Abstract
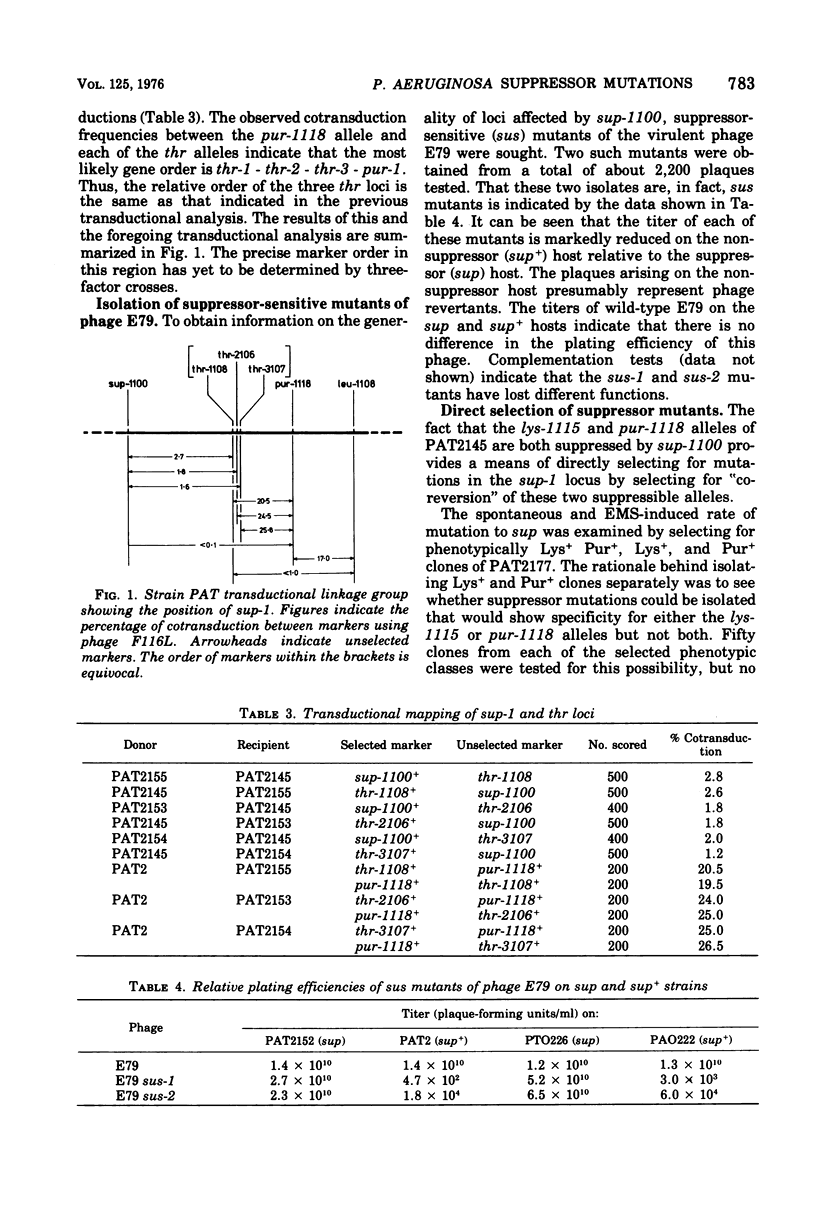
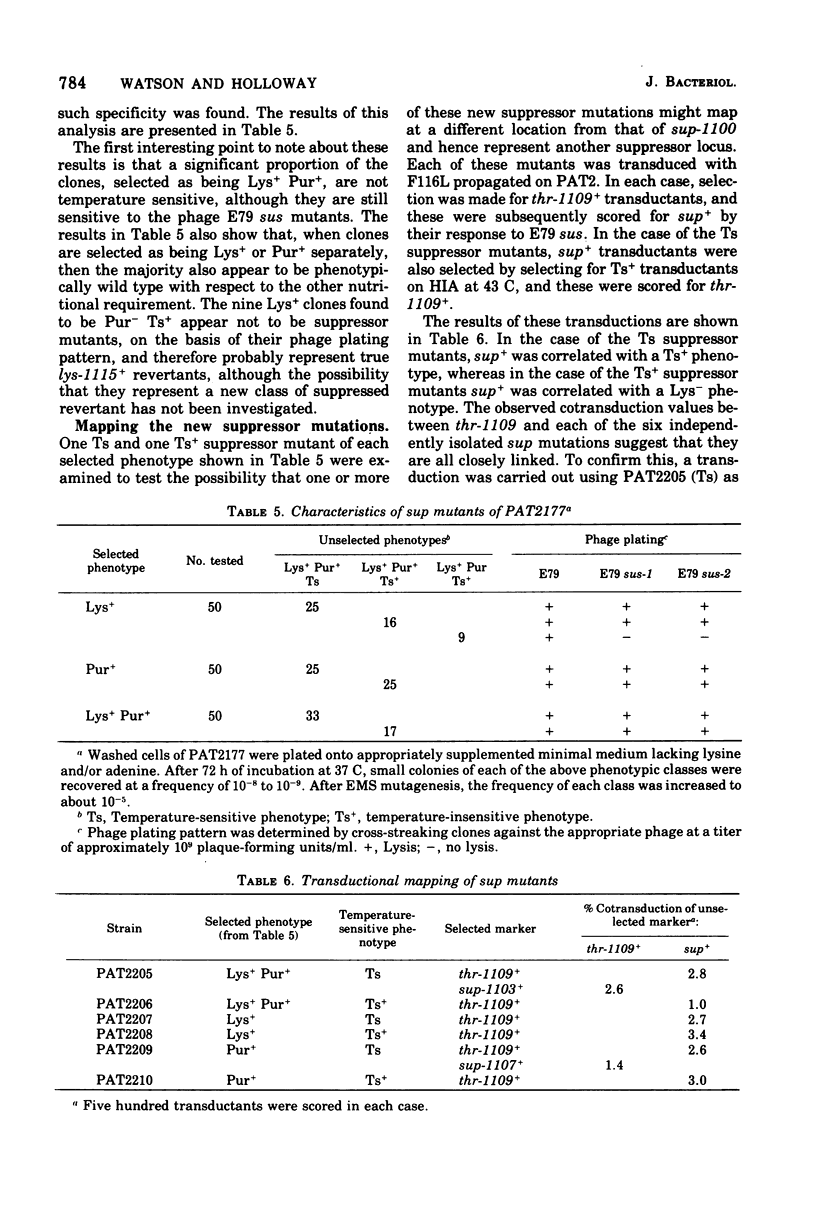
Suppressor mutations of Pseudomonas aeruginosa have been identified. An isolate of strain PAT, initially selected as being temperature sensitive for growth, was found to suppress two different auxotrophic mutations. A suppressor locus, designated sup-1, has been mapped and is co-transducible with three closely linked thr loci. The suppressor mutation has been used to isolate suppressor-sensitive (sus) mutants of the virulent phage E79 and the R factor R18. By selecting for revertants of auxotrophic markers, other sup mutants have been isolated and are found to be of two types, either temperature sensitive for growth like the original mutant or showing wild-type growth at 43 C. The mutations giving rise to both these classes of suppressor are very closely linked. One of the sup-1 alleles of strain PAT also shows suppressor activity when transferred into P. aeruginosa strain PAO. Escherichia coli strains carrying the nonsense suppressors supC, supD, or supF do not suppress the sus mutant of R18. This suggests that sup-1 is different from the amber and ochre suppressors of the enterobacteria.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Apirion D. Altered ribosomes in a suppressor strain of Escherichia coli. J Mol Biol. 1966 Apr;16(2):285–301. doi: 10.1016/s0022-2836(66)80173-0. [DOI] [PubMed] [Google Scholar]
- Chandler P. M., Krishnapillai V. Phenotypic properties of R factors of Pseudomonas aeruginosa: R factors readily transferable between Pseudomonas and the Enterobacteriaceae. Genet Res. 1974 Jun;23(3):239–250. doi: 10.1017/s0016672300014890. [DOI] [PubMed] [Google Scholar]
- Eggertsson G., Adelberg E. A. Map positions and specificities of suppressor mutations in Escherichia coli K-12. Genetics. 1965 Aug;52(2):319–340. doi: 10.1093/genetics/52.2.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOLLOWAY B. W., EGAN J. B., MONK M. Lysogeny in Pseudomonas aeruginosa. Aust J Exp Biol Med Sci. 1960 Aug;38:321–329. doi: 10.1038/icb.1960.34. [DOI] [PubMed] [Google Scholar]
- Hartman P. E., Roth J. R. Mechanisms of suppression. Adv Genet. 1973;17:1–105. doi: 10.1016/s0065-2660(08)60170-4. [DOI] [PubMed] [Google Scholar]
- Kennedy C., Crowlesmith I. A method for isolating nonsense suppressors in enterobacteriaceae using an amber mutant of the drug resistance factor R1. Mol Gen Genet. 1975 Jul 10;138(4):359–362. doi: 10.1007/BF00264806. [DOI] [PubMed] [Google Scholar]
- Krishnapillai V. A novel transducing phage. Its role in recognition of a possible new host-controlled modification system in Pseudomonas aeruginosa. Mol Gen Genet. 1972;114(2):134–143. doi: 10.1007/BF00332784. [DOI] [PubMed] [Google Scholar]
- Olsen R. H., Shipley P. Host range and properties of the Pseudomonas aeruginosa R factor R1822. J Bacteriol. 1973 Feb;113(2):772–780. doi: 10.1128/jb.113.2.772-780.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riddle D. L., Roth J. R. Frameshift suppressors. II. Genetic mapping and dominance studies. J Mol Biol. 1972 May 28;66(3):483–493. doi: 10.1016/0022-2836(72)90428-7. [DOI] [PubMed] [Google Scholar]
- Stanisich V., Holloway B. W. Genetic effects of acridines on Pseudomonas aeruginosa. Genet Res. 1969 Feb;13(1):57–70. doi: 10.1017/s0016672300002731. [DOI] [PubMed] [Google Scholar]
- VOGEL H. J., BONNER D. M. Acetylornithinase of Escherichia coli: partial purification and some properties. J Biol Chem. 1956 Jan;218(1):97–106. [PubMed] [Google Scholar]


