Abstract
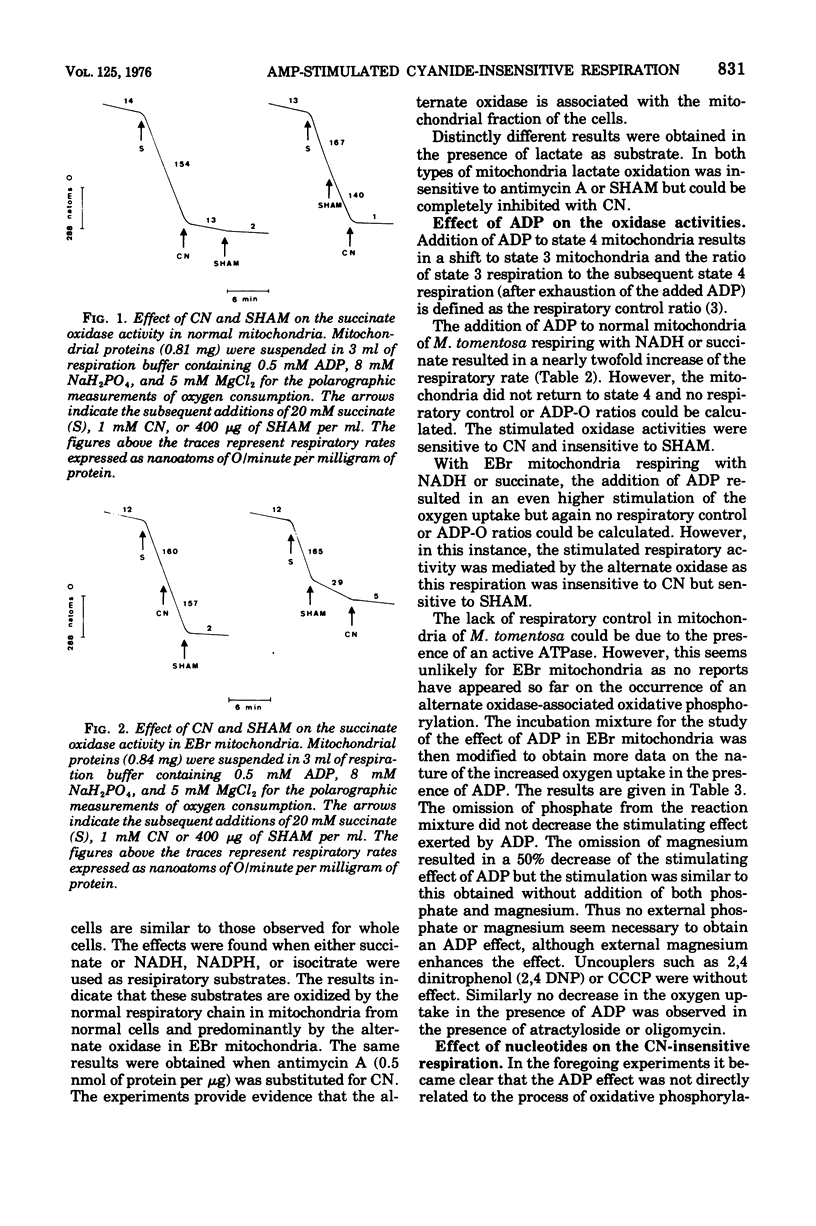
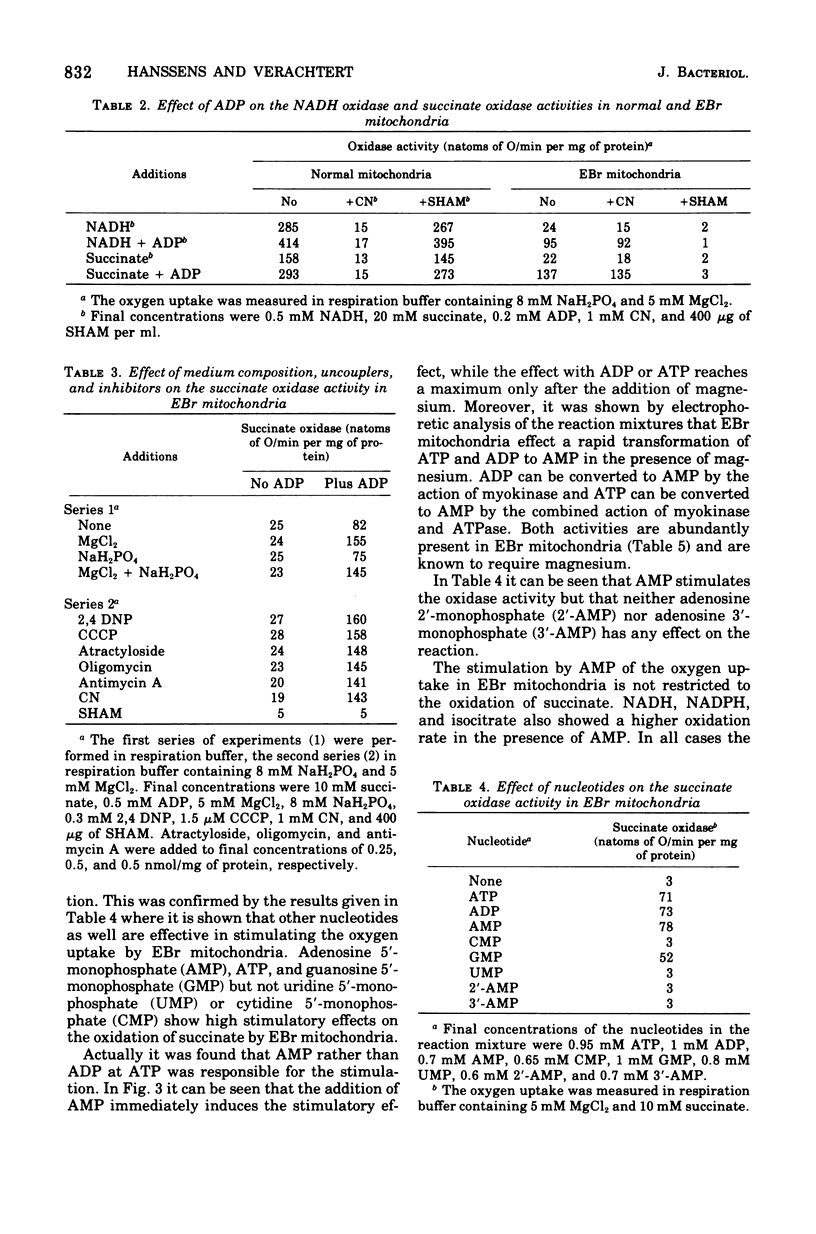
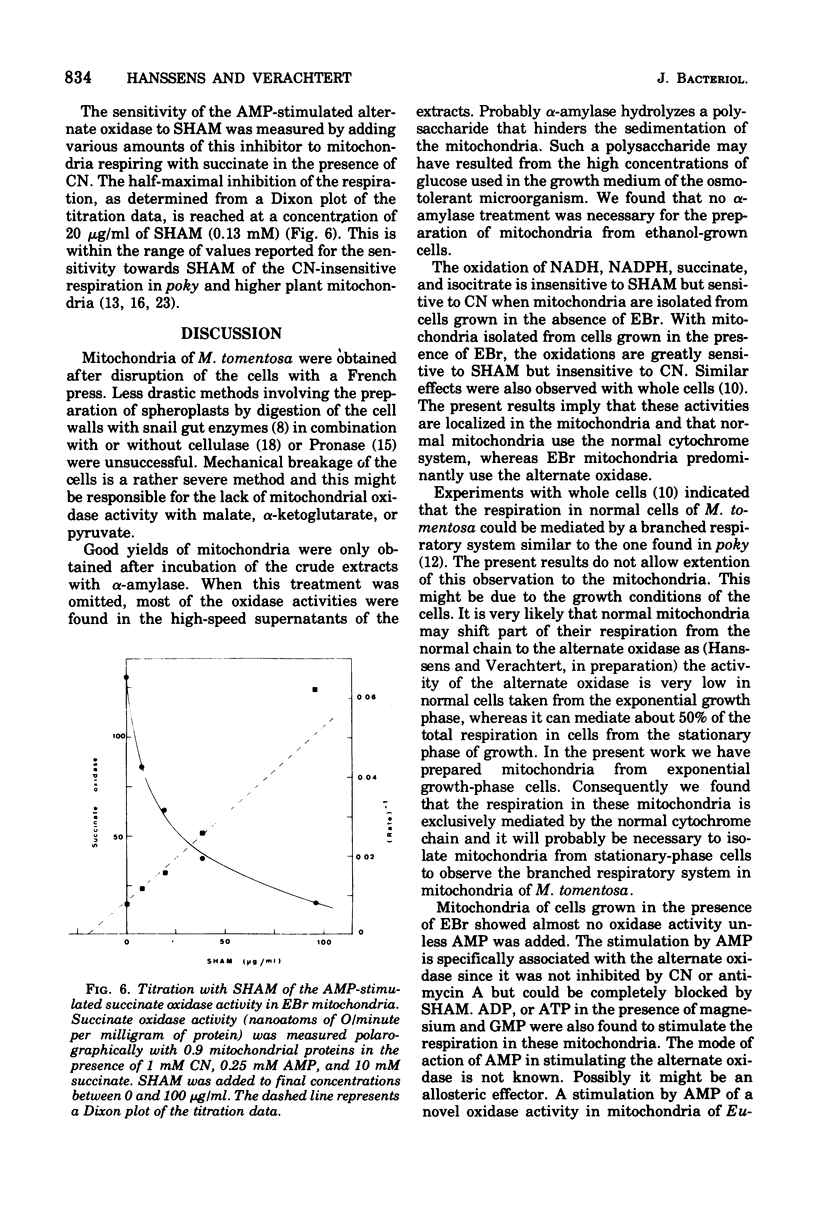
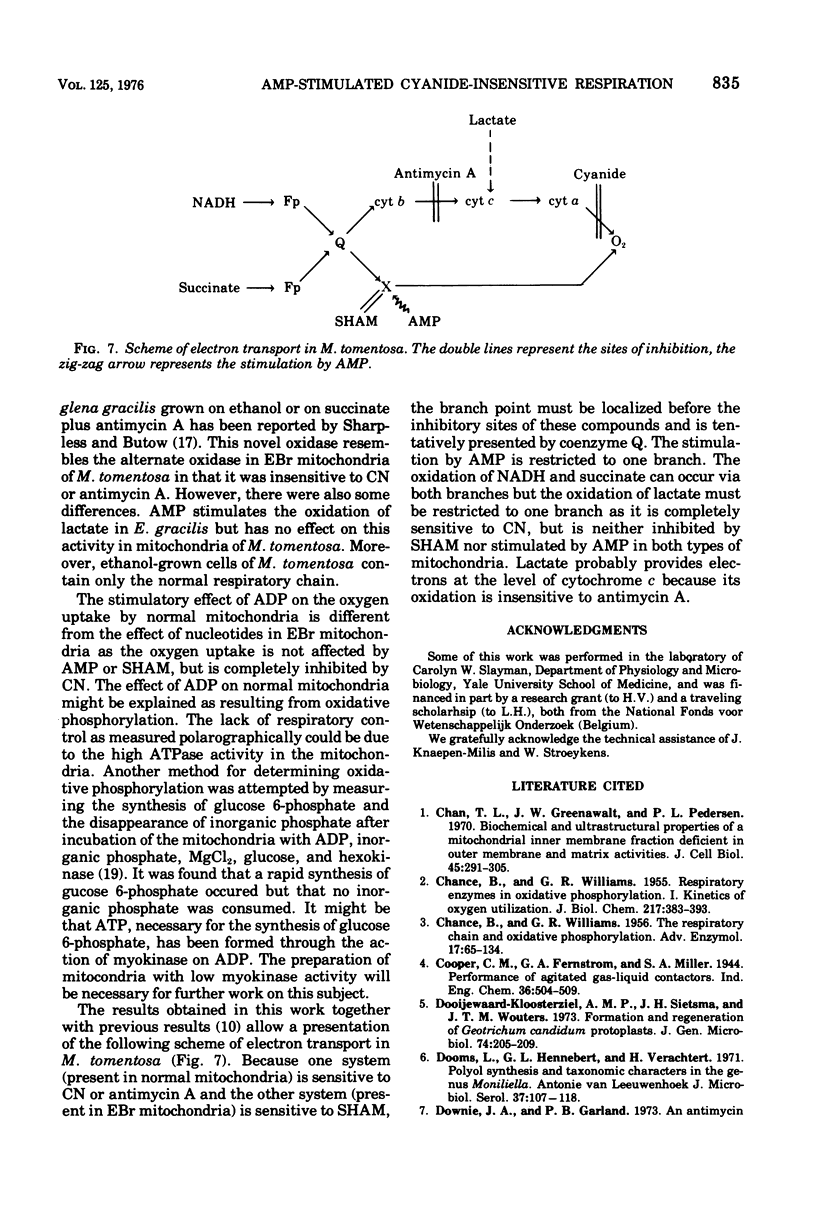
Mitochondria of the yeastlike fungus Moniliella tomentosa oxidize reduced nicotinamide adenine dinucleotide, reduced nicotinamide adenine dinucleotide phosphate, succinate, isocitrate, and lactate. These oxidations are completely inhibited by cyanide or antimycin A in mitochondria isolated from cells grown in the standard medium. On the other hand, the oxidation of all substrates, except lactate, is almost completely insensitive to cyanide or antimycin A in mitochondria from cells grown in the presence of ethidium bromide. In this instance, the oxidation is mainly mediated by an alternate oxidase which can be blocked by salicyl hydroxamic acid. The alternate oxidase can be specifically stimulated by adenosine 5'-monophosphate and this provides a new method for the characterization of the alternate oxidase in mitochondria of M. tomentosa.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- CHANCE B., WILLIAMS G. R. Respiratory enzymes in oxidative phosphorylation. I. Kinetics of oxygen utilization. J Biol Chem. 1955 Nov;217(1):383–393. [PubMed] [Google Scholar]
- CHANCE B., WILLIAMS G. R. The respiratory chain and oxidative phosphorylation. Adv Enzymol Relat Subj Biochem. 1956;17:65–134. doi: 10.1002/9780470122624.ch2. [DOI] [PubMed] [Google Scholar]
- Chan T. L., Greenawalt J. W., Pedersen P. L. Biochemical and ultrastructural properties of a mitochondrial inner membrane fraction deficient in outer membrane and matrix activities. J Cell Biol. 1970 May;45(2):291–305. doi: 10.1083/jcb.45.2.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DUELL E. A., INOUE S., UTTER M. F. ISOLATION AND PROPERTIES OF INTACT MITOCHONDRIA FROM SPHEROPLASTS OF YEAST. J Bacteriol. 1964 Dec;88:1762–1773. doi: 10.1128/jb.88.6.1762-1773.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dooijewaard-Kloosterziel, Sietsma J. H., Wouters J. T. Formation and regeneration of Geotrichum candidum protoplasts. J Gen Microbiol. 1973 Feb;74(2):205–209. doi: 10.1099/00221287-74-2-205. [DOI] [PubMed] [Google Scholar]
- Dooms L., Hennebert G. L., Verachtert H. Polyol synthesis and taxonomic characters in the genus Moniliella. Antonie Van Leeuwenhoek. 1971;37(1):107–118. doi: 10.1007/BF02218471. [DOI] [PubMed] [Google Scholar]
- Downie J. A., Garland P. B. An antimycin A- and cyanide-resistant variant of Candida utilis arising during copper-limited growth. Biochem J. 1973 Aug;134(4):1051–1061. doi: 10.1042/bj1341051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAJNY G. J., SMITH J. H., GARVER J. C. ERYTHRITOL PRODUCTION BY A YEASTLIKE FUNGUS. Appl Microbiol. 1964 May;12:240–246. doi: 10.1128/am.12.3.240-246.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanssens L., D'Hondt E., Verachtert H. Cyanide-insensitive respiration in Moniliella tomentosa and the effect of drugs on respiration and polyol biosynthesis. Arch Microbiol. 1974 Jul 22;98(4):339–349. doi: 10.1007/BF00425294. [DOI] [PubMed] [Google Scholar]
- Hanssens L., Van Regenmortel A., Verachtert H. pH-dependent polyol production in Moniliella tomentosa. Appl Microbiol. 1972 Nov;24(5):831–833. doi: 10.1128/am.24.5.831-833.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lambowitz A. M., Slayman C. W. Cyanide-resistant respiration in Neurospora crassa. J Bacteriol. 1971 Dec;108(3):1087–1096. doi: 10.1128/jb.108.3.1087-1096.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambowitz A. M., Smith E. W., Slayman C. W. Electron transport in Neurospora mitochondria. Studies on wild type and poky. J Biol Chem. 1972 Aug 10;247(15):4850–4858. [PubMed] [Google Scholar]
- Schnaitman C., Greenawalt J. W. Enzymatic properties of the inner and outer membranes of rat liver mitochondria. J Cell Biol. 1968 Jul;38(1):158–175. doi: 10.1083/jcb.38.1.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schonbaum G. R., Bonner W. D., Jr, Storey B. T., Bahr J. T. Specific inhibition of the cyanide-insensitive respiratory pathway in plant mitochondria by hydroxamic acids. Plant Physiol. 1971 Jan;47(1):124–128. doi: 10.1104/pp.47.1.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharpless T. K., Butow R. A. An inducible alternate terminal oxidase in Euglena gracilis mitochondria. J Biol Chem. 1970 Jan 10;245(1):58–70. [PubMed] [Google Scholar]
- von Jagow G., Weiss H., Klingenberg M. Comparison of the respiratory chain of Neurospora crassa wild type and the mi-mutants mi-1 and mi-3. Eur J Biochem. 1973 Feb 15;33(1):140–157. doi: 10.1111/j.1432-1033.1973.tb02665.x. [DOI] [PubMed] [Google Scholar]



