), all after a 12-h withdrawal of medication. We examined whether the differences between medication and unilateral STN DBS shown in Fig. 2 were due to the fact that medication affects both sides of the basal ganglia whereas unilateral STN DBS only acts on one side. If the positive and negative percent torque differences found between unilateral STN DBS and medication Fig. 2(c) were due to one-sided effects of unilateral STN DBS versus the bilateral effects of medication, then when on bilateral STN DBS one would expect the torque to decrease in a manner similar to that occurring with medication following visual feedback removal.
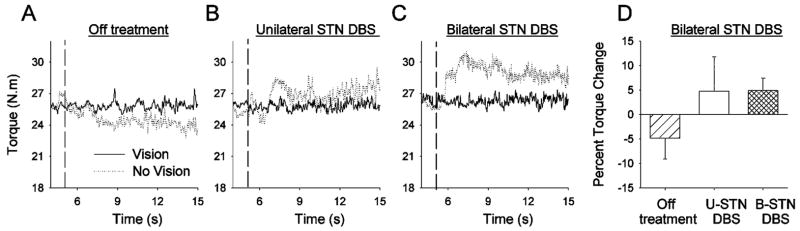
Fig. 3 shows the torque output from this PD patient either Off treatment, on U-STN DBS (left basal ganglia contralateral to right arm), or on B-STN DBS. Off treatment, the subject decreased torque output following visual feedback removal. In contrast, on U-STN DBS the subject increased torque output after visual feedback removal. On B-STN DBS there was also an increased torque output after visual feedback removal. Fig. 3(d) shows that the averaged percent torque change across the six trials was negative for Off treatment, positive for U-STN DBS, and also positive for B-STN DBS. The increased torque following visual feedback removal while on B-STN DBS was different to what was observed on medication alone (see Fig. 1, P1). This finding indicates that the different effects of medication and unilateral DBS when visual feedback was removed were not due to a unilateral versus bilateral basal ganglia mechanism.
Fig. 3.
(A–C) Individual force trial records from one subject who was tested again following implantation of a second STN DBS stimulator on the opposite side. During a separate testing session following the second STN DBS surgery, the patient was tested Off treatment (Part A), with one stimulator turned on (U-STN DBS) (Part B), and with both stimulators turned on (B-STN DBS) (Part C) with full visual feedback (solid lines) and visual feedback removed (dotted lines). (D) is the percent torque change in the Off treatment, U-STN DBS, and B-STN DBS conditions. Data are the means and standard deviations.
C. Pallidotomy and Unilateral STN DBS
A patient with a prior pallidotomy was studied to determine if the behavioral effects of STN DBS are similar to those of a lesion to GPi (Table I, P9). If the effects of STN DBS on force drift resemble those of a pallidotomy, this would suggest that the effects of STN DBS on force drift act through GPi. The subject was only examined following the pallidotomy with the STN DBS electrode implanted on the same side of the basal ganglia as the pallidotomy. The subject was examined in the Off treatment and STN DBS conditions.
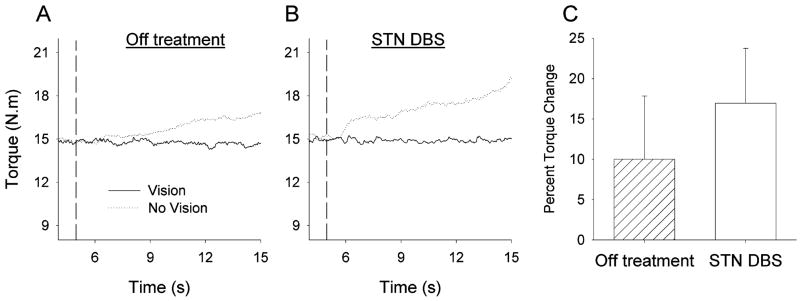
Fig. 4 shows that Off treatment, the patient increased torque output following visual feedback removal. When the stimulator was turned on, the torque output increased even higher after visual feedback removal. Fig. 4(c) indicates that the percent torque change was positive for both the Off treatment and STN DBS conditions, but that STN DBS had a greater percent torque change than the Off treatment condition. This finding suggests that pallidotomy has a similar effect to that of STN DBS on force drift (i.e., increased torque following visual feedback removal).
Fig. 4.
Individual force trial records from one subject who had undergone a prior pallidotomy before undergoing unilateral STN DBS on the same side: (A) is the Off treatment condition, and (B) the STN DBS condition with full visual feedback (solid lines) and visual feedback removed (dotted lines). (C) is the percent torque change in the Off treatment condition and the STN DBS condition. Data are the means and standard deviations.
IV. Discussion
This study examined the control of elbow force in patients with PD to determine how medication and STN DBS affected memory-guided force control. We have confirmed previous findings that in the absence of vision there is a reduction in force output in PD patients when on antiparkinsonian medication [12]. However, we have extended this to include a reduction of force in PD in the Off medicated state also. In sharp contrast, both unilateral and bilateral STN DBS led to increased force output following removal of visual feedback. One patient with a prior pallidotomy also showed increased force output following removal of visual feedback both when off all treatment and when on STN DBS. Taken together, these findings support the hypothesis that changing basal ganglia output in humans directly alters memory-guided force control and supports an effect of STN DBS on memory-guided force control in patients with PD.
There are several possible mechanisms regarding how STN DBS altered memory-guided force control, and we consider three possibilities. The first mechanism is that DBS stimulated the axonal projections from the subthalamic nucleus to GPi. In the rhesus monkey, it was shown that activated STN efferent neurons and resultant changes in the firing pattern of globus pallidus neurons underlie the beneficial effects of high frequency STN DBS [34]. Since efferent projections from the STN are directed to both segments of the globus pallidus, substantia nigra, striatum, pedunculopontine nucleus, and ventral tegmental area [35], [36], then each of these regions has the potential to change in activation with the stimulation. A second mechanism is that DBS stimulated lenticular fasciculus fibers from the internal segment of the globus pallidus, which pass dorsal to the STN. Based on recordings from a macaque rendered parkinsonian, Miocinovic and colleagues [37] created a 3-D simulation model of the effects of stimulation and these authors found that the DBS caused activation of the fibers in the lenticular fasciculus and STN neurons. Their findings support both of the above-mentioned mechanisms, although activation of STN neurons had a greater benefit for alleviating parkinsonism. The third mechanism is that DBS stimulated the thalamic fasciculus fibers that enter the thalamus. In our study, as part of the surgery protocol we recorded the pattern of firing of the cells within STN that changed with the electrode path and estimated the length of STN from the electrode path to always be greater than 4 mm for the subjects in this study. In addition, the contacts for all but one of the patients in this study were at 0 and 1 (monopolar), while patient #3 was stimulated through contact 2 (note: patient #3 had memory-guided force data similar to the other patients in the study). Since the DBS electrode is placed at the ventral border of STN and we stimulated at contacts 0 or 1, most estimates of current spread do not suggest that the thalamus could have been stimulated [38], [39]. In addition, we have previously found that the ventral thalamus is related to visually guided force control [13], but STN DBS did not alter the control of force when visual feedback was available [Fig. 2(a)], further suggesting that the thalamus may not have been the target explaining our effects. Therefore, we will base our discussion on the first two mechanisms (STN neurons to GPi; lenticular fasciculus from GPi) which would both lead to altered output from the GPi.
Support in this study for the hypothesis that GPi output was altered by STN DBS is provided by the single subject data from the patient with a prior pallidotomy (patient 9, Table I). Given that the effects of STN DBS on force drift resembled those of a pallidotomy, this suggests that STN DBS may have influenced force drift through the globus pallidus. While we do not have data prior to the pallidotomy in this patient, it is important to note that after testing over 35 healthy and Parkinsonian individuals across two previous studies which included over 150 trials [11], [12], no person has ever increased mean force output following visual feedback removal at 50% MVC. Findings from previous electrophysiological studies also support this hypothesis in that the STN has been shown to have connections to GPi and to play an important role in the modulation of the pattern of activity of pallidal neurons [40], [41].
The output from GPi has been suggested to play a functional role in memory-guided motor control [42]–[44]. A common task manipulation used to assess memory-guided motor control compares motor performance with full visual feedback to motor performance when visual feedback is unavailable. For instance, cooling GPi in Cebus monkeys produced a breakdown in the performance of elbow movements when the animals had no visual information but not when visual information was displayed to them [42]. In addition, muscimol or kainic acid induced lesions of the primate GPi caused a position drift toward flexion [45]. Inase and colleagues [46] observed a similar flexor drift following inactivation of GPi to that of Mink and Thach [45] but noted that the animals could overcome the drift when presented with an error signal. Furthermore, in human patients with PD, a position or force drift occurred more in patients compared to control subjects, but only during memory guided force control when visual information was not provided [12], [47]. The drift in force output which normally occurs following the removal of visual feedback has been attributed to decay in short-term motor memory [11]. In healthy individuals across a range of force levels, force drift has been shown to begin between 0.5–1.5 s following removal of visual feedback [11]. This time period is quite consistent with the drift time found in the present study and thus provides support for drift being due to decay of motor memory across all treatment conditions.
Memory-guided force control has both a motor and a cognitive component. Despite improvements in motor symptoms following STN DBS [48], [49], selected adverse effects of STN DBS on cognition have been reported (for a review see Temel et al. [22]). While STN DBS has been shown to impair cognitive performance during the cognitive based Stroop task [8, 50–52], medication has not been shown to lead to the same impairments in Stroop task performance [23]. This provides support for a differential effect of DBS and medication on cognitive function. Based on these adverse cognitive effects, it has been suggested that STN DBS may interfere with nonmotor pathways involving the dorsolateral, orbitofrontal, or anterior cingulate striato-thalamo-cortical circuits [6]. Since STN DBS affects the nonvisual control of force, the adverse effects of STN DBS on cognition may be extended to include adverse effects of STN DBS on memory guided force control.
A circuit for the retrieval of motor memory in the dorsolateral prefrontal cortex, ventral prefrontal cortex and the anterior cingulate cortex has previously been established in healthy individuals [13]. Given that impairment in cognitive performance during the Stroop task with DBS has been related to reduced activation in the anterior cingulate cortex and the ventral striatum [51], it is feasible that the retrieval of motor memory is also affected by STN DBS. It has been suggested that STN DBS may disrupt new and unexpected situations requiring nonautomated behavior [51]. This is consistent with the requirements of the memory guided force control task used in the present study.
In the STN-lesioned rat, reaction times were slowed following lesioning and the beneficial effect of advance information on reaction time was abolished [9]. Based on this, it has been suggested that STN lesions may impair the operation of a “motor” working memory which holds a selected response in readiness for performance until needed [9]. The control of force in the absence of vision has been linked to activation of brain areas associated with nonmotor pathways including the dorsolateral prefrontal cortex, the ventral prefrontal cortex, the anterior cingulate cortex, the basal ganglia, and the supplementary motor area [13], [53], [54]. The influence of basal ganglia output during memory-guided force control may most specifically come through the connections between the basal ganglia, the dorsolateral prefrontal cortex, the ventral prefrontal cortex, and the anterior cingulate cortex via dorsolateral and anterior cingulate striatal–thalamo-cortical circuits [55]–[57]. We suggest that it is through this circuitry that STN affects GPi, which in turn affects prefrontal areas leading to altered basal ganglia-thalamo-cortical output during memory guided force control.
In conclusion, STN DBS affects memory-guided force control in patients with PD whereas medication does not. Increased force output when on STN DBS may occur due to disruptions in the basal ganglia-thalamo-cortical circuity. Given that output from the GPi may play a functional role in motor control during memory guided force control, we hypothesize that modulation of GPi output by STN DBS may drive the differential response in force control in a task requiring motor memory. We suspect that increased torque output following the removal of visual feedback could affect behaviors such as driving an automobile. For instance, when the eyes are no longer watching the road (e.g., while changing the radio) a patient could potentially press on the accelerator with greater force and increase flexion of the right elbow, causing the car to increase speed and swerve to the right.
Acknowledgments
The authors would like to thank Medtronic for donating the Medtronic Program Consoler (Model 7432) for use in this study. They also thank the staff at the Section for Movement Disorders in the Department of Neurological Sciences at Rush University Medical Center for their help with patient recruitment and Dr. S. Leurgans for her assistance with some aspects of the statistical analyses.
This work was supported by the National Institutes of Health under Contract R01-NS-28127, Contract R01-NS-40902, and Contract R01-NS-52318. This paper was presented in part at the 13th annual meeting of the Society for the Neural Control of Movement, Santa Barbara, CA, 2003.
Biographies
Janey Prodoehl received the Dip. Phys. Ther. in physiotherapy from Leeds School of Physiotherapy, Leeds, U.K., in 1989, the M.S. degree in physical therapy from the Finch University of Health Sciences, the Chicago Medical School, Chicago, IL, in 1995, and the Ph.D. degree in movement sciences from the University of Illinois, Chicago, in 2005.
She is currently a Postdoctoral Research Associate in the Department of Movement Sciences at the University of Illinois, Chicago. Her research interests include the role of disordered sensorimotor integration and basal ganglia dysfunction in explaining pathological motor control. Her focus is on patients with dystonia and Parkinson’s disease.
Dr. Prodoehl is a member of the Society for Neuroscience and the American Physical Therapy Association.

Daniel M. Corcos received the Ph.D. degree in motor control from the University of Oregon, Eugene, in 1982.
He did a postdoctoral fellowship at Rush Medical Center, Chicago, IL, from 1983 to 1987. He was an Assistant Professor from 1987 to 1993, an Associate Professor with tenure from 1993 to 1997, and has been a Professor since 1997 at the University of Illinois, Chicago. He has served on several NIH study sections. He was Executive Editor of the Journal of Motor Behavior from 1996 to 2004 and is currently the Editor for Rapid Communications. He is also on the Editorial Board for the Journal of Neuroengineering and Rehabilitation.

John C. Rothwell, photograph and biography not available at the time of publication.
Leo Verhagen Metman received the M.D. and Ph.D. degrees from the University of Leiden, Leiden, The Netherlands. He completed his neurology residency at Thomas Jefferson University Hospital, Philadelphia, PA.
He is Medical Director of the Neurosurgery Program for Movement Disorders at Rush University Medical Center, Chicago, IL. He specializes in the medical and surgical management of patients with Parkinson’s disease and other movement disorders including tremor and dystonia. He completed a fellowship in the Experimental Therapeutics Branch, National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, MD, before joining Rush in 1999.

Roy A. E. Bakay received the M.D. degree from Northwestern University, Chicago, IL, after which he completed residency training in neurological surgery at the University of Washington School of Medicine, Seattle. Following his residency, Dr. Bakay completed a National Institutes of Health fellowship in neuronal plasticity.
He is currently the A. Watson Armour III and Sarah Armour Presidential Chair, Vice Chairman, and Residency Research Director in the Department of Neurosurgery at Rush University Medical Center, Chicago, IL. He enjoyed a successful career at Emory University School of Medicine in Atlanta, Georgia before joining Rush in 2000. A leading authority on surgery for movement disorders, he specializes in functional and stereotactic neurosurgery and has authored numerous journal articles. He has served as an editor for various journals including Neurosurgery. He has received funding from the NIH to study pallidotomy and deep brain stimulation. Hiss current research also includes neural tissue transplantation and gene therapy techniques.
Dr. Bakay is a member of numerous professional organizations including the American Association of Neurological Surgeons, the American Society for Neural Transplantation, the American Society of Stereotactic and Functional Neurosurgery and the Congress of Neurological Surgeons.

David E. Vaillancourt received the Ph.D. degree from Pennsylvania State University, State College, in 2001.
He then completed a postdoctoral fellowship at the University of Illinois, Chicago, in clinical neurophysiology and neuroimaging, and was promoted to Assistant Professor in 2005. His current interests include using systems neuroscience techniques to understand the role of the basal ganglia, cerebellum, and cortex in motor control and movement disorders in humans. He has served on several National Institutes of Health study sections. He is on the editorial board of the Journal of Motor Behavior.

References
- 1.Cotzias GC, Papavasiliou PS, Gellene R. Modification of Parkinsonism–Chronic treatment with L-dopa. New Engl J Med. 1969;280:337–345. doi: 10.1056/NEJM196902132800701. [DOI] [PubMed] [Google Scholar]
- 2.Yahr MD, Duvoisin RC, Schear MJ, Barrett RE, Hoehn MM. Treatment of Parkinsonism with levodopa. Arch Neurol. 1969;21:343–354. doi: 10.1001/archneur.1969.00480160015001. [DOI] [PubMed] [Google Scholar]
- 3.Fahn S. Parkinson disease, the effect of levodopa, and the ELLDOPA trial. Earlier versus Later L-DOPA. Arch Neurol. 1999;56:529–35. doi: 10.1001/archneur.56.5.529. [DOI] [PubMed] [Google Scholar]
- 4.Deuschl G, Wenzelburger R, Kopper F, Volkmann J. Deep brain stimulation of the subthalamic nucleus for Parkinson’s disease: A therapy approaching evidence-based standards. J Neurol. 2003;250:I43–6. doi: 10.1007/s00415-003-1109-8. [DOI] [PubMed] [Google Scholar]
- 5.Kumar R, Lozano AM, Sime E, Halket E, Lang AE. Comparative effects of unilateral and bilateral subthalamic nucleus deep brain stimulation. Neurology. 1999;53:561–6. doi: 10.1212/wnl.53.3.561. [DOI] [PubMed] [Google Scholar]
- 6.Trepanier LL, Kumar R, Lozano AM, Lang AE, Saint-Cyr JA. Neuropsychological outcome of GPi pallidotomy and GPi or STN deep brain stimulation in Parkinson’s disease. Brain Cogn. 2000;42:324–47. doi: 10.1006/brcg.1999.1108. [DOI] [PubMed] [Google Scholar]
- 7.Hershey T, Revilla FJ, Wernle A, Gibson PS, Dowling JL, Perlmutter JS. Stimulation of STN impairs aspects of cognitive control in PD. Neurology. 2004;62:1110–4. doi: 10.1212/01.wnl.0000118202.19098.10. [DOI] [PubMed] [Google Scholar]
- 8.Saint-Cyr JA, Trepanier LL, Kumar R, Lozano AM, Lang AE. Neuropsychological consequences of chronic bilateral stimulation of the subthalamic nucleus in Parkinson’s disease. Brain. 2000;123(pt 10):2091–108. doi: 10.1093/brain/123.10.2091. [DOI] [PubMed] [Google Scholar]
- 9.Baunez C, Humby T, Eagle DM, Ryan LJ, Dunnett SB, Robbins TW. Effects of STN lesions on simple vs choice reaction time tasks in the rat: Preserved motor readiness, but impaired response selection. Eur J Neurosci. 2001;13:1609–16. doi: 10.1046/j.0953-816x.2001.01521.x. [DOI] [PubMed] [Google Scholar]
- 10.Temel Y, Blokland A, Ackermans L, Boon P, van Kranen Mastenbroek VH, Beuls EA, Spincemaille GH, Visser-Vandewalle V. Differential effects of subthalamic nucleus stimulation in advanced Parkinson disease on reaction time performance. Exp Brain Res. 2006;169:389–99. doi: 10.1007/s00221-005-0151-6. [DOI] [PubMed] [Google Scholar]
- 11.Vaillancourt DE, Russell DM. Temporal capacity of short-term visuomotor memory in continuous force production. Exp Brain Res. 2002;145:275–85. doi: 10.1007/s00221-002-1081-1. [DOI] [PubMed] [Google Scholar]
- 12.Vaillancourt DE, Slifkin AB, Newell KM. Visual control of isometric force in Parkinson’s disease. Neuropsychologia. 2001;39:1410–8. doi: 10.1016/s0028-3932(01)00061-6. [DOI] [PubMed] [Google Scholar]
- 13.Vaillancourt DE, Thulborn KR, Corcos DM. Neural basis for the processes that underlie visually-guided and internally-guided force control in humans. J Neurophysiol. 2003;90:3330–40. doi: 10.1152/jn.00394.2003. [DOI] [PubMed] [Google Scholar]
- 14.Middleton FA, Strick PL. Basal-ganglia ‘projections’ to the prefrontal cortex of the primate. Cereb Cortex. 2002;12:926–35. doi: 10.1093/cercor/12.9.926. [DOI] [PubMed] [Google Scholar]
- 15.Brown RG, Dowsey PL, Brown P, Jahanshahi M, Pollak P, Benabid AL, Rodriguez-Oroz MC, Obeso J, Rothwell JC. Impact of deep brain stimulation on upper limb akinesia in Parkinson’s disease. Ann Neurol. 1999;45:473–488. doi: 10.1002/1531-8249(199904)45:4<473::aid-ana9>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 16.Vaillancourt DE, Prodoehl J, Sturman MM, Bakay RA, Metman LV, Corcos DM. Effects of deep brain stimulation and medication on strength, bradykinesia, and electromyographic patterns of the ankle joint in Parkinson’s disease. Mov Disord. 2006;21:50–8. doi: 10.1002/mds.20672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vaillancourt DE, Prodoehl J, Metman LV, Bakay RA, Corcos DM. Effects of deep brain stimulation and medication on bradykinesia and muscle activation in Parkinson’s disease. Brain. 2004;127:491–504. doi: 10.1093/brain/awh057. [DOI] [PubMed] [Google Scholar]
- 18.Tavares ALT, Jefferis GS, Koop M, Hill BC, Hastie T, Heit G, Bronte-Stewart HM. Quantitative measurements of alternating finger tapping in Parkinson’s disease correlate with UPDRS motor disability and reveal the improvement in fine motor control from medication and deep brain stimulation. Mov Disord. 2005;20:1286–98. doi: 10.1002/mds.20556. [DOI] [PubMed] [Google Scholar]
- 19.Rocchi L, Chiari L, Horak FB. Effects of deep brain stimulation and levodopa on postural sway in Parkinson’s disease. J Neurol Neurosurg Psychiatry. 2002;73:267–74. doi: 10.1136/jnnp.73.3.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rocchi L, Chiari L, Cappello A, Gross A, Horak FB. Comparison between subthalamic nucleus and globus pallidus internus stimulation for postural performance in Parkinson’s disease. Gait Posture. 2004;19:172–83. doi: 10.1016/S0966-6362(03)00059-6. [DOI] [PubMed] [Google Scholar]
- 21.Wenzelburger R, Kopper F, Zhang BR, Witt K, Hamel W, Weinert D, Kuhtz-Buschbeck J, Golge M, Illert M, Deuschl G, Krack P. Subthalamic nucleus stimulation for Parkinson’s disease preferentially improves akinesia of proximal arm movements compared to finger movements. Mov Disord. 2003;18:1162–9. doi: 10.1002/mds.10501. [DOI] [PubMed] [Google Scholar]
- 22.Temel Y, Blokland A, Steinbusch HW, Visser-Vandewalle V. The functional role of the subthalamic nucleus in cognitive and limbic circuits. Prog Neurobiol. 2005;76:393–413. doi: 10.1016/j.pneurobio.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 23.Alegret M, Valldeoriola F, Marti M, Pilleri M, Junque C, Rumia J, Tolosa E. Comparative cognitive effects of bilateral subthalamic stimulation and subcutaneous continuous infusion of apomorphine in Parkinson’s disease. Mov Disord. 2004;19:1463–9. doi: 10.1002/mds.20237. [DOI] [PubMed] [Google Scholar]
- 24.Hughes AJ, Daniel SE, Kilford L, Lees AJ. Accuracy of clinical diagnosis of idiopathic Parkinson’s disease: A clinico-pathological study of 100 cases [see comments] J Neurol Neurosurg Psychiatry. 1992;55:181–184. doi: 10.1136/jnnp.55.3.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hughes AJ, Ben-Shlomo Y, Daniel SE, Lees AJ. What features improve the accuracy of clinical diagnosis in Parkinson’s disease: A clinicopathologic study. Neurology. 1992;42:1142–6. doi: 10.1212/wnl.42.6.1142. [DOI] [PubMed] [Google Scholar]
- 26.D.-b. S. f. P. s. D. S. Group. Deep-brain stimulation of the subthalamic nucleus or the pars interna of the globus pallidus in Parkinson’s disease. N Engl J Med. 2001;345:956–63. doi: 10.1056/NEJMoa000827. [DOI] [PubMed] [Google Scholar]
- 27.Starr PA, Vitek JL, Bakay RA. Deep brain stimulation for movement disorders. Neurosurg Clin N Amer. 1998;9:381–402. [PubMed] [Google Scholar]
- 28.Cohn MC, Hudgins PA, Sheppard SK, Starr PA, Bakay RA. Pre- and postoperative MR evaluation of stereotactic pallidotomy. AJNR Amer J Neuroradiol. 1998;19:1075–80. [PMC free article] [PubMed] [Google Scholar]
- 29.Vitek JL, Bakay RA, Hashimoto T, Kaneoke Y, Mewes K, Zhang JY, Rye D, Starr P, Baron M, Turner R, DeLong MR. Microelectrode-guided pallidotomy: Technical approach and its application in medically intractable Parkinson’s disease. J Neurosurg. 1998;88:1027–43. doi: 10.3171/jns.1998.88.6.1027. [DOI] [PubMed] [Google Scholar]
- 30.Langston JW, Widner H, Goetz CG, Brooks D, Fahn S, Freeman T, Watts R. Core assessment program for intracerebral transplantation (CAPIT) Movement Disorders. 1992;7:2–13. doi: 10.1002/mds.870070103. [DOI] [PubMed] [Google Scholar]
- 31.Temperli P, Ghika J, Villemure JG, Burkhard PR, Bogous-slavsky J, Vingerhoets FJ. How do Parkinsonian signs return after discontinuation of subthalamic DBS? Neurology. 2003;60:78–81. doi: 10.1212/wnl.60.1.78. [DOI] [PubMed] [Google Scholar]
- 32.Kumar R, Lozano AM, Kim YJ, Hutchison WD, Sime E, Halket E, Lang AE. Double-blind evaluation of subthalamic nucleus deep brain stimulation in advanced Parkinson’s disease. Neurology. 1998;51:850–855. doi: 10.1212/wnl.51.3.850. [DOI] [PubMed] [Google Scholar]
- 33.Corcos DM, Chen CM, Quinn NP, McAuley J, Rothwell JC. Strength in Parkinson’s disease: Relationship to rate of force generation and clinical status. Ann Neurol. 1996;39:79–88. doi: 10.1002/ana.410390112. [DOI] [PubMed] [Google Scholar]
- 34.Hashimoto T, Elder CM, Okun MS, Patrick SK, Vitek JL. Stimulation of the subthalamic nucleus changes the firing pattern of pallidal neurons. J Neurosci. 2003;23:1916–23. doi: 10.1523/JNEUROSCI.23-05-01916.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hamani C, Saint-Cyr JA, Fraser J, Kaplitt M, Lozano AM. The subthalamic nucleus in the context of movement disorders. Brain. 2004;127:4–20. doi: 10.1093/brain/awh029. [DOI] [PubMed] [Google Scholar]
- 36.Parent A, Hazrati L. Functional anatomy of the basal ganglia. II. The place of subthalamic nucleus and external pallidum in basal ganglia circuitry. Brain Res Rev. 1995;20:128–154. doi: 10.1016/0165-0173(94)00008-d. [DOI] [PubMed] [Google Scholar]
- 37.Miocinovic S, Parent M, Butson CR, Hahn PJ, Russo GS, Vitek JL, McIntyre CC. Computational analysis of subthalamic nucleus and lenticular fasciculus activation during therapeutic deep brain stimulation. J Neurophysiol. 2006;96:1569–80. doi: 10.1152/jn.00305.2006. [DOI] [PubMed] [Google Scholar]
- 38.McIntyre CC, Mori S, Sherman DL, Thakor NV, Vitek JL. Electric field and stimulating influence generated by deep brain stimulation of the subthalamic nucleus. Clin Neurophysiol. 2004;115:589–95. doi: 10.1016/j.clinph.2003.10.033. [DOI] [PubMed] [Google Scholar]
- 39.Butson CR, McIntyre CC. Role of electrode design on the volume of tissue activated during deep brain stimulation. J Neural Eng. 2006;3:1–8. doi: 10.1088/1741-2560/3/1/001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Benazzouz A, Piallat B, Pollak P, Benabid AL. Responses of substantia nigra pars reticulata and globus pallidus complex to high frequency stimulation of the subthalamic nucleus in rats: Electrophysiological data. Neurosci Lett. 1995;189:77–80. doi: 10.1016/0304-3940(95)11455-6. [DOI] [PubMed] [Google Scholar]
- 41.Ni Z, Bouali-Benazzouz R, Gao D, Benabid AL, Benazzouz A. Changes in the firing pattern of globus pallidus neurons after the degeneration of nigrostriatal pathway are mediated by the subthalamic nucleus in the rat. Eur J Neurosci. 2000;12:4338–44. [PubMed] [Google Scholar]
- 42.Hore J, Meyer-Lohmann J, Brooks VB. Basal ganglia cooling disables learned arm movements of monkeys in the absence of visual guidance. Science. 1977;195:584–6. doi: 10.1126/science.402029. [DOI] [PubMed] [Google Scholar]
- 43.Glickstein M, Stein J. Paradoxical movement in Parkinson’s disease. Trends Neurosci. 1991;14:480–2. doi: 10.1016/0166-2236(91)90055-y. [DOI] [PubMed] [Google Scholar]
- 44.Flowers KA. Visual “closed loop” and “open loop” characteristics of voluntary movement in patients with parkinsonism and intention tremor. Brain. 1976;99:269–310. doi: 10.1093/brain/99.2.269. [DOI] [PubMed] [Google Scholar]
- 45.Mink JW, Thach WT. Basal ganglia motor control. III. Pallidal ablation: Normal reaction time, muscle cocontraction, and slow movement. J Neurophysiol. 1991;65:330–351. doi: 10.1152/jn.1991.65.2.330. [DOI] [PubMed] [Google Scholar]
- 46.Inase M, Buford JA, Anderson ME. Changes in the control of arm position, movement, and thalamic discharge during local inactivation in the globus pallidus of the monkey. J Neurophysiol. 1996;75:1087–1104. doi: 10.1152/jn.1996.75.3.1087. [DOI] [PubMed] [Google Scholar]
- 47.Cooke JD, Brown JD, Brooks V. Increased dependence on visual information for movement control in patients with Parkinson’s disease. Can J Neurological Sci. 1978;5:413–415. doi: 10.1017/s0317167100024197. [DOI] [PubMed] [Google Scholar]
- 48.Limousin P, Krack P, Pollak P, Benazzouz A, Ardouin C, Hoffmann D, Benabid AL. Electrical stimulation of the subthalamic nucleus in advanced Parkinson’s disease. New Engl J Med. 1998;339:1105–1111. doi: 10.1056/NEJM199810153391603. [DOI] [PubMed] [Google Scholar]
- 49.Krack P, Batir A, Van Blercom N, Chabardes S, Fraix V, Ardouin C, Koudsie A, Limousin PD, Benazzouz A, LeBas JF, Benabid AL, Pollak P. Five-year follow-up of bilateral stimulation of the subthalamic nucleus in advanced Parkinson’s disease. N Engl J Med. 2003;349:1925–34. doi: 10.1056/NEJMoa035275. [DOI] [PubMed] [Google Scholar]
- 50.Dujardin K, Blairy S, Defebvre L, Krystkowiak P, Hess U, Blond S, Destee A. Subthalamic nucleus stimulation induces deficits in decoding emotional facial expressions in Parkinson’s disease. J Neurol Neurosurg Psychiatry. 2004;75:202–8. [PMC free article] [PubMed] [Google Scholar]
- 51.Schroeder U, Kuehler A, Haslinger B, Erhard P, Fogel W, Tronnier VM, Lange KW, Boecker H, Ceballos-Baumann AO. Subthalamic nucleus stimulation affects striato-anterior cingulate cortex circuit in a response conflict task: A PET study. Brain. 2002;125:1995–2004. doi: 10.1093/brain/awf199. [DOI] [PubMed] [Google Scholar]
- 52.Jahanshahi M, Ardouin CM, Brown RG, Rothwell JC, Obeso J, Albanese A, Rodriguez-Oroz MC, Moro E, Benabid AL, Pollak P, Limousin-Dowsey P. The impact of deep brain stimulation on executive function in Parkinson’s disease. Brain. 2000;123(pt 6):1142–54. doi: 10.1093/brain/123.6.1142. [DOI] [PubMed] [Google Scholar]
- 53.Mushiake H, Inase M, Tanji J. Neuronal activity in the primate premotor, supplementary, and precentral motor cortex during visually guided and internally determined sequential movements. J Neurophysiol. 1991;66:705–718. doi: 10.1152/jn.1991.66.3.705. [DOI] [PubMed] [Google Scholar]
- 54.Jahanshahi M, Jenkins IH, Brown RG, Marsden CD, Passingham RE, Brooks DJ. Self-initiated versus externally triggered movements I. An investigation using measurement of regional cerebral blood flow with PET and movement-related potentials in normal and Parkinson’s disease subjects. Brain. 1995;118:913–933. doi: 10.1093/brain/118.4.913. [DOI] [PubMed] [Google Scholar]
- 55.Albin RL, Young AB, Penney JB. The functional anatomy of basal ganglia disorders. Trends Neurosci. 1989;12:366–375. doi: 10.1016/0166-2236(89)90074-x. [DOI] [PubMed] [Google Scholar]
- 56.Alexander GE, Crutcher MD, DeLong MR. Basal ganglia-thalamocortical circuits: Parallel substrates for motor, oculomotor, “prefrontal” and “limbic” functions. Progr Brain Res. 1990;85:119–146. [PubMed] [Google Scholar]
- 57.Young O, Peters J, editors. Plastics. 2. Vol. 3. New York: McGraw-Hill; 1964. Synthetic structure of industrial plastics; pp. 15–64. [Google Scholar]