Abstract
This prospective multicentre phase II study characterises the toxicity and activity of first-line capecitabine and oxaliplatin combination therapy (CAPOX) in advanced biliary system adenocarcinomas. Patients received oxaliplatin (130 mg m−2, day 1) plus capecitabine (1000 mg m−2 b.i.d., days 1–14) every 3 weeks. Patients were stratified prospectively into two groups based on location of the primary (gallbladder carcinoma (GBC) or extrahepatic cholangiocarcinoma (ECC) versus intrahepatic mass-forming type cholangiocarcinoma (ICC)). Sixty-five patients were evaluable. The response rate in 47 patients with GBC/ECC was 27% (4% complete responses), and in 23 patients (49%) stable disease (SD) was encountered. In 18 patients with ICC, we observed no objective responses, but 6 patients (33%) had SD. Median survival was 12.8 months (95% CI, 10.0–15.6) for patients with GBC or ECC (GBC: 8.2 months; 95% CI, 4.3–11.7; ECC: 16.8 months; 95% CI, 12.7–20.5), and 5.2 months (95% CI, 0.6–9.8) for ICC patients. In both cohorts, therapy was well tolerated. The most common grade 3–4 toxicity was peripheral sensory neuropathy (11 patients). Our data suggest that the CAPOX regimen is a well-tolerated and active treatment option for advanced ECC and GBC but might produce poorer results for ICC.
Keywords: capecitabine, chemotherapy, cholangiocarcinoma, gallbladder carcinoma, oxaliplatin
Biliary system adenocarcinomas include clearly defined gallbladder carcinomas (GBC) as well as two main types of cholangiocarcinomas (CCCs): an infiltrative ductal type involving extrahepatic (ECC) and in some cases intrahepatic bile ducts, and an intrahepatic mass-forming type (ICC). These morphologically distinct presentations have been shown not only to differ at the molecular level, but also appear to be distinct in clinical behaviour and with regard to response to cytotoxic drugs (Jarnagin et al, 2006).
To date, complete surgical resection provides the only curative treatment option in the early stages of these neoplasms irrespective of their origin within the biliary system. However, less than one-third of CCCs are resectable, and patients presenting with UICC stage IV GBC or with unresectable CCC treated only by best supportive care have a poor prognosis with a median survival time of less than 6 months (Parker et al, 1996; Pitt et al, 1997; Jarnagin et al, 2001; Weber et al, 2002).
At present, there is no established palliative standard of care for GBC and CCC, and the cytotoxic agents most extensively studied, as single agents are 5-fluorouracil (5-FU), gemcitabine, capecitabine, mitomycin C, cisplatin, doxorubicin, and methotrexate, resulting in partial responses (PRs) in about 10–20% of selected patients (Hejna et al, 1998; Penz et al, 2001). However, some phase-II trials indicate that the use of combination therapies with newer agents may boost response rates to 26% and higher (Knox et al, 2005; Harder et al, 2006).
To date, the only randomised trial for advanced biliary carcinomas, including an observational control arm, has shown an improvement of overall survival (OS) (6.5 versus 2.5 months) as well as quality of life, assessing a regimen of 5-FU plus leucovorin with or without etoposid, as compared to best supportive care (Glimelius et al, 1996).
In this setting, other treatment modalities, such as photodynamic therapy, have yielded preliminary beneficial results only for patients with ECC due to locoregional tumour control but not for those with metastatic disease (Ortner et al, 1998). A survival benefit of photodynamic therapy over best supportive care has only been demonstrated for patients not amenable to conventional stenting procedures (Ortner et al, 2003); therefore, its value relative to conventional stenting procedures remains unclear. Recently performed targeted therapy approaches with the Her/2-neu and/or EGFR inhibitors lapatinib or erlotinib have shown no or only modest activity (0 and 8% responses, respectively) in advanced biliary system adenocarcinomas (Philip et al, 2006; Ramanathan et al, 2006).
Thus, a rational basis for the treatment of advanced stages of biliary system adenocarcinomas might only be provided by cytotoxic treatment alone or in the context of a multimodality therapy strategy, including chemotherapy.
Oxaliplatin (l-OHP), an alkylating diaminocyclohexane platinum derivate, has been noted to display a marked cytotoxic synergism in combination with fluoropyrimidines against a variety of solid human tumour cells (Raymond et al, 1997).
Therefore, we recently have conducted a prospective phase II study of two-weekly oxaliplatin plus high-dose 5-FU/folinic acid in biliary system adenocarcinomas. The disease control rate (responses and stable disease (SD)) was 56%, and the median OS was 9.5 months (Nehls et al, 2001). To improve efficacy and to offer a more convenient treatment option for patients by reducing clinical visits and avoiding indwelling devices, we prospectively investigated the activity and toxicity profile of three-weekly intravenous oxaliplatin plus oral capecitabine (CAPOX) in two stratified cohorts with either advanced GBC and ECC (group A) or advanced ICC (group B).
PATIENTS AND METHODS
Eligibility criteria
This prospective, four-institutional study, which was officially accredited by the Arbeitsgemeinschaft Internistische Onkologie (AIO), enrolled 66 consecutive patients with the diagnosis of biliary system adenocarcinoma between February 2002 and January 2004.
Criteria for inclusion were histologically or cytologically confirmed adenocarcinomas of the gallbladder or the intrahepatic or extrahepatic biliary tract not amenable to curative surgical treatment strategies according to an Interdisciplinary Board of oncologists, surgeons, radiologists, and radiooncologists.
Other criteria for inclusion were at least one bidimensionally measurable lesion (ascites and pleural effusions were not considered measurable), Eastern Cooperative Oncology Group (ECOG) performance status (PS) ⩽2, age 18–75 years, adequate bone marrow (leucocyte count ⩾3.5 × 109 per l, platelet count ⩾100 × 109 per l), renal (creatinine-clearance ⩾60 ml min−1) and hepatic function (total serum bilirubin <2.0 mg per 100 ml, transaminases <2 × the upper limit of normal), and no prior cytotoxic treatment.
The trial protocol was approved by the local ethics review boards of all participating Hospitals, and written consent was obtained from all patients before any study-specific procedures.
Patient evaluation
Before entry onto study, all patients underwent a full medical history, physical examination, PS, and laboratory evaluation. In addition, baseline evaluation included electrocardiograms, chest X-rays, abdominal ultrasounds, and computed tomography scans (alternatively magnetic resonance imaging scans) of the abdomen. Further imaging investigations were added if clinically indicated or for disease assessment.
Objective tumour evaluation for response was performed according to World Health Organization (WHO) standard criteria (World Health Organization, 1979). A complete response (CR) was defined as disappearance of all measurable or evaluable disease with the absence of any new lesions for at least 4 weeks. A PR was defined as a >50% reduction in the sum of the products of the largest perpendicular diameters of all measurable lesions with the absence of any new lesions for at least 4 weeks. Stable disease was defined as a reduction of <50% or an increase of <25% of measurable lesions according to the previous method, with the absence of any new lesions. Progressive disease (PD) was characterised by an increase of >25% of measurable lesions according to the previous method or the appearance of new lesions. A baseline radiographic tumour evaluation was performed within 2 weeks before treatment started and repeated after two cycles, and again after every further three cycles by the same imaging method that was used for baseline measurement. Additional evaluations were performed if PD was suspected.
Clinical response assessment was determined separately for patients with either GBC/ECC or ICC, because these two distinct presentations have been supposed to differ substantially from each other according to clinical behaviour (hilar CCCs (Klatskin tumours) have been categorised as ECC). Accordingly, patients were entered prospectively into two nonrandomised parallel groups based on location of the primary (GBC/ECC versus ICC) in order to evaluate the outcome more accurately. Moreover, subgroup analysis of hilar versus distal CCCs was performed.
Toxicity was assessed using the National Cancer Institute Common Toxicity Criteria (NCI-CTC), except for neurologic toxicity, that was graded according to Lévis scale.
Treatment schedule
Patients received, on outpatient basis, the CAPOX regimen as reported previously (André et al, 1999). In brief, capecitabine 1000 mg per m2 per dose was administered orally twice a day on days 1–14, followed by a 7-day treatment-free period. Oxaliplatin, 130 mg m−2, was administered as a 2-h infusion on day 1, in a 3-week cycle. All patients received, as concomitant antiemetic prophylaxis, a 5-HT 3 antagonist intravenously prior to each oxaliplatin dose.
Treatment was terminated in the case of disease progression, intolerable side effects, or patient's choice to discontinue the therapy.
To prevent oxaliplatin-induced neurotoxicity, all patients received carbamazepine (initially 100 mg b.i.d. to achieve a serum-level of 3–6 mg l−1) according to the preliminary results of a pilot study (Lersch et al, 2002).
Toxicity and dosage modification guidelines
The toxicities were graded according to NCI-CTC (version 2). Capecitabine doses should be interrupted in cases of grade 2 or higher events (except alopecia) and treatment should be delayed until complete recovery or until the adverse event improved to grade 0 or 1. Capecitabine was decreased by 25% in subsequent cycles at the first occurrence of a grade 2 or 3 toxicity, and it was reduced by 50% at the second occurrence of a given grade 2 or grade 3 toxicity or at the first occurrence of a grade 4 event. Treatment with capecitabine was permanently stopped if, despite dose reduction, a given toxicity occurred for the third time at grade 2 or grade 3, or a second time at grade 4.
Oxaliplatin was reduced by 25% in subsequent cycles for grade 3 or 4 neutropenia or thrombocytopenia, grade 3 diarrhoea or mucositis, any other drug-related grade 3 toxicity or painful paresthesias lasting 8–14 days (without functional impairment) (grade 2 according to Lévis scale), whereas oxaliplatin was diminished by 50% if persistent (>14 days) paresthesias (without functional impairment) emerged (grade 3). Oxaliplatin was stopped if a beginning functional impairment occurred (grade 4).
If more than two dose reductions were indicated and/or treatment was delayed for more than 3 weeks, patients were removed from the study.
Statistical considerations
The main objective of the study was the response rate (CR and PR) according to WHO criteria. Both cohorts (groups A and B) were analysed separately according to Simon's two-stage design (Simon, 1989). If two or less CR or PR were noted in the first 18 patients of a group, accrual would stop in this cohort, otherwise it was planned to accrue at least 43 patients in this group to determine outcome more accurately. Assuming that about 5% of patients could not be evaluable, the accrual was planned to continue to a total of 47 patients for the second step of a group. The significance level was 5% and the power was 80%.
Secondary objectives were safety, OS, and time to progression (TTP). Overall survival or TTP were calculated both from the initiation of chemotherapy until death and from the date of objectively measured disease progression till the occurrence of death from any cause. The cutoff was 30 April 2007. Statistical analysis was performed using SPSS for Windows 11.5 (SPSS Inc., Chicago, IL, USA). Median OS and TTP were estimated according to the Kaplan–Meier method (Kaplan and Maier, 1959).
RESULTS
Patient characteristics
A total of 66 patients (27 men and 39 women) were enrolled onto this multicenter trial at the University Hospitals of Berlin, Frankfurt, Mannheim and Tübingen, Germany, and 65 patients were evaluable for efficacy and safety. One patient was retrospectively found to have a diagnosis of pancreatic adenocarcinoma and has been disclosed from the study. Patient characteristics are detailed in Table 1.
Table 1. Patient characteristics by treatment group and site of the primary.
|
Group A
|
Group B | ||
|---|---|---|---|
| Characteristic | Gallbladder | Extrahepatic bile duct | Intrahepatic bile duct (mass-forming-type) |
| No. of patients | 27 | 20 | 18 |
| Age (years) | |||
| Median | 61 | 66 | 52 |
| Range | 49–73 | 49–74 | 28–74 |
| Sex (%) | |||
| Male | 9 (33) | 10 (50) | 8 (44) |
| Female | 18 (67) | 10 (50) | 10 (56) |
| PS, ECOG (%) | |||
| 0 | 8 (30) | 7 (35) | 7 (39) |
| 1 | 15 (55) | 11 (55) | 9 (50) |
| 2 | 4 (15) | 2 (10) | 2 (11) |
| Sites of disease (%) | |||
| Locoregional | 0 (0) | 3 (15) | 0 (0) |
| Liver | 25 (93) | 14 (70) | 18 (100) |
| Lymph node | 14 (52) | 5 (25) | 12 (67) |
| Lung | 2 (7) | 2 (10) | 6 (33) |
| Peritoneum | 1 (4) | 1 (5) | 0 (0) |
| Other | 6 (22) | 7 (35) | 2 (11) |
| No. of metastatic sites (%) | |||
| 1 | 10 (37) | 6 (30) | 4 (22) |
| ⩾2 | 17 (63) | 11 (70) | 14 (78) |
| Prior surgery (%) | |||
| Yes | 12 (44) | 9 (45) | 5 (28) |
| No | 15 (56) | 11 (55) | 13 (72) |
| CEA elevated (%) | |||
| Yes | 6 (22) | 6 (30) | 7 (39) |
| No | 21 (78) | 13 (65) | 9 (50) |
| Not done | 0 (0) | 1 (5) | 2 (11) |
| CA 19-9 elevated (%) | |||
| Yes | 19 (70) | 16 (80) | 10 (56) |
| No | 8 (30) | 4 (20) | 6 (33) |
| Not done | 0 (0) | 0 (0) | 2 (11) |
The median age of the study population was 61 years (range, 28–74). Twenty-seven, 20, and 18 had adenocarcinoma of the gallbladder, ECC, or ICC, respectively.
In group A (GBC or ECC), three patients (6%) had locoregional advanced disease. Forty-four patients (94%) had metastatic disease. Sixteen patients had one metastatic site (34%), and 28 patients (60%) had two affected sites and more. Liver involvement was noted in 39 patients (83%). In group B (ICC), four patients (22%) had one metastatic site, and 14 patients (78%) had two affected sites and more (Table 1).
In groups A and B, 21 and 5 patients, respectively, experienced disease recurrence or metastatic spread to distant organs after they had undergone curatively intended surgery previously.
Efficacy
In group A, 2 of the 47 patients (4%) achieved a complete remission, 11 patients (23%) experienced a partial remission, and SD was noted in 23 patients (49%). Accordingly, the overall disease control rate (CR, PR, or SD) on 47 patients with GBC or ECC was 77%, and PD was found in 11 patients (23%). From initiation of chemotherapy, actuarial estimation of the median TTP and median OS were 6.5 months (95% CI, 5.3–7.7 months), and 12.8 months (95% CI, 10.0–15.6 months), respectively. At the cutoff of the study, two patients of group A were still alive.
In group B, no CR or PR was observed in 18 patients with ICC, but six patients (33%) had SD, and 12 patients (68%) experienced PD. Median TTP and OS were 2.2 months (95% CI, 1.4–3.0 months) and 5.2 months (95% CI, 0.6–9.8 months), respectively. At the cutoff of the study, all patients of group B have died.
Subgroup analysis for efficacy
The results of the response rates for patients with GBC versus ECC are detailed in Table 2.
Table 2. Best response to treatment.
| Group A | |||||
|---|---|---|---|---|---|
|
Extrahepatic bile duct carcinoma (n=20)
|
|||||
| Outcome | Gallbladder carcinoma (n=27) | Hilar (n=6) | Distal (n=14) No. of patients (%) | Hilar and distal (n=20) | Group B Intrahepatic bile duct carcinoma (n=18) |
| CR | 1 (4) | 0 (0) | 1 (7) | 1 (5) | — |
| PR | 7 (26) | 2 (33) | 2 (14) | 4 (20) | — |
| SD | 9 (33) | 3 (50) | 11 (79) | 14 (70) | 6 (33) |
| PD | 10 (37) | 1 (17) | 0 (0) | 1 (5) | 12 (67) |
| Disease control rate (CR or PR or SD) | 36 (77) | 6 (33) | |||
Abbreviations: CR=complete remission; PD=progressive disease; PR=partial remission; SD=stable disease.
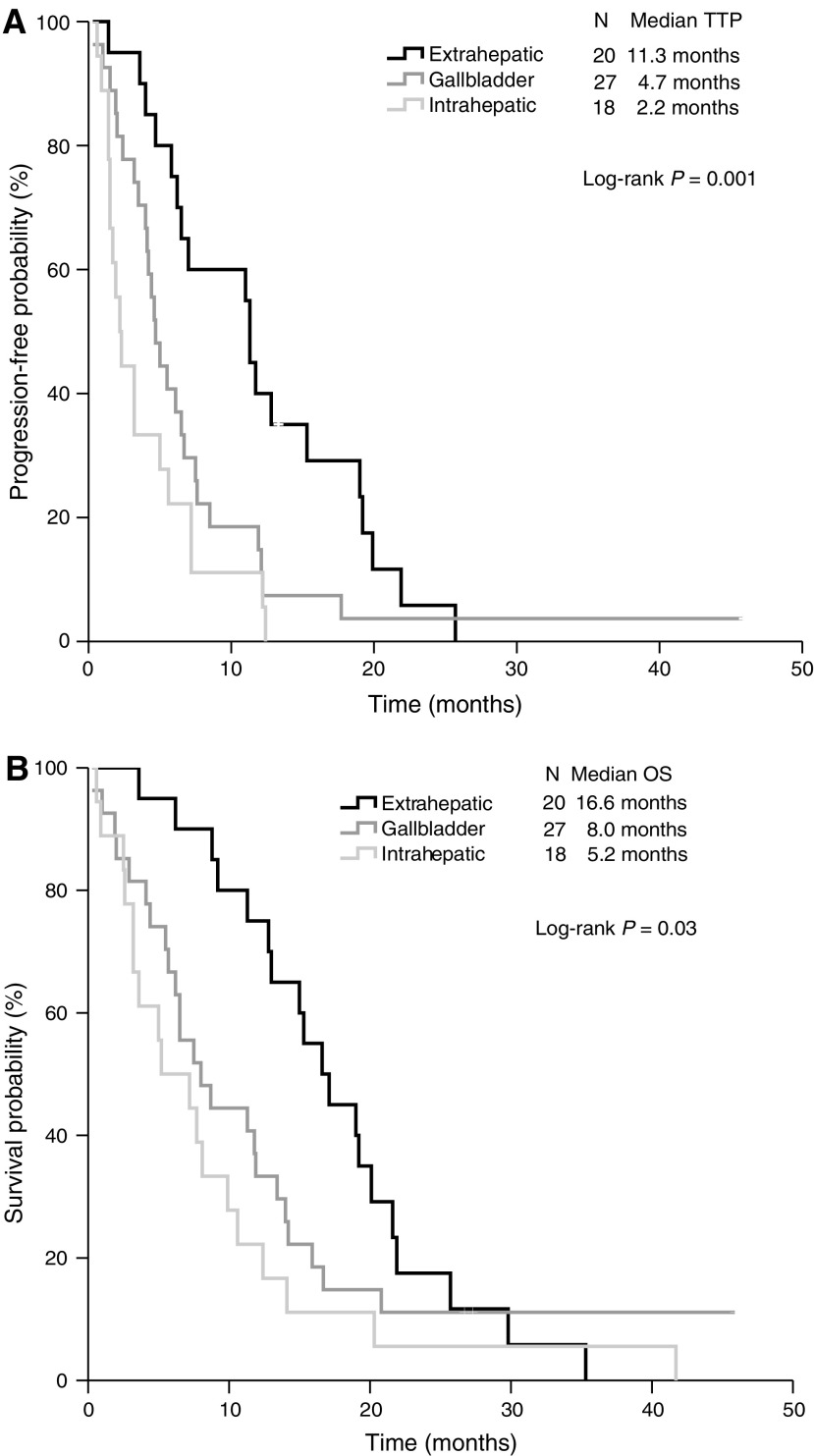
Furthermore, survival analysis for patients with GBC versus ECC revealed markedly differences on both TTP (Figure 1A; Kaplan–Meier curves for TTP) and OS (Figure 1B; Kaplan–Meier curves for OS). Thus, TTP or OS in GBC and ECC were 4.7 months (95% CI, 3.7–5.7) and 11.3 months (95% CI, 10.6–12.0), respectively, or 8.0 months (95% CI, 4.3–11.7) and 16.6 months (95% CI, 12.7–20.5), respectively. Subgroup analysis of patients with hilar versus distal CCC showed no significant differences on TTP or OS. Thus, TTP or OS in hilar and distal CCC were 6.5 months (95% CI, 5.1–7.9) and 11.3 months (95% CI, 10.4–12.2), respectively, or 20.1 months (95% CI, 12.2–28) and 15.3 months (95% CI, 8.7–21.9), respectively (log-rank test, P=0.85, or P=0.51).
Figure 1.
The Kaplan–Meier curves of (A) TTP and (B) OS.
Subgroup analysis of patients from groups A and B revealed that TTP or OS was worst for patients with ICC compared to patients with ECC or GBC (log-rank test; P=0.01, or P=0.03, respectively) (Figure 1A and B).
Safety
All 65 patients were evaluable for toxic effects. A total of 367 courses of treatment have been administered (median, five courses per patient; range, 1–17). The median cumulative doses for oxaliplatin and capecitabine were 560 mg m−2 (range, 130–1.945) and 8.500 mg m−2 (range, 2.000–30.500) corresponding to a relative dose intensity of 86% for oxaliplatin and 85% for capecitabine, respectively. In 46.2% of the patients, at least one cycle of treatment was delayed due to oxaliplatin and/or capecitabine-related side effects resulting in dose reductions for oxaliplatin and capecitabin in 30.8 and 46.2% of the patients, respectively. The recorded toxicities are summarised in Table 3.
Table 3. Toxicity in any cycle (worst per patient) (both patient series (groups A and B), n=65).
|
Grade 2
|
Grade 3
|
Grade 4
|
||||
|---|---|---|---|---|---|---|
| No. of patients | % | No. of patients | % | No. of patients | % | |
| Neutropenia | 9 | 14 | 0 | 0 | 1 | 2 |
| Febrile neutropenia | 0 | 0 | 0 | 0 | 1 | 2 |
| Thrombocytopenia | 13 | 20 | 6 | 9 | 1 | 2 |
| Anemia | 12 | 18 | 0 | 0 | 0 | 0 |
| Nausea/vomiting | 19 | 29 | 4 | 6 | 0 | 0 |
| Diarrhoea | 12 | 18 | 3 | 5 | 1 | 2 |
| Mucositis | 1 | 2 | 0 | 0 | 0 | 0 |
| Hand–foot syndrome | 6 | 9 | 3 | 5 | NA | NA |
| Infection | 10 | 15 | 0 | 0 | 2 | 3 |
| Thrombembolic events | 0 | 0 | 0 | 0 | 1 | 2 |
Abbreviation: NA=not applicable.
The National Cancer Institute Common Toxicity Criteria grade 4 thrombocytopenia or febrile leukopenia was observed in one patient each (2% of patients, respectively). Two patients (3%) experienced grade 4 infection and a thrombembolic event grade 4 was reported in a single patient (2%). Grade 3 adverse events were thrombocytopenia (9% of patients), nausea/vomiting (6%), diarrhoea (5%), and hand–foot syndrome (5%). We observed no line infections. The most common toxicity was peripheral neuropathy observed in 54 of the 65 patients (83%). However, grade 3 or 4 peripheral sensory neuropathy was encountered only in 11 of the patients (17%) and 11% of courses (Table 4).
Table 4. Incidence of neurosensory symptoms according to Lévís scalea (worst per patient) (both patient series, group A and B), n=65.
| Paresthesia a | Grade 1 | Grade 2 | Grade 3 | Grade 4 | ||||
|---|---|---|---|---|---|---|---|---|
| No of patients (%) | 20 | 31 | 23 | 35 | 10 | 15 | 1 | 2 |
| No of cycles (%) | 53 | 14 | 48 | 13 | 39 | 11 | 1 | 0.3 |
Lévís scale: grade 1, paresthesias of moderate intensity lasting less than 7 days; grade 2, painful paresthesias lasting 8–14 days (without functional impairment); grade 3, persistent (>14 days) paresthesias (without functional impairment); grade 4, beginning functional impairment.
In two patients, study medication has been stopped due to oxaliplatin-related allergic reactions occurring in cycles 1 and 6. Generalised rash and fever have been recovered completely.
In one patient with ICC, chemotherapy was stopped after the first treatment cycle due to progression-related cholangiosepsis not amenable to stenting procedures. Despite treatment with antibiotics, he died from septic shock.
Another patient with a history of long-standing insulin-dependent diabetes mellitus died due to a cerebral ischaemic insult after the first treatment cycle. This event has been considered unlikely related to study treatment, but a causal relation with the study medication cannot be excluded entirely.
Second-line treatment
Thirty-three patients (51%) underwent second-line chemotherapy after treatment with the CapOx regimen failed (31, gemcitabine monotherapy; 1, gemcitabine plus capecitabine; 1, mitomycin C plus capecitabine). None of the patients achieved a PR or a CR after second-line therapy.
DISCUSSION
Although there is no standard of care in advanced biliary system adenocarcinoma, palliative chemotherapy to date remains the primary therapeutic approach. Nevertheless, the search continues for an appropriate cytotoxic treatment protocol for biliary tract carcinoma. Moreover, it remains unclear if patients with GBC or ECC may respond similarly to cytotoxic treatment as those with ICC.
In the present prospective phase II trial, stratified prospectively into two groups based on location of the primary (GBC or ECC versus ICC), a response rate of 27% was achieved in 47 patients with GBC or ECC (group A) (including 4% complete remissions) adding to a disease-control rate (responses and SD) of 77%. Moreover, response rates were comparable for patients with ECC and GBC (30 and 25%).
In patients with ICC, SD was found in 33% of 18 cases, whereas no objective tumour response could be detected in this subgroup.
Our data show that the CAPOX regimen may be active, particularly in the subset of ECC and GBC, although it has only modest activity in ICC.
This agrees with data from a recent trial using gemcitabine combined with platinum-based cytotoxic treatment in 42 patients with biliary system adenocarcinomas, which also demonstrated both comparable response rates for patients with ECC and GBC and a marked difference in response rates between GBC and ECC versus ICC (40 and 43 versus 7%, respectively) (Table 5) (Harder et al, 2006). The relatively higher response rates in the latter study as compared to our data may reflect the use of a different evaluation system (RECIST versus WHO criteria). In addition, accumulating data suggest that GBC in comparison to ICC responds noticeably better to various cytotoxic protocols (Table 5) (Kim et al, 2003; André et al, 2004; Patt et al, 2004; Alberts et al, 2005). These differences may reflect site-related distinct biologic features (Jarnagin et al, 2006).
Table 5. Summary of phase II trials of biliary system adenocarcinoma (trials selected differ between GBC, ECC, and/or ICC).
|
No. of patients RR (%)
|
Median survival (months)
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Author (year) | Regimen | Metastatic disease % | Total | GBC | ECC | ICC | GBC | ECC | ICC |
| André et al (2004) | Gemcitabine Oxaliplatin | 94 | 31† | 11 (54) | 4 (25) | 16 (21) | 16 | NA | 14.5 |
| Kim et al (2003) | Capecitabine Cisplatin | 67 | 38* | 19 (32) | 9 (11) | 14 (14) | 9.1 | ||
| Patt et al (2004) | Capecitabine | NA | 26† | 8 (50) | 18 (6) | 9.9 | 8.1 | ||
| Knox et al (2005) | Gemcitabine Capecitabine | 89 | 42* | 22 (28) | 23 (34) | 6.6 | 19 | ||
| Alberts et al (2005) | Gemcitabine 5-FU/LV | 83 | 42* | 14 (21) | 9 (7) | 19 | 7.2 | 9.9 | |
| Verderame et al (2006) | Gemcitabine Oxaliplatin | 67 | 24* | 9 (44) | 15 (53) | 6.0 | 12.0 | ||
| Harder et al (2006) | Gemcitabine Oxaliplatin | 90 | 31* | 10 (40) | 7 (43) | 14 (7) | NA | 13.3 | 8.4 |
| This study | Capecitabine Oxaliplatin | 95 | 65† | 27 (30) | 20 (25) | 18 (0) | 8.2 | 16.8 | 5.2 |
Abbreviations: ECC=extrahepatic cholangiocarcinoma; GBC=gallbladder carcinoma; ICC=intrahepatic mass-forming type cholangiocarcinoma; NA=data not available; RR=response rate; †Response evaluation according to WHO (World Health Organization) criteria; *Response evaluation according to RECIST (Response evaluation in Solid Tumors) criteria.
Of note, only in studies evaluating ICC and ECC as one entity, classified as CCC, no obvious differences in response rates have been found between CCC and GBC. It may be hypothesised that the majority of responders in these studies were patients with ECC (Table 5) (Knox et al, 2005; Verderame et al, 2006). In this context, it should be emphasised that Klatskin tumours are misclassified as ICC in a substantial number of cases (Welzel et al, 2006).
In our series, it was most striking that median OS for patients with ECC was about twice as long than for patients with GBC (16.8 versus 8.1 months). Although the comparison of results of non-randomised phase II trials regarding survival may be inconclusive, that is, due to patient selection bias, our observation of an OS of 8.1 months for GBC was in good accordance with other phase II trials using fluoropyrimidin-based therapies, platin-analogues, or gemcitabine-based schedules (Table 5). A possible explanation for a shorter survival for GBC compared to ECC may be the more aggressive natural course of GBC, and that patients with ECC might also benefit from local treatment (i.e., stenting procedures). Conversely, one study found very long median survival times for GBC (16 months) using a combination chemotherapy of oxaliplatin plus gemcitabine (André et al, 2004). However, two other studies using this combination protocol could not reproduce this result, possibly due to differences in patient selection, inclusion criteria, and/or applications of the drugs (Harder et al, 2006; Verderame et al, 2006).
Therefore, randomised trials are warranted in biliary system adenocarcinomas, stratified according to the three entities, GBC, ICC, and ECC, to draw definitive conclusions about differences in response rates and survival.
Fifty-one percent of our patients received second-line treatment, but no complete or partial responses were seen in this setting. Accordingly, an influence of second-line treatment on outcome seems to be inferior.
The toxicity profile of the CAPOX regimen was generally favourable. Gastrointestinal grade 3/4 toxicities were nausea/vomiting and diarrhoea, each of which affected four patients (6%) including grade 4 diarrhoea in only one patient (2%) (Table 3), comparing favourably to other trials using the CAPOX regimen for treatment of gastrointestinal tumours other than biliary (Park et al, 2006). The most common grade 3/4 haematological event was thrombocytopenia (11% of patients), including a grade 4 event in a single patient (2%). No bleeding disorders have been seen.
In the current study, we observed grade 2 infection in 10 patients and grade 4 infection in 2 patients, respectively, causing treatment delays in only 4% of the courses. In contrast, Harder et al (2006) found treatment delays in 9% of cycles using a regimen with gemcitabine combined with oxaliplatin. Of note, in four of our patients, infection was based on occlusion of biliary stents, and in eight patients, no such association was found. Furthermore, in one patient with and in one patient without stent insertion, cholangiosepsis occurred.
However, no line infections were observed. In contrast, in a previous study on advanced biliary cancers using a schedule based on protracted infusion of 5-FU, rates of central line infection and venous thrombosis were 19 and 7% (Knox et al, 2004) indicating the risks of implantable devices.
Two patients who were withdrawn from our study due to an allergic reaction to oxaliplatin recovered fully. This oxaliplatin-related toxicity, typically occurring after application of 7–12 treatment cycles, was estimated to affect about 2% of patients (Tournigand et al, 1998). In addition, three patients (5%) developed grade 3 hand–foot syndrome.
The main grade 3/4 toxic effect was peripheral sensory neuropathy, which was noted in 11 patients (17%) (Table 4). However, this oxaliplatin-related side effect was reported to be completely reversible after omission of oxaliplatin in more than 80% of the patients within 8 months (Gamelin et al, 2002).
In conclusion, the CAPOX regimen has a favourable safety profile and is more convenient than infusional regimens, avoiding the need for indwelling devices and frequent hospital visits. Finally, the prospective data presented here may open a triad perspective on adenocarcinomas of the biliary system after platinum-based cytotoxic treatment. First, response rates and outcome seem worst for patients with ICC compared to patients with ECC or GBC, indicating that tumour growth in these neoplasms may reflect other biological mechanisms than in GBC and/or ECC; Second, GBC may have an intermediate prognosis with response rates very similar to ECC, whereas outcome might be poorer. Third, ECC, including perihilar CCC, may have high response rates and median survival times that clearly tend to exceed those of GBC and ICC. To prove these hypotheses, prospective randomised trials should be undertaken.
Acknowledgments
This study was supported in part by Roche Pharma AG, Grenzach-Whylen.
References
- Alberts SR, Al-Khatib H, Mahoney MR, Burgart L, Cera PJ, Flynn PJ, Finch TR, Levitt R, Windschitl HE, Knost JA, Tschetter LK (2005) Gemcitabine, 5-fluorouracil and leucovorin in advanced biliary tract and gallbladder carcinoma. Cancer 103: 111–118 [DOI] [PubMed] [Google Scholar]
- André T, Bensmaine MA, Louvet C, Francois E, Lucas V, Desseigne F, Beerblock K, Bouché O, Carola E, Merrouche Y, Morvan F, Dupont-André G, de Gramont A (1999) Multicenter phase II study of bimonthly high-dose leucovorin, fluorouracil infusion, and oxaliplatin for metastatic colorectal cancer resistant to the same leucovorin and fluorouracil regimen. J Clin Oncol 17: 3560–3568 [DOI] [PubMed] [Google Scholar]
- André T, Tournigand C, Rosmorduc O, Provent S, Maindrault-Goebel F, Avenin D, Selle F, Paye F, Hannoun L, Houry S, Gyet B, Lotz JP, de Gramont A, Louvet C (2004) Gemcitabine combined with oxaliplatin (GEMOX) in advanced biliary tract adenocarcinoma: a GERCOR study. Ann Oncol 15: 1339–1343 [DOI] [PubMed] [Google Scholar]
- Gamelin E, Gamelin L, Bossi L, Quiasthoff S (2002) Clinical aspects and molecular basis of oxaliplatin neurotoxicity: current management and development of preventive measures. Sem Oncol 29(5 Suppl 15): 21–33 [DOI] [PubMed] [Google Scholar]
- Glimelius B, Hoffman K, Sjöden PO, Jacobsson G, Sellstrom H, Enander LK, Linne T, Svensson C (1996) Chemotherapy improves survival and quality of life in advanced pancreatic and biliary cancer. Ann Oncol 7: 593–600 [DOI] [PubMed] [Google Scholar]
- Harder J, Riecken B, Kummer O, Lohrmann C, Otto F, Usadel H, Geissler M, Opitz O, Henß H (2006) Outpatient chemotherapy with gemcitabine and oxaliplatin in patients with biliary tract cancer. Br J Cancer 95: 848–852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hejna M, Pruckmayer M, Raderer M (1998) The role of chemotherapy and radiation in the management of biliary cancer: a review of the literature. Eur J Cancer 34: 977–986 [DOI] [PubMed] [Google Scholar]
- Jarnagin WR, Fong Y, DeMatteo RP, Gonen M, Burke EC, Bodniewicz J, Youssef M, Klimstra D, Blumgart LH (2001) Staging, resectability, and outcome in 225 patients with hilar cholangiocarcinoma. Ann Surgery 234: 507–519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarnagin WR, Klimstra DS, Hezel M, Gonen M, Fong Y, Roggin K, Cymes K, DeMatteo RP, D́Angelica M, Blumgart LH, Singh B (2006) Differential cell-cycle-regulatory protein expression in biliary tract adenocarcinoma: correlation with anatomic site, pathologic variables, and clinical outcome. J Clin Oncol 24: 1152–1160 [DOI] [PubMed] [Google Scholar]
- Kaplan EL, Maier P (1959) Non-Parametric estimation from incomplete observations. J Am Stat Assoc 53: 457–481 [Google Scholar]
- Kim TW, Chang HM, Kang HJ, Lee JR, Ryu MH, Ahn JH, Kim JH, Lee JS, Kang YK (2003) Phase II study of capecitabine plus cisplatin as first-line chemotherapy in advanced biliary cancer. Ann Oncol 14: 1115–1120 [DOI] [PubMed] [Google Scholar]
- Knox JJ, Hedley D, Oza A, Feld R, Siu LL, Chen E, Nematollahi M, Pond GR, Zhang J, Moore MJ (2005) Combining gemcitabine and capecitabine in patients with advanced biliary cancer: a phase II trial. J Clin Oncol 23: 2332–2338 [DOI] [PubMed] [Google Scholar]
- Knox JJ, Hedley D, Oza A, Siu LL, Pond GR, Moore MJ (2004) Gemcitabine concurrent with continuous infusional 5-fluoprouracil in advanced biliary cancers: a review of the Princess Margaret Hospital experience. Ann Oncol 15: 770–774 [DOI] [PubMed] [Google Scholar]
- Lersch C, Schmelz R, Eckel F, Erdmann J, Mayr M, Schulte-Frohlinde E, Quasthoff S, Grosskreutz J, Adelsberger H (2002) Prevention of oxaliplatin-induced peripheral sensory neuropathy by carbamazepine in patients with advanced colorectal cancer. Clin Colorectal Cancer 2: 54–58 [DOI] [PubMed] [Google Scholar]
- Nehls O, Klump B, Arkenau HT, Hass HG, Gregor M, Porschen R (2001) A phase II trial of oxaliplatin, fluorouracil and leucovorin for advanced biliary system adenocarcinomas. Br J Cancer 87: 702–704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortner MAEJ, Liebetruth J, Schreiber S, Hanft M, Wruck U, Fusco V, Müller JM, Hörtnagl H, Lochs H (1998) Photodynamic therapy in nonresectable cholangiocarcinoma. Gastroenterology 114: 536–542 [DOI] [PubMed] [Google Scholar]
- Ortner ME, Caca K, Berr F, Lieberuth J, Mansmann U, Huster D, Voderholzer W, Schachschal G, Mössner J, Lochs H (2003) Successful photodynamic therapy for nonresectable cholangiocarcinoma: a randomized prospective study. Gastroenterology 125: 1355–1363 [DOI] [PubMed] [Google Scholar]
- Park YH, Kim BS, Ryoo BY, Yang SH (2006) A phase II study of capecitabine plus 3-weekly oxaliplatin as first-line therapy for patients with advanced gastric cancer. Br J Cancer 94: 959–963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker SL, Tong T, Bolden S, Wingo PA (1996) Cancer statistics 1996. CA Cancer J Clin 65: 5–27 [DOI] [PubMed] [Google Scholar]
- Patt YZ, Hassan MM, Aguayo A, Nooka AK, Lozano RD, Curley SA, Vauthey JN, Ellis LM, Schnirer II, Wolff RA, Charnsanavej C, Brown TD (2004) Oral capecitabine for the treatment of hepatocellular carcinoma, cholangiocarcinoma, and gallbladder carcinoma. Cancer 101: 578–586 [DOI] [PubMed] [Google Scholar]
- Penz M, Kornek GV, Raderer M, Ulrich-Pur H, Fiebiger W, Lenauer A, Depisch D, Krauss G, Schneeweiss B, Scheithauer W (2001) Phase II trial of two-weekly gemcitabine in patients with advanced biliary tract cancer. Ann Oncol 12: 183–186 [DOI] [PubMed] [Google Scholar]
- Philip PA, Mahoney MR, Allmer C, Thomas J, Pitot HC, Kim G, Donehower RC, Fitch T, Picus J, Erlichman C (2006) Phase II study of erlotinib in patients with advanced biliary cancer. J Clin Oncol 24: 3069–3074 [DOI] [PubMed] [Google Scholar]
- Pitt HA, Grochow LB, Abrams RA (1997) Cancer of the biliary tree. In Cancer: Principles and Practice of Oncology, DeVita VT, Hellman S, Rosenberg SA (eds) 5th edn, pp 1114–1128. Philadelphia, Pennsylvania/New York: Lippincott-Raven [Google Scholar]
- Ramanathan RK, Belani CP, Singh DA, Tanaka M, Lenz HJ, Yen Y, Kindler HL, Iqbal S, Longmate J, Gandara DR (2006) Phase II study of lapatinib, a dual inhibitor of epidermal growth factor receptor (EGFR) tyrosine kinase 1 and 2 (Her/2-neu) in patients (pts) with advanced biliary tree cancer (BTC) or hepatocellular cancer (HCC). A california consortium (CCC-P) trial. In Proc Am Soc Clin Oncol 24: 18S (abstract 4010) [Google Scholar]
- Raymond E, Buquet-Fagot C, Djelloul S, Mester J, Cvitcovic E, Allain P, Louvet C, Gespach C (1997) Antitumoral activity of oxaliplatin in combination with 5-fluorouracil and the thymidylate synthase inhibitor AG337 in human colon, breast, and ovarian cancers. Anticancer Drugs 8: 876–885 [DOI] [PubMed] [Google Scholar]
- Simon R (1989) Optimal two-stage design for phase II clinical trials. Control Clin Trials 10: 1–10 [DOI] [PubMed] [Google Scholar]
- Tournigand C, Maindrault-Goebel F, Louvet A, de Gramont A, Krulik M (1998) Severe anaphylactic reactions to Oxaliplatin. Eur J Cancer 34: 1297–1298 [DOI] [PubMed] [Google Scholar]
- Verderame F, Russo A, Di Leo R, Badalamenti G, Santagelo D, Cicero G, Valerio MR, Gulotta G, Tomasello G, Gebbia N, Fulfaro F (2006) Gemcitabine and oxaliplatin combination chemotherapy in advanced biliary tract cancers. Ann Oncol 17(Suppl 7): vii68–vii72 [DOI] [PubMed] [Google Scholar]
- Weber SM, DeMatteo RP, Fong Y, Blumgart LH, Jarnagin WR (2002) Staging laparoscopy in patients with extrahepatic biliary carcinoma. Ann Surgery 235: 392–399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welzel TM, McGlynn KA, Hsing AW, ÓBrien TR, Pfeiffer RM (2006) Impact of classification of hilar cholangiocarcinomas (Klatskin tumors) on the incidence of intra- and extrahepatic cholangiocarcinomas in the United States. J Natl Cancer Inst 98: 873–875 [DOI] [PubMed] [Google Scholar]
- World Health Organization (1979) WHO Handbook for Reporting Results of Cancer Treatment WHO Offset Publication No 48. World Health Organization: Geneva [Google Scholar]