Abstract
Previous studies suggest that the activation (autophosphorylation) of dsRNA-dependent protein kinase (PKR) can stimulate protein degradation, and depress protein synthesis in skeletal muscle through phosphorylation of the translation initiation factor 2 (eIF2) on the α-subunit. To understand whether these mediators are important in muscle wasting in cancer patients, levels of the phospho forms of PKR and eIF2α have been determined in rectus abdominus muscle of weight losing patients with oesophago-gastric cancer, in comparison with healthy controls. Levels of both phospho PKR and phospho eIF2α were significantly enhanced in muscle of cancer patients with weight loss irrespective of the amount and there was a linear relationship between phosphorylation of PKR and phosphorylation of eIF2α (correlation coefficient 0.76, P=0.005). This suggests that phosphorylation of PKR led to phosphorylation of eIF2α. Myosin levels decreased as the weight loss increased, and there was a linear relationship between myosin expression and the extent of phosphorylation of eIF2α (correlation coefficient 0.77, P=0.004). These results suggest that phosphorylation of PKR may be an important initiator of muscle wasting in cancer patients.
Keywords: muscle atrophy, cancer patients, PKR, eIF2α, p70S6k
Patients with cancer, especially those of the gastrointestinal tract, show a progressive wasting of skeletal muscle, which reduces both their quality of life and survival time (DeWys et al, 1980). Skeletal muscle atrophy is characterised by a decreased protein content, fibre diameter, force production and fatigue resistance. Muscle wasting is due to a combination of depressed protein synthesis (Emery et al, 1984), and elevated endogenous protein breakdown, with oxidation of the resultant amino acids (O'Keefe et al, 1990). In cancer patients the mechanism for the depression in protein synthesis is not known, while the increased protein degradation has been attributed to an increased expression of the ubiquitin–proteasome proteolytic pathway (Khal et al, 2005).
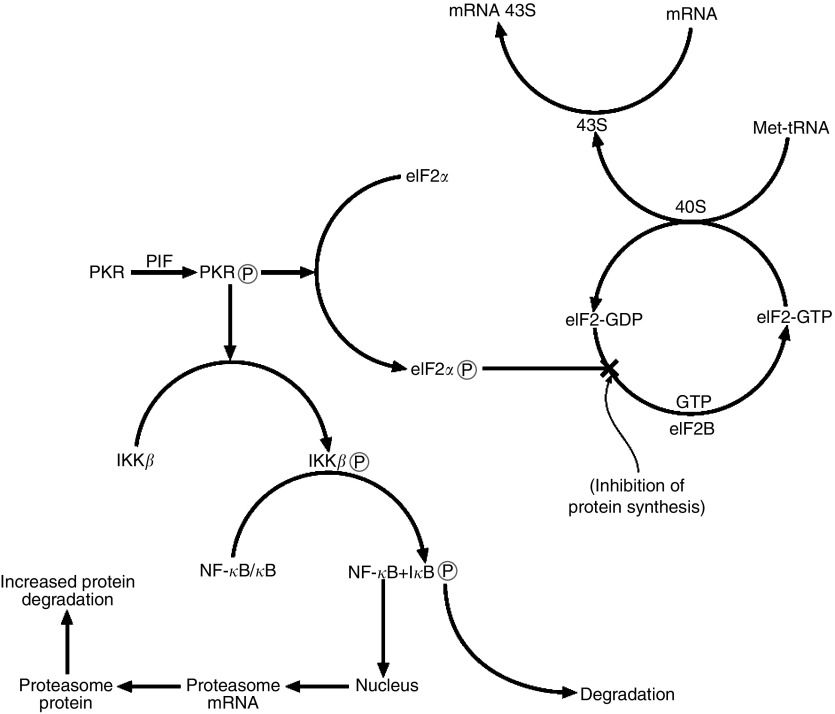
Several potential mediators of the cachectic process, including proteolysis-inducing factor (PIF) and angiotensin II (Ang II), inhibit protein synthesis in skeletal muscle (Lorite et al, 1997; Russell et al, 2006a), and also stimulate degradation, through increased activity and expression of the ubiquitin–proteasome pathway (Lorite et al, 2001; Sanders et al, 2005). We have recently shown (Eley and Tisdale, 2007) a link between the ability of PIF and Ang II to inhibit protein synthesis and increase protein degradation in murine myotubes through the dsRNA-dependent protein kinase (PKR). dsRNA-dependent protein kinase is a serine/threonine-specific protein kinase, which undergoes autophosphorylation at multiple serine and threonine residues, causing activation, in the presence of double-stranded (ds)RNA, in response to viral attack (Jammi and Beal, 2001). Both PIF and Ang II were shown to induce autophosphorylation of PKR. Activated PKR can phosphorylate several protein substrates including the α-subunit of the heterotrimeric translation initiation factor 2 (eIF2α) (deHaro et al, 1996). Phosphorylation of eIF2α inhibits continued initiation of protein synthesis by the eIF2 complex, which initiates Met tRNA binding to the 40S ribosomal subunit. During this process GTP associated with eIF2 is hydrolysed to GDP, and recycling of eIF2-GDP to eIF2-GTP requires a guanine nucleotide exchange factor eIF2B (Price and Proud, 1994). Phosphorylation of eIF2 on the α-subunit causes it to act as an inhibitor of eIF2B and the reduction in eIF2-GTP levels reduces general translation (Rowlands et al, 1998) (Figure 1). Thus activation of PKR by PIF and Ang II was responsible for the depression in protein synthesis, since transfection of myotubes with a mutant PKR incapable of autophosphorylation and induction of phosphorylation of eIF2α, completely attenuated the depression in protein synthesis by both agents (Eley and Tisdale, 2007). Mutation of PKR also completely attenuated the induction of protein degradation and upregulation of the ubiquitin–proteasome pathway. Induction of the ubiquitin–proteasome pathway by both PIF (Wyke and Tisdale, 2005) and Ang II (Russell et al, 2006b) requires activation of the transcription factor nuclear factor-κB (NF-κB). dsRNA-dependent protein kinase has been shown to activate the upstream kinase IκB kinase (IKK) leading to degradation of the inhibitor protein IκB, leading to release of NF-κB, which migrates to the nucleus and induces transcriptional activation of specific genes (Zamanian-Daryoush et al, 2000). Myotubes containing mutant PKR showed no activation of NF-κB in response to either PIF or Ang II, and no induction of the ubiquitin–proteasome pathway (Eley and Tisdale, 2007), suggesting that NF-κB activity is required for the induction of ubiquitin–proteasome pathway by PKR (Figure 1). Thus activation of PKR leads potentially to both a depression of protein synthesis and an increase in protein degradation in skeletal muscle. These studies in vitro were also reflected by changes in vivo in gastrocnemius muscle of mice bearing a cachexia-inducing tumour, where levels of phosphorylated PKR and eIF2α were found to increase with increasing weight loss by as much as 18-fold for PKR at 25% weight loss (Eley and Tisdale, 2007).
Figure 1.
Summary of pathways leading to a depression in protein synthesis and an increase in protein degradation in skeletal muscle through activation of PKR.
To determine whether changes similar to those induced by PIF and Ang II also occur in human cancer cachexia, the present study examines the levels of phosphorylation of PKR and eIF2α in skeletal muscle of weight-losing patients with upper gastrointestinal cancer, in comparison with healthy, weight-stable subjects undergoing minor elective surgery.
PATIENTS AND METHODS
Cancer patients and controls
Patients provided written, informed consent, and the study was approved by the Lothian Research Ethics Committee. Twenty-nine patients with newly-diagnosed oesophago-gastric adenocarcinoma who were undergoing elective resection of their primary cancer were recruited for the study. Oesophago-gastric cancer patients have a high incidence of weight loss (DeWys et al, 1980) and were therefore chosen as a representative group of patients who develop cancer cachexia. Muscle biopsies were also collected from 10 healthy, weight-stable volunteers who were undergoing elective hernia surgery and who served as controls.
Muscle biopsy
A sample of rectus abdominis muscle was obtained from the edge of the patients abdominal wound within 10 min of induction of general anaesthesia. The sample was obtained without the use of diathermy and was frozen immediately in liquid nitrogen using liquid nitrogen-resistant tubes (Corning BV, Netherlands). Samples were frozen at −70°C until analysis.
Nutritional assessment
At the preoperative assessment, preillness stable weight was self-reported by the patient. Height was measured using a wall-mounted stadiometer with the patient standing erect without shoes. Patients were weighed on spring balance scales without shoes and wearing light clothing, and body mass index was calculated. Mid arm circumference (MAC) was measured at the midpoint between the acromion and olecranon processes. Triceps skinfold thickness (TSF) was measured with Harpenden skin callipers (Holtain, Crymych, UK). Mid arm muscle circumference (MAMC) was calculated according to the formula: MAC-[π × TSF]=MAMC. Karnofsky performance score was documented by the recruiting physician.
Molecular biology materials
Rabbit polyclonal antibody to phospho PKR (pThr 446), was purchased from Insight Biotechnology, (Wembley, Middlesex, UK) and to total PKR (C terminus) from New England Biolabs, (Herts, UK). Rabbit polyclonal antisera to total eIF2α was purchased from Santa Cruz Biotechnology (CA, USA) and rabbit polyclonal antisera to phospho eIF2α was purchased from Abcam (Cambridge, UK). Mouse monoclonal antibody to myosin heavy chain was from Novocastra (Newcastle, UK). Rabbit polyclonal antiserum to actin was from Sigma Aldridge (Dorset, UK). Peroxidase-conjugated goat anti-rabbit antibody and peroxidase-conjugated rabbit anti-mouse antibody were purchased from Dako Ltd (Cambridge, UK). PhosphoSafe™ extraction reagent was obtained from Merck Biosciences, (Nottingham, UK). Hybond A nitrocellulose membranes and enhanced chemiluminescence (ECL) development kits were from Amersham Biosciences Ltd (Bucks, UK).
Western blot analysis
Samples (approximately 10 mg) of muscle were homogenised in 500 μl of PhosphoSafe™ Extraction Reagent and centrifuged at 15 000 g for 15 min. Samples of cytosolic protein (10 μg) were resolved on 10% sodium dodecylsulphate polyacrylamide gels (6% for eIF2α) and transferred to 0.45 μm nitrocellulose membranes, which had been blocked with 5% marvel in Tris-buffered saline, pH 7.5, at 4°C for 1–2 h. Membranes were then washed for 15 min in 0.5% Tween-buffered saline or TBS Tween prior to adding the primary antibodies. The primary antibodies were used at a dilution of 1 : 1000 except for actin (1 : 250) and anti-myosin (1 : 100). Incubation was at 4°C overnight, except for total eIF2α (1–2 h at room temperature). The primary antibodies were washed off the membranes for 15 min (changing the wash every 5 min), except for actin, which was washed for 45 min (changing the wash every 15 min). TBS Tween (0.1%) was used for washing phospho antibodies and total antibodies. The secondary antibodies were used at a dilution of 1 : 1000, and were washed off after 45 min. Development was by ECL and films were developed for 3–6 min. Blots were scanned by a densitometer to quantify differences.
Statistical analysis
Western blot densitometry results are presented as means±s.e.m. for at least three replicate experiments. Differences in means between groups were determined by one-way analysis of variance, followed by Tukey–Kramer multiple comparison test. Significance level was set at P<0.05.
RESULTS
The characteristics of the patients in this study is shown in Table 1. Muscle biopsies from healthy subjects undergoing elective surgery for hernia served as weight stable controls. The weight losing subjects had oesophago-gastric cancer and had a weight loss at the time of operation between 2.4 and 27.5%. As a comparison some patients with oesophago-gastric cancer without weight loss were also included.
Table 1. Demographics of the weight-losing cancer patients and weight-stable, healthy, noncancer controls.
| Healthy controls | Cancer patients | |
|---|---|---|
| Number (n) | 9 | 15 |
| Sex | ||
| Male | 9 | 13 |
| Female | 0 | 2 |
| Age (years) | 56 (41–86) | 66 (49–83) |
| Tumour site | ||
| Oesophageal | N/A | 6 |
| Gastric | 8 | |
| Histology | ||
| ACC | N/A | 14 |
| SCC | 1 | |
| Stage | ||
| I | 3 | |
| II | N/A | 3 |
| III | 6 | |
| IV | 3 | |
| BMI (kg/m2) | 28.5 (19.6–35.2) | 26.1 (20.1–34.4) |
| MAC (cm) | 32.6 (25.5–35.2) | 28.9 (23.0–40.0) |
| TSF (mm) | 15.5 (5.0–29.4) | 14.4 (7.8–37.4) |
| MAMC (cm) | 25.8 (23.8–30.5) | 25.3 (18.3–30.0) |
| KPS | 100 (100–100) | 90a (60–100) |
| Weight loss (%) | 0 | 7.8b (0.0–27.5) |
Abbreviations: BMI=body mass index; KPS=Karnofsky performance score; MAC=mid-arm circumference; MAMC=mid-arm muscle circumference; TSF=triceps skinfold thickness.
Data are presented as medians with ranges in parentheses. Differences are shown from healthy controls as aP<0.05 and bP<0.001.
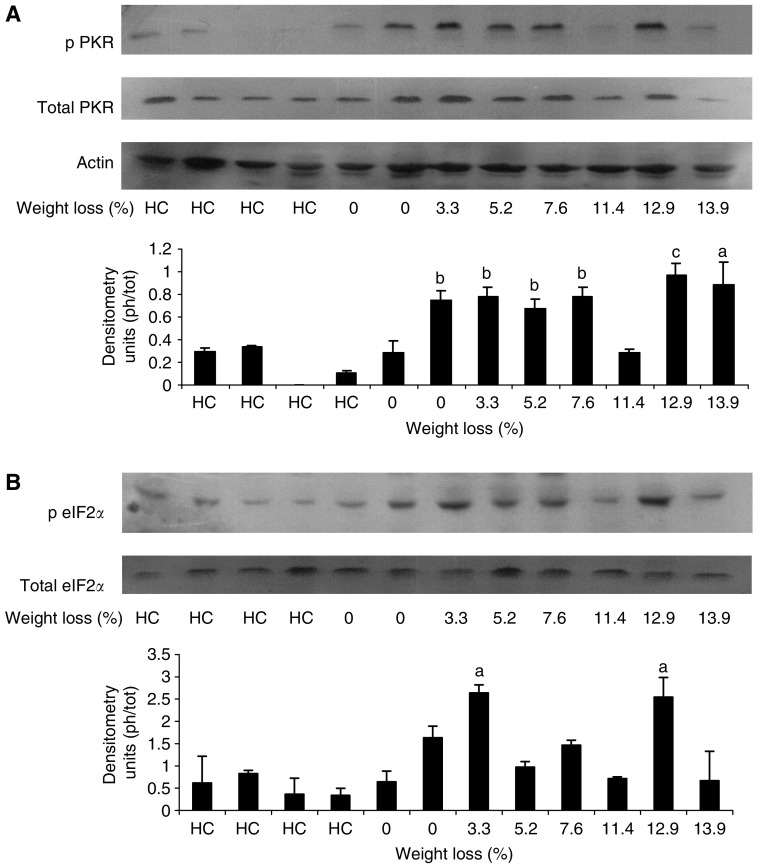
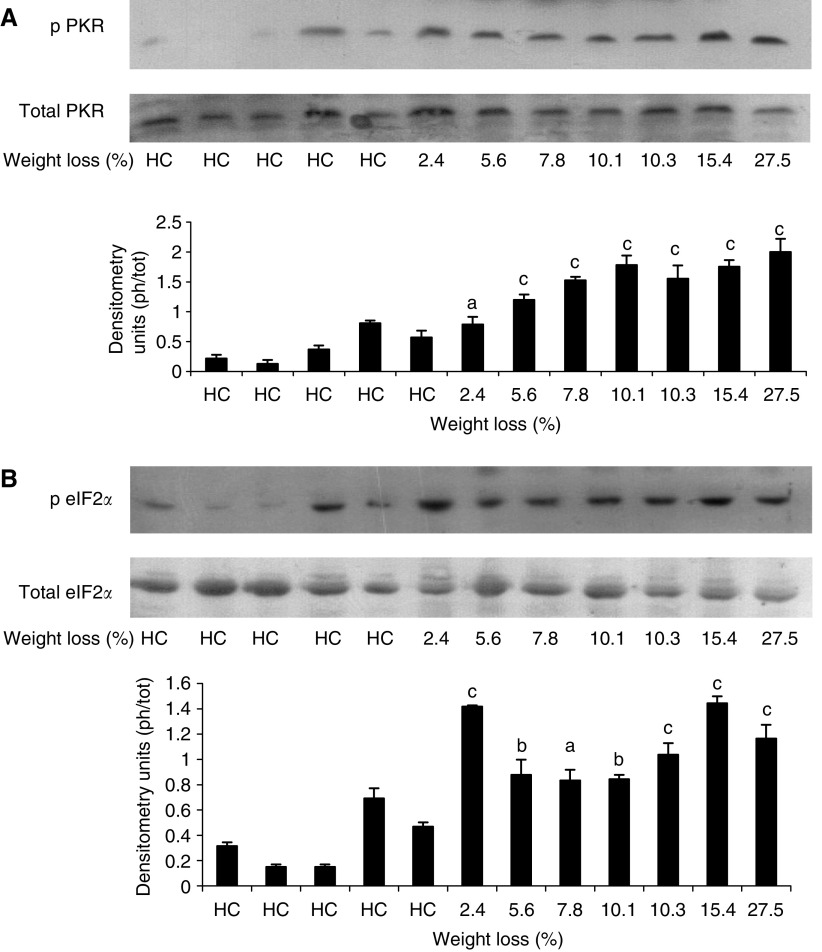
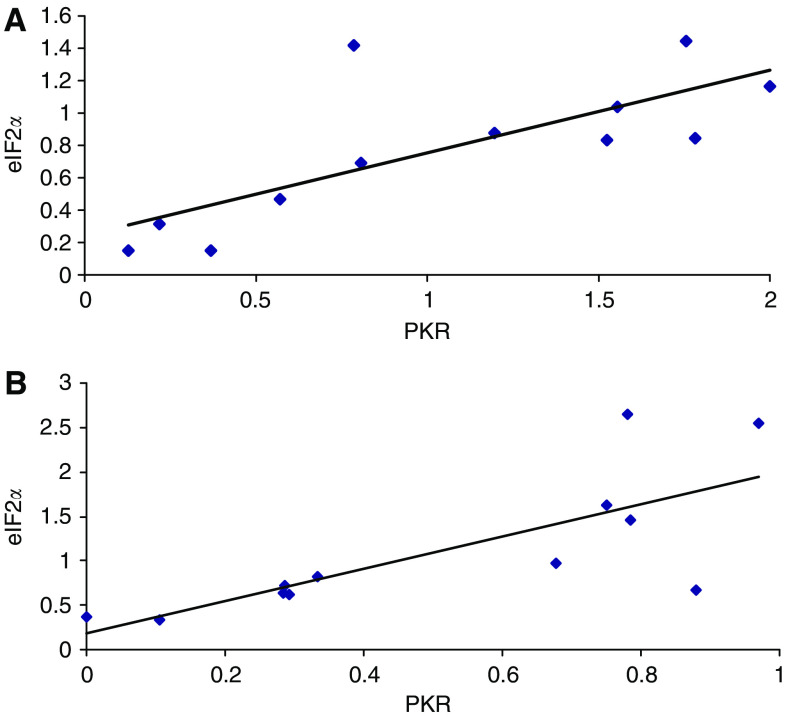
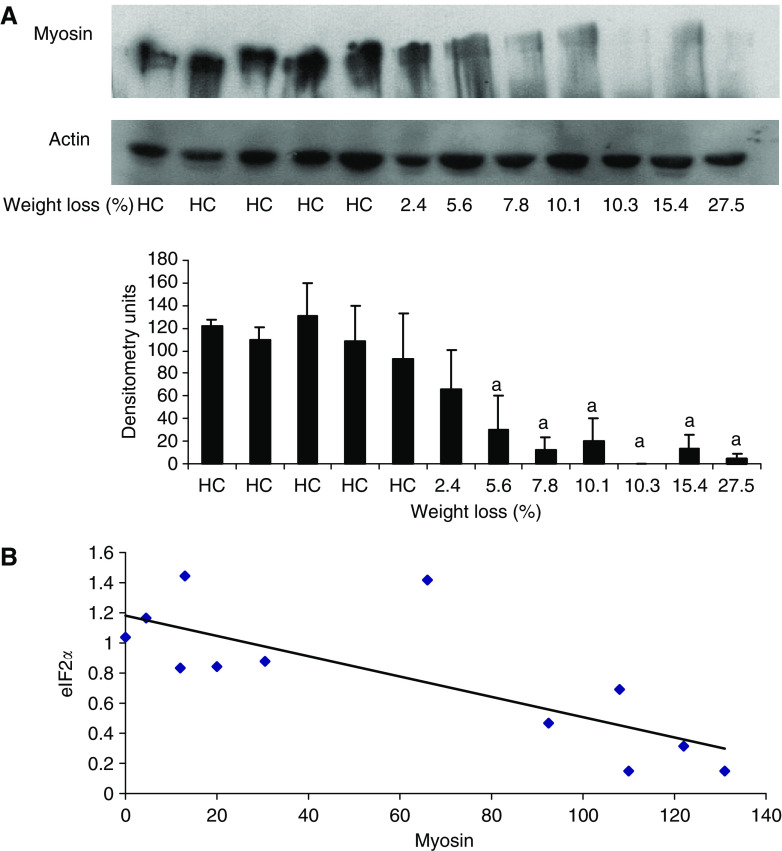
Western blots for the phospho and dephospho forms of PKR and eIF2α in rectus abdominus muscle as a function of weight loss is shown in Figures 2 and 3, which display values for different patients. While there was no major change in total PKR or eIF2α with weight loss, there was a significant increase in the phosphorylated forms in all patients with weight loss, which, however, did not show a tendency for increased expression with increasing weight loss. Cancer patients with no weight loss, or weight gain (Figure 2) showed the same low expression of phosphorylated PKR and eIF2α, as nonweight-losing normal subjects. There was a linear correlation between expression of phosphorylated PKR and phosphorylated eIF2α (correlation coefficient 0.76, P=0.005), consistent with increased PKR activity being responsible for the increased phosphorylation of eIF2 on the α-subunit (Figure 4). Myosin levels decreased as the weight loss increased (Figure 5A), and there was an inverse relationship between the expression of myosin in rectus abdominus muscle and the extent of phosphorylation of eIF2α (correlation coefficient 0.77, P=0.004) (Figure 5B). As previously reported (Acharyya et al, 2004) in skeletal muscle of mice bearing a cachexia-inducing tumour (colon 26) myosin levels decreased, while actin levels remained constant. This has been attributed to specific targeting of myosin by the ubiquitin–proteasome pathway.
Figure 2.
Western blots of phospho PKR (A) and eIF2α (B) in comparison with total PKR and eIF2α in rectus abdominus muscle of healthy controls (HC) and cancer patients as a function of weight loss. Actin was used as a loading control. Each lane represents muscle from an individual patient. The specificity of the antibodies is given in Patients and Methods section. A densitometric analysis of the ratio of phospho to total forms is given underneath and represents the average of three separate blots. Differences from healthy controls are shown as a, P<0.05 or b, P<0.01. c, P<0.001.
Figure 3.
Western blots of phospho PKR (A) and eIF2α (B) in comparison with total PKR and eIF2α in rectus abdominus muscle of healthy controls (HC) and cancer patients as a function of weight loss. Actin was used as a loading control. Each lane represents muscle from an individual patient. A densitometric analysis of the ratio of phospho to total forms is given underneath and represents the average of three separate blots. Differences from healthy controls are shown as a, P<0.05, b, P<0.01 or c, P<0.001.
Figure 4.
Relationship between the ratios of autophosphorylation of PKR and phospho eIF2α to total PKR and eIF2α in skeletal muscle samples from patients shown in either Figure 2A (A) or Figure 3B (B). The correlation coefficient in each case is 0.76, P=0.005.
Figure 5.
(A) Western blot of myosin expression in comparison with actin as a loading control in rectus abdominis muscle of the patients shown in Figure 3 as a function of weight loss. Each lane represents muscle from an individual patient. The densitometric analysis represents the average of three separate blots. Differences from healthy controls are shown as a, P<0.05. (B) Ratio of phosphorylated to total PKR vs the myosin levels of the samples shown in A. The correlation coefficient is 0.77, P<0.0004.
DISCUSSION
This is the first report to show an increased expression of phosphorylated PKR and eIF2α in the skeletal muscle of weight-losing cancer patients compared with healthy weight-stable controls. As found in the gastrocnemius muscle of weight-losing mice bearing the MAC16 tumour (Eley and Tisdale, 2007), expression of both phospho PKR and eIF2α increased in patients with weight loss, although there was no trend to increased expression with increasing weight loss. This suggests that the same signalling mechanism is operative in the skeletal muscle of cachectic cancer patients as that in mice with experimental cancer cachexia. Similar findings have been observed in murine myotubes in the presence of PIF or Ang II, and are thought to be responsible for the depression in protein synthesis and increase in degradation (Eley and Tisdale, 2007). dsRNA-dependent protein kinase is normally activated in response to viral attack, and the depression of protein synthesis resulting from phosphorylation of eIF2α constitutes one of the major ways in which viral replication is impaired (Clemens, 1997). However, PKR can also exert effects in uninfected cells and can be a potent growth inhibitory protein when activated (Chong et al, 1992). dsRNA-dependent protein kinase is also linked to the induction of pro-apoptotic genes by dsRNA, and may trigger cell death in response to viral infection and possible tumorigenesis (Balachandran et al, 1998). Activation of PKR by PIF may be responsible for its ability to induce apoptosis in murine myotubes (Smith and Tisdale, 2003). Increased apoptosis has also been observed in the skeletal muscle of rats bearing the cachexia-inducing Yoshida AH-130 ascites hepatoma (van Royen et al, 2000) and in the early stage of weight loss in rabbits bearing the VX2 carcinoma (Yoshida et al, 2001). In addition, muscle biopsies from weight losing patients with upper gastro-intestinal cancer showed a threefold increase in DNA fragmentation compared with control subjects, together with an increased PARP cleavage and decrease in MyoD protein content (Busquets et al, 2007). Thus activation of PKR might be responsible for the increased apoptosis in the skeletal muscle of weight-losing cancer patients contributing to muscle atrophy.
The increased phosphorylation of eIF2α is likely to contribute to the depression of protein synthesis in the skeletal muscle of cancer patients, through the inhibition of eIF2B and subsequent translational repression (Rowlands et al, 1998), as was previously observed in murine myotubes treated with PIF and Ang II (Eley and Tisdale, 2007). Phosphorylation of eIF2α has also been shown to be responsible for the inhibition of protein synthesis in rat liver by vasopressin (Kimball and Jefferson, 1990), and rat skeletal muscle by interleukin-1 (Cooney et al, 1999). Phosphorylation of PKR would also be expected to lead to an increased breakdown of myofibrillar proteins in skeletal muscle by induction of the ubiquitin–proteasome pathway (Wyke and Tisdale, 2005; Russell et al, 2006b) through activation of NF-κB (Zamanian-Daryoush et al, 2000) analogous to the effect of PIF and Ang II (Eley and Tisdale, 2007).
In the current study, there was a linear relationship between activation (autophosphorylation) of PKR and phosphorylation of eIF2α, suggesting that PKR is responsible for this effect rather than general control of gene expression, nondepressing 2, which is expected to be activated (Anthony et al, 2004) by the reduction in plasma levels of amino acids in cachectic subjects (Norton et al, 1985). Moreover, in myotubes expressing mutant PKR there was no increase in phosphorylation of eIF2α in response to catabolic stimuli (Eley and Tisdale, 2007) suggesting that PKR is the major eIF2α kinase under such conditions.
In mice bearing the MAC16 tumour, which show a similar elevation of phospho PKR in skeletal muscle with weight loss (Eley and Tisdale, 2007), treatment with a PKR inhibitor, at a concentration which reduced levels of phospho PKR down to that found in non tumour-bearing animals, effectively attenuated the depression of body weight, through an increase in lean body mass (Eley et al, 2007). This was achieved through attenuation in both the depression in protein synthesis and the increase in protein degradation, as observed in murine myotubes exposed to either PIF or Ang II (Eley and Tisdale, 2007). This suggests that muscle atrophy in cachectic cancer patients may also be responsive to inhibitors of PKR.
In addition to attenuation of cachexia, treatment of mice bearing the MAC16 tumour with a PKR inhibitor also inhibited tumour growth (Eley et al, 2007). In vitro studies (unpublished) also showed sensitisation of MAC16 cells to the growth inhibitory effects of gemcitabine and 5-fluoroacil. The MAC16 tumour also shows elevated autophosphorylation of PKR and phosphorylation of eIF2α, which has been linked to constitutive activation of NF-κB and chemoresistance (unpublished). Other studies have also shown an increased autophosphorylation of PKR and phosphorylation of eIF2α in human breast carcinoma cell lines, compared with nontransformed epithelial cells (Kim et al, 2000), and in human melanoma cells compared with nontransformed melanocytes in culture (Kim et al, 2002). In addition analysis of colon cancer specimens showed that transformation from normal mucosa to adenomas and carcinomas coincided with an increase in PKR expression (Kim et al, 2002). These results suggest a positive role of PKR in cancer progression and growth control of tumour cells. This suggests that inhibitors of PKR may not only be effective in attenuating muscle wasting in cancer patients, but may also induce antitumour effects or synergise with existing chemotherapy.
The changes that are seen may be part of a common signalling mechanism found in conditions where muscle atrophy occurs. Thus PIF has been found in the urine of weight-losing cancer patients, and when purified and administered to mice causes muscle atrophy (Cariuk et al, 1997). Ang II has also been linked with muscle wasting in congestive heart failure (Onder et al, 2002), and tumour necrosis factor-α, which may be linked to muscle wasting in sepsis, AIDS and parasitic infections, as well as cancer, has also been shown to activate PKR (Jeffrey et al, 2002). Burn injury, which also causes muscle atrophy, is also associated with an increased phosphorylation of eIF2α as a result of a 274% increase in phosphorylation of PKR (Kaneki et al, 2004). This suggests that inhibitors of PKR autophosphorylation may have a general role in the treatment of muscle atrophy.
Acknowledgments
This work has been supported by a grant from Novartis Medical Nutrition. RJES is supported by the Maurice Wohl Fellowship in Surgery/Dental Surgery, and a Small Projects Grant from the Royal College of Surgeons in Edinburgh. Mr Simon Paterson-Brown, Mr Andrew de Beaux and Mr Graeme Couper, Consultant Surgeons, Royal Infirmary of Edinburgh, were essential for the recruitment of patients and the provision of tissue used in this study.
References
- Acharyya S, Ladner KJ, Nelsen LL, Damrauer J, Reiser PJ, Swoap S, Guttridge DC (2004) Cancer cachexia is regulated by selective targeting of skeletal muscle gene products. J Clin Invest 114: 370–378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anthony TG, McDaniel BJ, Byerley RL, McGrath BC, Cavener DR, McNurlan MA, Wek RC (2004) Preservation of liver protein synthesis during dietary leucine deprivation occurs at the expense of skeletal muscle mass in mice deleted for eIF2 kinase GCN2. J Biol Chem 279: 36553–36561 [DOI] [PubMed] [Google Scholar]
- Balachandran S, Kim CN, Yeh W-C, Mak TW, Bhalla K, Barber GN (1998) Activation of the dsRNA-dependent protein kinase, PKR, induces apoptosis through FADD-mediated death signalling. EMBO J 17: 6888–6902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busquets S, Deans C, Figueras M, Moore-Carrasco R, Lopez-Soriano FJ, Fearon KCH, Argiles JM (2007) Apoptosis is present in skeletal muscle of cachectic gastro-intestinal cancer patients. Clin Nutr 26: 614–618 [DOI] [PubMed] [Google Scholar]
- Cariuk P, Lorite MJ, Todorov PT, Field WN, Wigmore SJ, Tisdale MJ (1997) Induction of cachexia in mice by a product isolated from the urine of cachectic cancer patients. Br J Cancer 76: 606–613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong KL, Feng L, Schappert K, Meurs E, Donahue TF, Friesen JD, Hovanessian AG, Williams BR (1992) Human p68 kinase exhibits growth suppression in yeast and homology to the translational regulator GCN2. EMBO J 11: 1553–1562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clemens MJ (1997) PKR – A protein kinase regulated by double-stranded RNA. Int J Biochem Cell Biol 29: 945–949 [DOI] [PubMed] [Google Scholar]
- Cooney RN, Maish III GO, Gilpin T, Shumate ML, Lang CH, Vary TC (1999) Mechanism of IL-1 induced inhibition of protein synthesis in skeletal muscle. Shock II: 235–241 [DOI] [PubMed] [Google Scholar]
- deHaro C, Mendez R, Santogo J (1996) The eIF-2α kinases and the control of protein synthesis. FASEB J 10: 1378–1387 [DOI] [PubMed] [Google Scholar]
- DeWys WD, Begg C, Laven PT, Band PR, Bennett JM, Bertino JR, Cohen MH, Douglass Jr HO, Engstrom PF, Edzini EZ, Horton J, Johnson GJ, Moertel CG, Oken MM, Perlia C, Rosenbaum C, Silverstein MN, Skeel RT, Sponzo RW, Tormey DC (1980) Prognostic effect of weight loss prior to chemotherapy in cancer patients. Eastern Cooperative Oncology Group. Am J Med 69: 491–497 [DOI] [PubMed] [Google Scholar]
- Eley HL, Russell ST, Tisdale MJ (2007) Attenuation of muscle atrophy in a murine model of cachexia by inhibition of the dsRNA-dependent protein kinase. Br J Cancer 96: 1216–1222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eley HL, Tisdale MJ (2007) Skeletal muscle atrophy: a link between depression of protein synthesis and increase in degradation. J Biol Chem 282: 7087–7097 [DOI] [PubMed] [Google Scholar]
- Emery PW, Edwards RHT, Rennie MJ, Souhami RL, Halliday D (1984) Protein synthesis in muscle measured in vivo in cachectic patients with cancer. Br Med J 289: 584–586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jammi NV, Beal PA (2001) Phosphorylation of the RNA-dependent protein kinase regulates its RNA-binding activity. Nucleic Acids Res 29: 3020–3029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffrey IW, Bushell M, Tilleray VJ, Morley S, Clemens MJ (2002) Inhibition of protein synthesis in apoptosis: differential requirements by the tumor-necrosis factor α family and a DNA-damaging agent for caspases and the double-stranded RNA-dependent protein kinase. Cancer Res 62: 2272–2280 [PubMed] [Google Scholar]
- Kaneki M, Kunii K, Chang K, Martyn J (2004) Inhibitory phosphorylation of translation initiation factors, eIF2α and eIF2Bε in skeletal muscle of burned rats. Anaesthesiology 101: A421 [Google Scholar]
- Khal J, Hine AV, Fearon KCH, Dejong CHC, Tisdale MJ (2005) Increased expression of proteasome subunits in skeletal muscle of cancer patients with weight loss. Int J Biochem Cell Biol 37: 2196–2206 [DOI] [PubMed] [Google Scholar]
- Kim SH, Forman AP, Matthews MB, Gunnery S (2000) Human breast cancer cells contain elevated levels and activity of the protein kinase, PKR. Oncogene 19: 3086–3094 [DOI] [PubMed] [Google Scholar]
- Kim SH, Gunnery S, Choe JK, Matthews MB (2002) Neoplastic progression in melanoma and colon cancer is associated with increased expression and activity of the interferon-inducible protein kinase PKR. Oncogene 21: 8741–8748 [DOI] [PubMed] [Google Scholar]
- Kimball SR, Jefferson LS (1990) Mechanism of the inhibition of protein synthesis by vasopressin in rat liver. J Biol Chem 265: 16794–16798 [PubMed] [Google Scholar]
- Lorite MJ, Caruik P, Tisdale MJ (1997) Induction of muscle protein degradation by a tumour factor. Br J Cancer 76: 1035–1040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorite MJ, Smith HJ, Arnold JA, Morris A, Thompson MG, Tisdale MJ (2001) Activation of ATP-ubiquitin-dependent proteolysis in skeletal muscle in vivo and murine myoblasts in vitro by a proteolysis-inducing factor (PIF). Br J Cancer 85: 297–302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norton JA, Gorschboth CM, Wesley RA (1985) Fasting plasma amino acid levels in cancer patients. Cancer 56: 1181–1186 [DOI] [PubMed] [Google Scholar]
- O'Keefe SJD, Ogden J, Ramjee G, Rund J (1990) Contribution of elevated protein turnover and anorexia to cachexia in patients with hepatocellular carcinoma. Cancer Res 50: 1226–1230 [PubMed] [Google Scholar]
- Onder G, Penninx BWJH, Balkrishnan R, Fried LP, Chaves PHM, Williamson J, Carter C, DiBari M, Guralnik JM, Pahor M (2002) Relation between use of angiotensin-converting inhibitors and muscle strength and physical function in older women: an observational study. Lancet 359: 926–930 [DOI] [PubMed] [Google Scholar]
- Price N, Proud C (1994) The guanine nucleotide-exchange factor, eIF-2B. Biochimie 76: 748–760 [DOI] [PubMed] [Google Scholar]
- Rowlands AG, Panniers R, Henshaw EC (1998) The catalytic mechanism of guanine nucleotide exchange factor action and competitive inhibition by phosphorylated eukaryotic initiation factor 2. J Biol Chem 263: 5526–5533 [PubMed] [Google Scholar]
- Russell ST, Sanders PM, Tisdale MJ (2006a) Angiotensin II directly inhibits protein synthesis in murine myotubes. Cancer Lett 231: 290–294 [DOI] [PubMed] [Google Scholar]
- Russell ST, Wyke SM, Tisdale MJ (2006b) Mechanism of induction of muscle protein degradation by angiotensin II. Cell Sig 18: 1087–1096 [DOI] [PubMed] [Google Scholar]
- Sanders PM, Russell ST, Tisdale MJ (2005) Angiotensin II directly induces muscle protein catabolism through the ubiquitin-proteasome pathway and may play a role in cancer cachexia. Br J Cancer 93: 425–434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith HJ, Tisdale MJ (2003) Induction of apoptosis by a cachectic-factor in murine myotubes and inhibition by eicosapentaenoic acid. Apoptosis 8: 161–169 [DOI] [PubMed] [Google Scholar]
- Van Royen M, Carbo N, Busquets S, Alvarez B, Quin LS, Lopez-Soriano FJ, Argiles JM (2000) DNA fragmentation occurs in skeletal muscle during tumour growth: a link with cachexia? Biochem Biophys Res Commun 270: 533–537 [DOI] [PubMed] [Google Scholar]
- Wyke SM, Tisdale MJ (2005) NF-κB mediates proteolysis-inducing factor induced protein degradation and expression of the ubiquitin-proteasome system in skeletal muscle. Br J Cancer 92: 711–721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida H, Ishiko O, Sumi T, Honda K, Hirai K, Ogita S (2001) Expression of apoptosis regulatory proteins in the skeletal muscle of tumour-bearing rabbits. Jpn J Cancer Res 92: 1135–1140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamanian-Daryoush M, Mogensen TH, DiDonato JA, Williams BRG (2000) NF-κB activation by double-stranded-RNA-activated protein kinase (PKR) is mediated through NF-κB-inducing kinase and IκB kinase. Mol Cell Biol 20: 1278–1290 [DOI] [PMC free article] [PubMed] [Google Scholar]