Abstract
This review explores possible mechanisms by which the neurofibromatosis type-2 tumour suppressor Merlin regulates contact-dependent inhibition of proliferation. Starting from an evolutionary perspective, the concurrent emergence of intercellular contacts and proliferation control in multicellular organisms is first considered. Following a brief survey of the molecular and subcellular milieus in which merlin performs its function, the importance of different cellular and biological contexts in defining the function of merlin is discussed. Finally, an integrated model for merlin and the Ezrin, Radixin, and Moesin (ERM) proteins functioning in the regulation of cellular interfaces is proposed.
Keywords: Merlin, Ezrin, EGFR, contact-inhibition, adherens junctions, cytoskeleton
All cancers result from the cumulative acquisition of molecular defects within a basic core of cellular functions that control proliferation, apoptosis, and invasiveness (Hanahan and Weinberg, 2000). Regardless of the specific alterations, their combined effects must also disable the mechanism of contact-dependent inhibition of proliferation, namely the cell's ability to prevent activation of the proliferation machinery in response to contact with neighbouring cells. Indeed, loss of contact-dependent inhibition of proliferation is an absolute conditio sine qua non for the development and progression of any solid tumour. This phenomenon was first described in the early 1960s with the observation that normal cultured diploid cells can reach a maximum saturation density beyond which cell number and DNA synthesis are unaffected by media replenishment (Levine et al, 1965). This finding also introduced the idea that upon adhering to each other, cells can enter and maintain a quiescent state despite the unlimited availability of nutrients and growth-promoting factors – a phenomenon that occurs in vivo in all adult solid tissues. Conversely, the uncoupling of mitogenic signals from the blockage imposed by cell:cell adhesion is a physiological event during embryogenesis and tissue homoeostasis and regeneration. Therefore, highly regulated molecular machinery must exist to conditionally establish and resolve the link between intercellular contacts and mitogenic signalling. Although still poorly understood, the molecular mechanisms that coordinate intercellular adhesion and cell proliferation are beginning to emerge (Brunton et al, 2004). A detailed understanding of the molecular components and events that govern this phenomenon will be essential to understanding and intercepting the process of tumourigenesis.
Mounting evidence indicates that the neurofibromatosis type-2 (NF2) tumour suppressor, Merlin (also known as schwannomin) is a critical regulator of contact-dependent inhibition of proliferation. Mutations in the NF2 tumour suppressor gene underlie the familial cancer syndrome NF2, which is characterised by the development of tumours of the nervous system such as bilateral vestibular schwannomas and meningiomas (Baser et al, 2002). Biallelic NF2 inactivation also underlies the development of many sporadic schwannomas and meningiomas, as well as malignant mesothelioma, an essentially untreatable cancer. Despite the restricted spectrum of tumours associated with loss of NF2, Merlin is a widely expressed protein and studies from several model organisms have shown that Merlin plays an important role in the function of many different cell types (McClatchey and Giovannini, 2005). Although its molecular function has been elusive, recent studies suggest that Merlin can coordinate the establishment of intercellular contacts with the concomitant suppression of mitogenic signals at the membrane.
EVOLUTION
The transition from prokaryotes to early unicellular eukaryotes was accompanied by the generation of a variety of membrane subdomains including cortical membrane patches, protrusions and ‘landmarks’ utilised during locomotion, budding, or conjugation. This increased complexity in cellular polarisation was likely facilitated by the acquisition of flexible membranes and a primordial cytoskeleton (endoskeleton), together with the emergence of small GTPases that enabled directional (exo- and endocytic) membrane flow, and localised cytoskeleton assembly (Jekely, 2003; Nelson, 2003). On the eve of multicellular life, unicellular polarity had evolved a sophisticated membrane complexity with specialised subdomains of disparate forms and functions such as cilia, flagella, cytostomata, and pseudopodia. Unicellular choanoflagellates, the most likely cenancestor of metazoa, already utilised adhesion machineries for food capture or colony formation, and transmembrane receptors that can respond to environmental cues. In fact, it has been recently discovered that proteins (spectrin, cadherins, G protein-coupled receptors) and interaction motifs (SH2, EGF) thought to be unique to metazoa were already in place and cell proliferation was already under the control of receptor and non-receptor tyrosine kinases (King et al, 2003; King, 2004).
Unicellular organisms were capable of organizing their membranes and pacing proliferation in response to extracellular signals, but the transition to multicellularity necessitated the structural and functional coordination of these membrane subdomains at the interface between cells. This might have occurred via the molecular rewiring of existing adhesive and transmembrane signalling apparata utilising intracellular proteins/modules capable of orchestrating multiple interactions at the plasma membrane. Such a function could have been carried out by scaffolding proteins containing, for example, PDZ and LIM domains but these were already found in prokaryotes and likely regulated polarity (Te Velthuis et al, 2007). The further increase in membrane complexity inherent in the transition to multicellularity was accompanied by the emergence of new protein domains, several including the four-point-one, ezrin, radixin, moesin (FERM) domain. Indeed, 14 orthologous groups of FERM domain-containing proteins can be identified in worms, flies, and mammals suggesting that a dramatic expansion of this superfamily occurred in the earliest metazoa (Bretscher et al, 2002). The trilobed FERM domain is generally thought to mediate membrane association and can be tethered to a number of different functional domains. In a subset of proteins, including Merlin and the ERM proteins, the FERM domain is appended to modules that link it to the cytoskeleton, and poise these proteins to coordinate multiple signalling events at the membrane with specific architectural features of the cell. Thus, the FERM domains appear to have evolved within an already polarised cellular context to further organise membrane domains in response to the requirements imposed by multicellularity.
Nutrient availability is the major regulator of proliferation in unicellular organisms; under conditions of unlimited availability proliferation appears to be the default. The complex morphogenetic processes and compartmentalised tissue functions that evolved in metazoa necessitated the ability to inhibit cell proliferation, effectively disengaging the proliferative machinery from that which senses and responds to surrounding conditions (Edgar, 2006). Among FERM domain-containing proteins, the function of the tumour suppressor Merlin is most clearly linked to the inhibition of cell proliferation. In fact, accumulating evidence suggests that Merlin physically and functionally links cell:cell communication with negative regulation of proliferation, a conclusion drawn from molecular, cellular, and biological studies (reviewed in McClatchey and Giovannini, 2005).
MOLECULAR-CELLULAR CONTEXT
Studies of the closely related ERM proteins have made important contributions to our understanding of the molecular function of merlin. The term ‘membrane:cytoskeleton linker’ used to describe both Merlin and the ERM proteins highlights the bipartite subcellular context in which they operate. A standard view of ERM function depicts cycling between ‘active’ and ‘inactive’ states and considers ‘activity’ to be the tethering of certain membrane proteins to the cytoskeleton (Bretscher et al, 2002). Although studies of Merlin yield many parallels, it is increasingly apparent that more sophisticated models of Merlin (and ERM) function must be considered. More refined models would consider the possibility that Merlin can exist in more than two states and can bring together and regulate multiple partners. Indeed, an increasing number of proteins have been reported to physically interact with Merlin and an increasing number of functional activities have been ascribed to Merlin (McClatchey and Giovannini, 2005). Collectively, these studies suggest a physical and functional role for Merlin in regulating cell:cell adhesion, transmembrane signalling receptors, Rho-GTPase signalling, and in modulating actin cytoskeleton dynamics. Could Merlin do all this?
The membrane
Most studies conclude that membrane association is necessary for the growth-suppressing function of Merlin. Mutant versions of Merlin that cannot localise to the membrane cannot inhibit cell proliferation. In addition, Merlin can associate with several membrane proteins, including both adhesion receptors and membrane receptors that regulate cell proliferation and differentiation.
In normal cells, Merlin is recruited to cadherin-containing complexes at nascent cell:cell contacts and is regulated by cell:cell adhesion. Conversely, primary Nf2−/− cells of several types do not form stable adherens junctions (AJs) (Lallemand et al, 2003). Merlin has also been reported to associate with other types of adhesion receptors, including the cell:extracellular matrix receptor CD44 and β1-integrin, perhaps reflecting alternative ways for merlin to coordinate the receipt of information from the extracellular environment with the proliferative machinery (Morrison et al, 2001; Fernandez-Valle et al, 2002). Indeed, in some cells the association of Merlin with CD44 has been shown to be required for contact-dependent inhibition of proliferation (Morrison et al, 2001).
Recent studies suggest that Merlin directly controls the surface availability and function of membrane receptors that regulate proliferation and differentiation in both Drosophila and mammalian cells (Figure 1). In the fly, loss of both Merlin and the related tumour suppressor, Expanded, leads to increased surface levels and altered distribution of certain membrane receptors including epidermal growth factor receptor (EGFR), Notch, Patched, and Fat, suggesting that Merlin normally promotes the clearance of receptors from the plasma membrane. This was shown to be accompanied by increased signalling output for some receptors (Maitra et al, 2006). In cultured mammalian cells, Merlin inhibits the internalisation, effector complexing, and downstream signalling of activated EGFR upon cell:cell contact, consistent with the idea that Merlin normally sequesters EGFR into a non-signalling plasma membrane compartment; as follows, proliferation and EGFR activation are inhibited at high cell density in wild-type but not Nf2−/− cells (Curto et al, 2007). Although these two studies seem to reach differing views of how Merlin regulates membrane receptor surface availability/function – in one case promoting turnover and the other stabilising surface receptors – it is possible that the primary function of Merlin in both cases is to retain receptors in a certain membrane compartment and that the consequence of that is dependent upon species, cell context, cell type or even the identity of the receptor itself. Alternatively, these differences could reflect the concomitant loss of Expanded in the fly studies (Maitra et al, 2006).
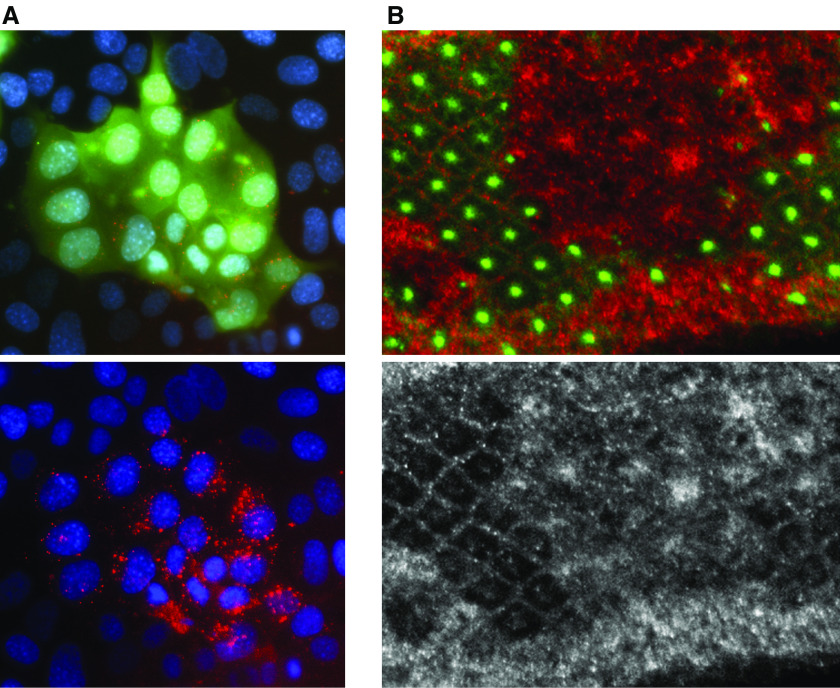
Figure 1.
Merlin differentially controls the membrane distribution of EGFR in mammalian vs insect cells. (A) Mosaic culture of wild-type mouse epithelial cells surrounding a clone of Nf2-deficient cells marked with a green fluorescent protein-expressing adenovirus (green). Upon stimulation (30 min), fluorescent EGF (Texas Red conjugated) is internalised and concentrated within intracellular vesicles in cells that do not express Merlin, but not in wild-type cells (Curto et al, 2007). Bottom panel, Texas Red EGF. (B) Drosophila eye imaginal disc. Mutant clones containing mutations in both merlin and Expanded are identified by the loss of Merlin staining (green). In these cells, the steady-state levels of EGFR are increased (Maitra et al, 2006) (figure kindly provided by Sushmita Maitra and Richard Fehon, University of Chicago). Bottom panel, EGFR.
Increasing evidence suggests that Merlin mediates contact-dependent inhibition of proliferation by both sensing cell:cell contact and intercepting mitogenic signalling initiated at the plasma membrane. A simultaneous, contact-dependent complexing of Merlin to both signalling and adhesion receptors would be a simple strategy for accomplishing this (Figure 2). In mammalian cells Merlin associates with EGFR in a contact-dependent manner; this association requires the tandem PDZ domain-containing adaptor Na+–H+ exchanger regulatory factor 1; ERM-binding protein of 50 kDa (NHE-RF1/EBP50; hereafter referred to as NHE-RF1), which can associate with both Merlin and EGFR (Lazar et al, 2004; Curto et al, 2007). In fact, NHE-RF1 can also associate with the ERM proteins and, either directly or indirectly with a variety of receptors, including ErbB2 and the platelet-derived growth factor receptor (PDGFR) (James et al, 2004; Rangwala et al, 2005). The latter is particularly notable given the recent report that Merlin can also negatively regulate signalling downstream of PDGFR (Morrison et al, 2007); this study, which did not examine NHE-RF1, concluded that Merlin interfered with PDGFR signalling downstream of its immediate effectors, in contrast to the aforementioned studies of Merlin:EGFR. Notably, however, NHE-RF1 has been reported to associate with internal PDZ binding sites in the EGFR cytoplasmic domain that are in close proximity to the major effector binding sites (Lazar et al, 2004); in contrast, NHE-RF1 associates with the extreme C-terminus of PDGFR and therefore may not alter effector association. Interestingly, a role for the closely related NHE-RF2 adaptor in facilitating an association between N-cadherin and PDGFR has recently been reported (Theisen et al, 2007). Perhaps the nature of the NHE-RF:receptor association, along with the spatiotemporal specificity achieved by associating with Merlin and/or the ERM proteins, can impose differential physical and signalling regulation upon different membrane receptors. It will be interesting to see whether the association of Merlin with analogous PDZ adaptors is important for receptor surface abundance in Drosophila. The output of a variety of receptors could be regulated by Merlin using this general mechanism, potentially explaining the many intracellular pathways that have been reported to be affected by Merlin.
Figure 2.
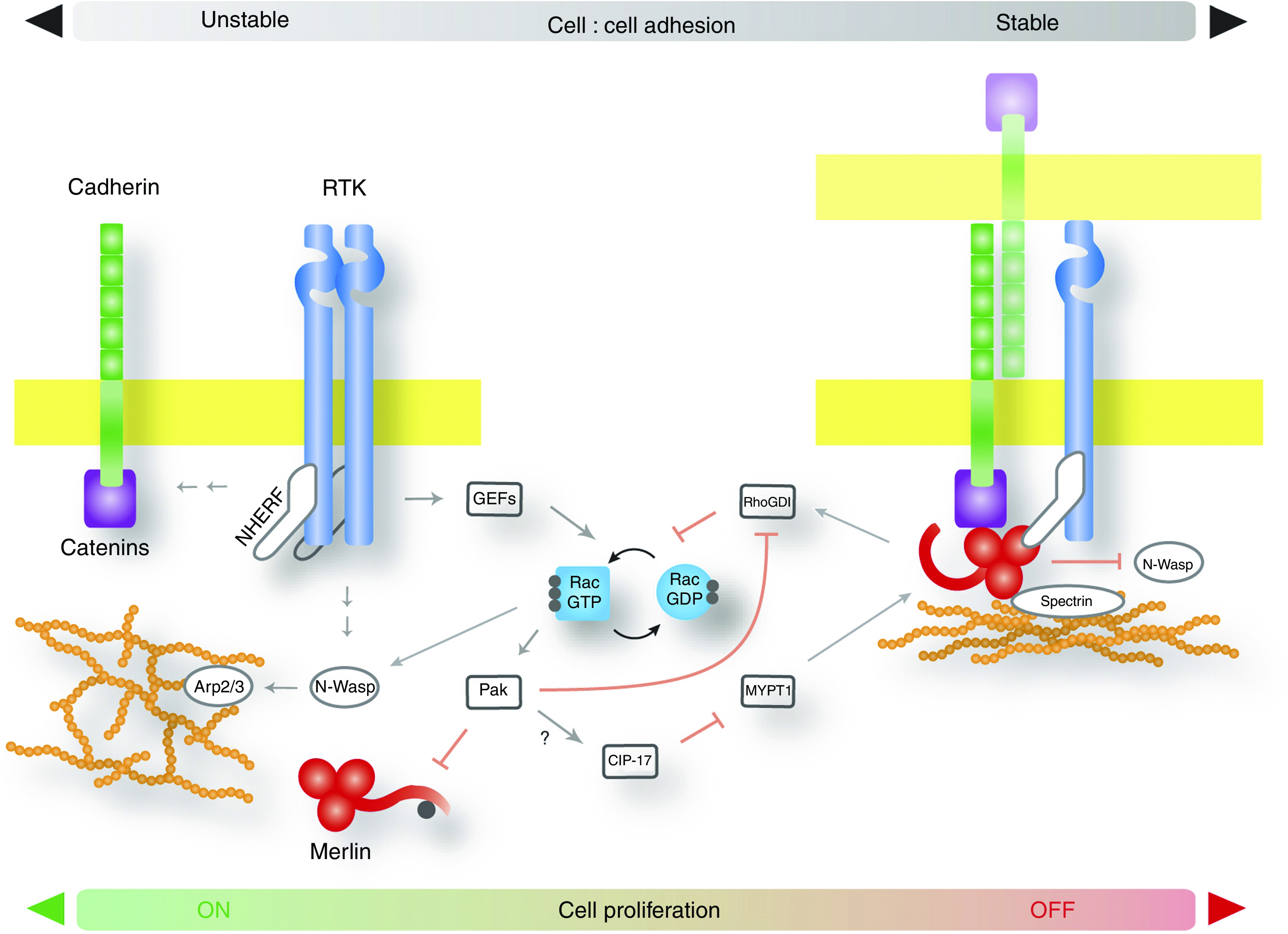
Merlin coordinates membrane receptor signalling and cell:cell contact. Schematic depiction of the basic molecular circuitry utilised by Merlin to link intercellular adhesion with the regulation of membrane receptor signalling. As an example, the relationships among Merlin, Cadherin and the mitogenic receptor tyrosine kinase (RTK) EGFR, are shown here. (Left) In conditions where cell:cell contacts are minimal or unstable, such as remodelling tissues or sparse cultured cells, signals from activated RTKs lead to instability of cadherin-catenin-containing complexes, highly dynamic actin cytoskeleton, and increased activation of Rac which, in turn, keeps Merlin inactive. Proliferative signals are transduced unopposed. (Right) The progressive recruitment of Merlin to newly formed cell:cell contacts is accompanied by a decline in Rac activity, decreased turnover of the cortical actin cytoskeleton, and AJ stabilisation. At the same time, complexing of Merlin to EGFR:E-cadherin could concomitantly tether it to the more stable actin network and restrict EGFR to an insoluble adhesive compartment from which it can neither signal nor internalise (see also Figure 1). At late confluence, EGFR signalling induced by serum or exogenous EGF is disabled and proliferative signals are silenced. These events do not occur in the absence of Merlin.
The actin cytoskeleton
Many studies indicate a functional association between Merlin and the actin cytoskeleton. Biochemically, Merlin can clearly associate with actin in vitro. Merlin does not localise to stress fibres and instead, like the ERM proteins, appears to decorate the cortical actin network (James et al, 2001). Although lacking the bona fide carboxyl-C-terminal actin-binding site found in the ERM proteins, Merlin may instead directly bind F-actin via its N-terminal domain (Brault et al, 2001; James et al, 2001). Alternatively, indirect tethering of Merlin to the cytoskeleton may occur via actin-binding interactors such as βII-spectrin/fodrin, or heterodimerisation with other ERMs (reviewed in McClatchey and Giovannini, 2005). The importance of actin cytoskeleton association is underscored by the fact that association of Merlin with the cortical actin network is required for growth suppression and inhibition of EGFR signalling (B Cole, MC, and AIM, unpublished data).
A functional association between Merlin and the cortical actin cytoskeleton is also suggested by the fact that loss of merlin is accompanied by disruption of cortical actin structures in some cell types (Bashour et al, 2002; Lallemand et al, 2003). The disorganised actin cytoskeleton observed in some Nf2−/− cells could reflect the ability of Merlin to anchor actin to the membrane, to inhibit signals that maintain a highly dynamic actin cytoskeleton, or both. For example, Merlin can inhibit both Arp2/3- (Manchanda et al, 2005) and Rac-induced actin assembly (Pelton et al, 1998).
Regulation
Beyond its physical identity, the membrane:cytoskeleton interface is a dynamic compartment in which Merlin, the ERM proteins, and the molecular circuitry that controls their localisation and function operate. A central node of this circuitry is occupied by the Rho family of small GTPases, well-established regulators of actin cytoskeleton remodelling and membrane trafficking. In contrast to the Rho-dependent activation of the ERM proteins, Rac-dependent phosphorylation of a particular C-terminal serine residue (S518) leads to Merlin inactivation (Shaw et al, 2001). This is generally thought to involve the phosphorylation-dependent transition from a ‘closed’ to an ‘open’ conformation; however, while the lack of S518 phosphorylation correlates well with the growth-suppressing function of Merlin, it has not been definitively shown that active Merlin is necessarily ‘closed’. Moreover, Merlin can be phosphorylated at residues other than S518 but the functional consequences of such regulation are not yet known (McClatchey and Giovannini, 2005). Notably, S518 phosphorylation can be reversed by the myosin phosphatase MYPT-1-PP1δ (Jin et al, 2006, and references therein). This provides a potentially interesting point of regulatory convergence between Merlin and the ERM proteins given that myosin phosphatase has also been shown to associate with and induce dephosphorylation of the ERM proteins. Another point is provided by recent studies indicating that the Ste-20 kinase Slik can induce phosphorylation of both Merlin and the ERM proteins in the fly (Hughes and Fehon, 2006). Importantly, by analogy to the ability of the ERM proteins to negatively regulate Rho signalling, Merlin can negatively regulate Rac signalling (Shaw et al, 2001), perhaps through an association with the Rac regulator Rho-GDI or the Rac effector Pak or by directly controlling membrane association of Rac in some cells (Kissil et al, 2002; Okada et al, 2005). These core regulatory loops underlie the complex nature of Merlin/ERM-regulated signalling at the membrane:cytoskeleton interface.
BIOLOGICAL CONTEXTS
The ability of Merlin to physically coordinate multiple signalling entities as described above only hints at the combinatorial level of complexity that is possible, given that Merlin can sense different types of cell contact and regulate multiple membrane receptors. The outcome of this coordinated regulation is likely to be context dependent in vivo. The exquisite requirement for regulating cell:cell contact and proliferation during tissue morphogenesis in both developmental and regenerative processes is likely to make these contexts critically dependent upon Merlin function.
For example, Nf2 is an essential gene in Drosophila since null mutations result in embryonic lethality. Hypomorphic mutants display tissue overgrowth consistent with its anti-proliferative function. Recent studies have used clonal deletion/somatic recombination in developing imaginal discs to generate cells lacking both Merlin and the related tumour suppressor Expanded (Mer/Ex). The hyperplastic outgrowth of the mutant clones in several adult structures (eye, wing, leg) appears to result from the combined effect of increased proliferation and reduced apoptosis during development of these structures and is associated with increased surface abundance of several signalling and adhesion receptors (Edgar, 2006; Maitra et al, 2006). In this regard, persistent EGFR signalling in Mer/Ex mutants is likely to antagonise normal apoptosis-inducing signals within the developing imaginal discs and results in increased cell survival (Yang and Baker, 2003).
Signalling via the recently characterised Salvador–Warts–Hippo (SWH) pathway is also elevated in Mer/Ex double mutant cells (Edgar, 2006; Harvey and Tapon, 2007). It has been proposed that Merlin and Expanded are upstream regulators of the SWH pathway, which suppresses Yki-dependent transcription of CycE, DIAP, and Mer/Ex themselves. Direct molecular links between Merlin and components of this pathway have not yet been found and the identity of the upstream signal(s) that are regulated by Mer/Ex is still unknown. The extent of these phenotypes might also reflect a synergistic effect of the combined Mer/Ex mutation. However, consistent with the theme derived from studies of cultured mammalian cells, the adhesion molecule Fat appears to confer contact-dependent regulation of the pathway (Harvey and Tapon, 2007).
Merlin is also required for embryonic development in zebrafish, as embryos fail to develop after anti-sense-mediated inactivation of the nf2 gene. Loss-of-function nf2 mutants display biliary hyperplasia and formation of choledochal cysts indicating that Merlin is an important regulator of biliary tract development in zebrafish (Sadler et al, 2005). These findings complement the previous observations that both hepatocellular and cholangiocellular neoplasia develop in the liver of Nf2 heterozygous mutant mice (McClatchey et al, 1998).
The generation of Nf2-mutant mouse models has contributed important information regarding Merlin function in development and tumourigenesis. Merlin is required for the normal development of several tissues; Nf2-null embryos fail to gastrulate due to extra-embryonic defects (McClatchey and Giovannini, 2005). This defect is rescued in mosaic Nf2+/↔Nf2−/− embryos which go on to develop a number of additional abnormalities, including in heart and skeletal development and in neural tube closure (AIM unpublished data). In fact, recent studies have shown that targeted deletion of Nf2 in the developing nervous system causes neural tube defects from impaired tissue fusion (McLaughlin et al, 2007). Abnormal cell detachment and apoptosis are found at the leading front of unfused folds and the apical side of ventricular neuroepithelia. These phenotypes appear to derive from the inability to form apico-junctional complexes (McLaughlin et al, 2007), consistent with the role of Merlin in controlling formation and stabilisation of AJs (Lallemand et al, 2003).
Heterozygous Nf2+/−-mutant mice develop a spectrum of unusually highly metastatic tumours, mainly osteosarcomas, fibrosarcomas, and liver carcinomas, that exhibit loss of the wild-type allele. Although these mice do not spontaneously develop schwannomas, meningiomas, or mesotheliomas, mouse models for each have been developed through the use of conditional mutant Nf2 alleles (Giovannini et al, 2000). Altogether, these studies from different organisms and biological settings reveal that perturbing Merlin function yields dramatic consequences over a biological continuum from development to cancer.
PERSPECTIVES
In simple two-dimensional cell culture models the establishment of cell:cell contact is progressive. Despite a similar morphological appearance, the cellular physiologies of early vs late confluent cultures are remarkably different and signalling that occurs in non-contacting cells can still operate in early but not late stages of confluence (Rothen-Rutishauser et al, 1998). Silencing of proliferative signals from the time of initial closure of a monolayer requires a few days to complete (Curto et al, 2007). Indeed, our understanding of membrane receptor signalling in cultured cells may be frequently confounded by inadequately defined cell densities. An example of how Merlin could mediate the progressive coordination of cadherin-based contact with the inhibition of EGFR signalling is presented in the model in Figure 2. By operating in a reverse fashion, this molecular circuitry could also control the disassembly of cell:cell contacts in response to morphogenetic or regenerative stimuli. For example, receptor and/or non-receptor tyrosine kinase activity can destabilise cell adhesions directly by phosphorylating cadherin-catenin complexes (Brunton et al, 2004), or indirectly by promoting Rac/Pak-mediated inactivation of Merlin (Kissil et al, 2002; Shaw et al, 2001). In this scenario, Merlin could serve as a three-channel rheostat linking, (1) the metering of lateral surface area engaged in intercellular contacts, to (2) the localised modulation of specific signalling receptors at the interfacing membranes, to (3) the tensegrity of the cortical actin cytoskeleton. Thresholds of receptor activity could be affected by normal or pathological changes in the stoichiometry of these interactions. Through association with the cortical cytoskeleton and a reciprocal functional relationships with Rho-GTPases that regulate actomyosin contractility, Merlin and the ERM proteins seem poised to play an important role in linking changes in cellular tensegrity to mitogenic signalling output.
Increasing evidence suggest that the function of Merlin in stabilising AJs could be opposed by that of the ERM proteins. Despite their similarity, the ERM proteins cannot compensate for Merlin loss and vice versa; moreover, Merlin and the ERM proteins can form heterodimers and exhibit overlapping but distinct membrane localisations. In mature epithelia, the ERM proteins are largely restricted to the apical domain where they are required for proper localisation and regulation of apical surface proteins, including NHE-RF1 itself (reviewed in Fievet et al, 2007). The coexistence of contacting (junctional) and non-contacting (apical) surfaces within the same epithelial cell necessitates mechanisms for forming, maintaining, and reversing these compartments. In this context, Merlin and the ERM proteins could play opposing functions to partition junctional and apical membrane domains in mature epithelia.
Interestingly, a series of in vitro studies also support a role for active Ezrin in destabilising cell contacts (Pujuguet et al, 2003; Elliott et al, 2004, 2005). Loss-of-function studies in Caenorhabditis elegans, Drosophila, and mouse reveal defects specifically in morphogenetic processes involving epithelial remodelling and dynamic instability of intercellular contacts (Fievet et al, 2007). These studies suggest that the ERM proteins are necessary for proper AJ remodelling during epithelial morphogenesis. Notably, in some developing epithelia, including the mouse blastomere, intestinal epithelium and epidermis, Ezrin is transiently and markedly concentrated along cell:cell boundaries where Merlin normally localises (Dard et al, 2004; Saotome et al, 2004; A Gladden and AIM, unpublished data). An intriguing possibility is that the coexistence of Merlin and Ezrin within the same membrane domain could maintain a dynamic state of the cell:cell interface (Figure 3). This could be particularly important in proliferating epithelial tissues where morphogenetic processes involving the establishment or remodelling of cell contacts are coordinated with proliferative and differentiation signals. The progressive partitioning of these proteins into junctional and apical domains, respectively, would then yield a mature stable epithelium. Given the prominent role of ERM proteins in maintaining organised apical surfaces (ie plasma membrane domains devoid of intercellular contacts), and that of Merlin in stabilising cell:cell contacts, localised intermixing could counteract the function of each, promoting a dynamic state of intercellular adhesion. A compelling parallel is provided by recent studies linking Ezrin over-expression to tumour metastasis – a fundamental property of which is junctional instability (Khanna et al, 2004; Yu et al, 2004; Elliott et al, 2005).
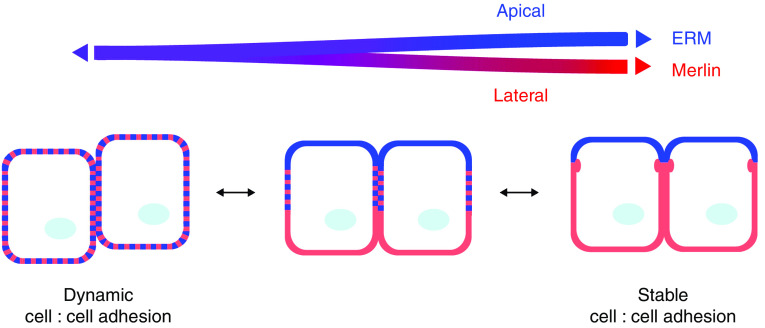
Figure 3.
Merlin and the Ezrin, Radixin, and Moesin (ERM) proteins as regulators of cellular interfaces. In multicellular organisms, a regulated instability of specific intercellular contacts is required during morphogenesis and tissue regeneration. While normally localised in the apical domain of stable epithelia (right), the ERM protein Ezrin can also be found within dynamic cell:cell contacts (left), where Merlin also localises. As contacts stabilise to form mature epithelia, this temporary intermixing of Merlin and Ezrin at membrane surfaces is resolved upon their partitioning into junctional and apical domains, respectively (right). Given the prominent role of the ERM proteins in maintaining organised apical surfaces (ie plasma membrane domains devoid of intercellular contacts), and that of Merlin in stabilising cell:cell contacts, localised intermixing could counteract the function of each, promoting a dynamic state of intercellular adhesion. Ezrin, blue; Merlin, red.
While more detailed investigations are necessary to verify such a model, an essential function of Merlin and the ERM proteins may be to work in concert to physically and functionally regulate the cellular interfaces of metazoa. This possibility has also a fascinating, yet unsettling, implication: that cancers may arise from deregulating the very same mechanisms upon which multicellular life is built.
Acknowledgments
We thank Rick Fehon for discussions and the images in Figure 1b, Jessica Casaletto and other members of the McClatchey laboratory for discussions and comments on the manuscript. AIM is funded by the National Institutes of Health and Department of Defense Neurofibromatosis Research Program.
References
- Baser ME, Friedman JM, Wallace AJ, Ramsden RT, Joe H, Evans DG (2002) Evaluation of clinical diagnostic criteria for neurofibromatosis 2. Neurology 59: 1759–1765 [DOI] [PubMed] [Google Scholar]
- Bashour AM, Meng JJ, Ip W, MacCollin M, Ratner N (2002) The neurofibromatosis type 2 gene product, merlin, reverses the F-actin cytoskeletal defects in primary human Schwannoma cells. Mol Cell Biol 22: 1150–1157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brault E, Gautreau A, Lamarine M, Callebaut I, Thomas G, Goutebroze L (2001) Normal membrane localization and actin association of the NF2 tumor suppressor protein are dependent on folding of its N-terminal domain. J Cell Sci 114: 1901–1912 [DOI] [PubMed] [Google Scholar]
- Bretscher A, Edwards K, Fehon RG (2002) ERM proteins and merlin: integrators at the cell cortex. Nat Rev Mol Cell Biol 3: 586–599 [DOI] [PubMed] [Google Scholar]
- Brunton VG, MacPherson IR, Frame MC (2004) Cell adhesion receptors, tyrosine kinases and actin modulators: a complex three-way circuitry. Biochim Biophys Acta 1692: 121–144 [DOI] [PubMed] [Google Scholar]
- Curto M, Cole BK, Lallemand D, Liu CH, McClatchey AI (2007) Contact-dependent inhibition of EGFR signaling by Nf2/Merlin. J Cell Biol 177: 893–903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dard N, Louvet-Vallee S, Santa-Maria A, Maro B (2004) Phosphorylation of ezrin on threonine T567 plays a crucial role during compaction in the mouse early embryo. Dev Biol 271: 87–97 [DOI] [PubMed] [Google Scholar]
- Edgar BA (2006) From cell structure to transcription: Hippo forges a new path. Cell 124: 267–273 [DOI] [PubMed] [Google Scholar]
- Elliott BE, Meens JA, SenGupta SK, Louvard D, Arpin M (2005) The membrane cytoskeletal crosslinker ezrin is required for metastasis of breast carcinoma cells. Breast Cancer Res 7: R365–R373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott BE, Qiao H, Louvard D, Arpin M (2004) Co-operative effect of c-Src and ezrin in deregulation of cell–cell contacts and scattering of mammary carcinoma cells. J Cell Biochem 92: 16–28 [DOI] [PubMed] [Google Scholar]
- Fernandez-Valle C, Tang Y, Ricard J, Rodenas-Ruano A, Taylor A, Hackler E, Biggerstaff J, Iacovelli J (2002) Paxillin binds schwannomin and regulates its density-dependent localization and effect on cell morphology. Nat Genet 31: 354–362 [DOI] [PubMed] [Google Scholar]
- Fievet B, Louvard D, Arpin M (2007) ERM proteins in epithelial cell organization and functions. Biochim Biophys Acta 1773: 653–660 [DOI] [PubMed] [Google Scholar]
- Giovannini M, Robanus-Maandag E, van der Valk M, Niwa-Kawakita M, Abramowski V, Goutebroze L, Woodruff JM, Berns A, Thomas G (2000) Conditional biallelic Nf2 mutation in the mouse promotes manifestations of human neurofibromatosis type 2. Genes Dev 14: 1617–1630 [PMC free article] [PubMed] [Google Scholar]
- Hanahan D, Weinberg RA (2000) The hallmarks of cancer. Cell 100: 57–70 [DOI] [PubMed] [Google Scholar]
- Harvey K, Tapon N (2007) The Salvador–Warts–Hippo pathway – an emerging tumour-suppressor network. Nat Rev Cancer 7: 182–191 [DOI] [PubMed] [Google Scholar]
- Hughes SC, Fehon RG (2006) Phosphorylation and activity of the tumor suppressor Merlin and the ERM protein Moesin are coordinately regulated by the Slik kinase. J Cell Biol 175: 305–313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- James MF, Beauchamp RL, Manchanda N, Kazlauskas A, Ramesh V (2004) A NHERF binding site links the beta PDGFR to the cytoskeleton and regulates cell spreading and migration. J Cell Sci 117: 2951–2961 [DOI] [PubMed] [Google Scholar]
- James MF, Manchanda N, Gonzalez-Agosti C, Hartwig JH, Ramesh V (2001) The neurofibromatosis 2 protein product merlin selectively binds F-actin but not G-actin, and stabilizes the filaments through a lateral association. Biochem J 356: 377–386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jekely G (2003) Small GTPases and the evolution of the eukaryotic cell. Bioessays 25: 1129–1138 [DOI] [PubMed] [Google Scholar]
- Jin H, Sperka T, Herrlich P, Morrison H (2006) Tumorigenic transformation by CPI-17 through inhibition of a merlin phosphatase. Nature 442: 576–579 [DOI] [PubMed] [Google Scholar]
- Khanna C, Wan X, Bose S, Cassaday R, Olomu O, Mendoza A, Yeung C, Gorlick R, Hewitt SM, Helman LJ (2004) The membrane-cytoskeleton linker ezrin is necessary for osteosarcoma metastasis. Nat Med 10: 182–186 [DOI] [PubMed] [Google Scholar]
- King N (2004) The unicellular ancestry of animal development. Dev Cell 7: 313–325 [DOI] [PubMed] [Google Scholar]
- King N, Hittinger CT, Carroll SB (2003) Evolution of key cell signaling and adhesion protein families predates animal origins. Science 301: 361–363 [DOI] [PubMed] [Google Scholar]
- Kissil JL, Johnson KC, Eckman MS, Jacks T (2002) Merlin phosphorylation by p21-activated kinase 2 and effects of phosphorylation on merlin localization. J Biol Chem 277: 10394–10399 [DOI] [PubMed] [Google Scholar]
- Lallemand D, Curto M, Saotome I, Giovannini M, McClatchey AI (2003) NF2 deficiency promotes tumorigenesis and metastasis by destabilizing adherens junctions. Genes Dev 17: 1090–1100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazar CS, Cresson CM, Lauffenburger DA, Gill GN (2004) The Na+/H+ exchanger regulatory factor stabilizes epidermal growth factor receptors at the cell surface. Mol Biol Cell 15: 5470–5480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine EM, Becker Y, Boone CW, Eagle H (1965) Contact inhibition, macromolecular synthesis, and polyribosomes in cultured human diploid fibroblasts. Proc Natl Acad Sci USA 53: 350–356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maitra S, Kulikauskas RM, Gavilan H, Fehon RG (2006) The tumor suppressors Merlin and expanded function cooperatively to modulate receptor endocytosis and signaling. Curr Biol 16: 702–709 [DOI] [PubMed] [Google Scholar]
- Manchanda N, Lyubimova A, Ho HY, James MF, Gusella JF, Ramesh N, Snapper SB, Ramesh V (2005) The NF2 tumor suppressor Merlin and the ERM proteins interact with N-WASP and regulate its actin polymerization function. J Biol Chem 280: 12517–12522 [DOI] [PubMed] [Google Scholar]
- McClatchey AI, Giovannini M (2005) Membrane organization and tumorigenesis – the NF2 tumor suppressor, Merlin. Genes Dev 19: 2265–2277 [DOI] [PubMed] [Google Scholar]
- McClatchey AI, Saotome I, Mercer K, Crowley D, Gusella JF, Bronson RT, Jacks T (1998) Mice heterozygous for a mutation at the Nf2 tumor suppressor locus develop a range of highly metastatic tumors. Genes Dev 12: 1121–1133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin ME, Kruger GM, Slocum KL, Crowley D, Michaud NA, Huang J, Magendantz M, Jacks T (2007) The Nf2 tumor suppressor regulates cell–cell adhesion during tissue fusion. Proc Natl Acad Sci USA 104: 3261–3266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison H, Sherman LS, Legg J, Banine F, Isacke C, Haipek CA, Gutmann DH, Ponta H, Herrlich P (2001) The NF2 tumor suppressor gene product, merlin, mediates contact inhibition of growth through interactions with CD44. Genes Dev 15: 968–980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison H, Sperka T, Manent J, Giovannini M, Ponta H, Herrlich P (2007) Merlin/neurofibromatosis type 2 suppresses growth by inhibiting the activation of Ras and Rac. Cancer Res 67: 520–527 [DOI] [PubMed] [Google Scholar]
- Nelson WJ (2003) Adaptation of core mechanisms to generate cell polarity. Nature 422: 766–774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada T, Lopez-Lago M, Giancotti FG (2005) Merlin/NF-2 mediates contact inhibition of growth by suppressing recruitment of Rac to the plasma membrane. J Cell Biol 171: 361–371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelton PD, Sherman LS, Rizvi TA, Marchionni MA, Wood P, Friedman RA, Ratner N (1998) Ruffling membrane, stress fiber, cell spreading and proliferation abnormalities in human Schwannoma cells. Oncogene 17: 2195–2209 [DOI] [PubMed] [Google Scholar]
- Pujuguet P, Del Maestro L, Gautreau A, Louvard D, Arpin M (2003) Ezrin regulates E-cadherin-dependent adherens junction assembly through Rac1 activation. Mol Biol Cell 14: 2181–2191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rangwala R, Banine F, Borg JP, Sherman LS (2005) Erbin regulates mitogen-activated protein (MAP) kinase activation and MAP kinase-dependent interactions between Merlin and adherens junction protein complexes in Schwann cells. J Biol Chem 280: 11790–11797 [DOI] [PubMed] [Google Scholar]
- Rothen-Rutishauser B, Kramer SD, Braun A, Gunthert M, Wunderli-Allenspach H (1998) MDCK cell cultures as an epithelial in vitro model: cytoskeleton and tight junctions as indicators for the definition of age-related stages by confocal microscopy. Pharm Res 15: 964–971 [DOI] [PubMed] [Google Scholar]
- Sadler KC, Amsterdam A, Soroka C, Boyer J, Hopkins N (2005) A genetic screen in zebrafish identifies the mutants vps18, nf2 and foie gras as models of liver disease. Development 132: 3561–3572 [DOI] [PubMed] [Google Scholar]
- Saotome I, Curto M, McClatchey AI (2004) Ezrin is essential for epithelial organization and villus morphogenesis in the developing intestine. Dev Cell 6: 855–864 [DOI] [PubMed] [Google Scholar]
- Shaw RJ, Paez JG, Curto M, Yaktine A, Pruitt WM, Saotome I, O'Bryan JP, Gupta V, Ratner N, Der CJ, Jacks T, McClatchey AI (2001) The Nf2 tumor suppressor, merlin, functions in Rac-dependent signaling. Dev Cell 1: 63–72 [DOI] [PubMed] [Google Scholar]
- Te Velthuis AJ, Isogai T, Gerrits L, Bagowski CP (2007) Insights into the molecular evolution of the PDZ/LIM family and identification of a novel conserved protein motif. PLoS ONE 2: e189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theisen CS, Wahl III JK, Johnson KR, Wheelock MJ (2007) NHERF links the N-cadherin/catenin complex to the platelet-derived growth factor receptor to modulate the actin cytoskeleton and regulate cell motility. Mol Biol Cell 18: 1220–1232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L, Baker NE (2003) Cell cycle withdrawal, progression, and cell survival regulation by EGFR and its effectors in the differentiating Drosophila eye. Dev Cell 4: 359–369 [DOI] [PubMed] [Google Scholar]
- Yu Y, Khan J, Khanna C, Helman L, Meltzer PS, Merlino G (2004) Expression profiling identifies the cytoskeletal organizer ezrin and the developmental homeoprotein Six-1 as key metastatic regulators. Nat Med 10: 175–181 [DOI] [PubMed] [Google Scholar]