Abstract
We aimed to create a prognostic model in metastatic melanoma based on independent prognostic factors in 321 patients receiving interleukin-2 (IL-2)-based immunotherapy with a median follow-up time for patients currently alive of 52 months (range 15–189 months). The patients were treated as part of several phase II protocols and the majority received treatment with intermediate dose subcutaneous IL-2 and interferon-α. Neutrophil and monocyte counts, lactate dehydrogenase (LDH), number of metastatic sites, location of metastases and performance status were all statistically significant prognostic factors in univariate analyses. Subsequently, a multivariate Cox's regression analysis identified elevated LDH (P<0.001, hazard ratio 2.8), elevated neutrophil counts (P=0.02, hazard ratio 1.4) and a performance status of 2 (P=0.008, hazard ratio 1.6) as independent prognostic factors for poor survival. An elevated monocyte count could replace an elevated neutrophil count. Patients were assigned to one of three risk groups according to the cumulative risk defined as the sum of simplified risk scores of the three independent prognostic factors. Low-, intermediate- and high-risk patients achieved a median survival of 12.6 months (95% confidence interval (CI), 11.4–13.8), 6.0 months (95% CI, 4.8–7.2) and 3.4 months (95% CI, 1.2–5.6), respectively. The low-risk group encompassed the majority of long-term survivors, whereas the patients in the high-risk group with a very poor prognosis should probably not be offered IL-2-based immunotherapy.
Keywords: interleukin-2, prognostic factors, prognostic model, metastatic melanoma, LDH, neutrophils, monocytes
The prognosis for patients with metastatic melanoma is poor with a median survival time between 4 and 12 months (Barth et al, 1995). Interleukin-2 (IL-2) has resulted in durable responses and cure in 5–7% of the patients (Rosenberg et al, 1998; Atkins et al, 2000). Meta-analyses on patients receiving IL-2-based immunotherapy have identified independent prognostic factors of survival such as serum lactate dehydrogenase (LDH), number of metastatic sites and performance status (Eton et al, 1998; Keilholz et al, 1998, 2002; Manola et al, 2000). Presently, the strongest prognostic factor in metastatic melanoma is LDH, which is used in the American Joint Committee on Cancer (AJCC) stage IV classification in combination with site of metastases (Balch et al, 2001a).
The new paradigm in tumour immunology states that tumour-infiltrating inflammatory cells such as macrophages and neutrophils play a pivotal role in tumour progression and dissemination in a wide range of cancers (Balkwill and Mantovani, 2001; Coussens and Werb, 2002; Pollard, 2004). Recently, baseline elevated neutrophil and monocyte counts in peripheral blood were proposed as prognostic factors for poor survival in patients with metastatic renal cell carcinoma undergoing immunotherapy with IL-2 or α-interferon-2b (IFN) (Negrier et al, 2002; Atzpodien et al, 2003; Donskov et al, 2004). Similar data are not available in metastatic melanoma.
We have examined the prognostic impact of neutrophils and monocytes in peripheral blood together with other potentially prognostic factors in 321 patients with metastatic melanoma receiving IL-2-based immunotherapy in consecutive clinical trials between 1990 and 2003. Based on independent prognostic factors, we propose a prognostic model for metastatic melanoma.
MATERIAL AND METHODS
Patients
Between April 1990 and December 2003, 321 patients with metastatic melanoma were entered on consecutive clinical trials with IL-2-based immunotherapy. Data were analysed per 1 February 2005, that is, more than 1 year after the last patient had received IL-2-based immunotherapy. The main inclusion criteria consisted of biopsy-verified stage IV disease, a WHO performance status of 2 or better and age above 18 years. The main exclusion criteria were brain metastases, a previous malignant disease other than nonmelanoma skin cancer, symptomatic heart or lung disease, seizure disorders, serious autoimmune disorders, concomitant corticosteroid therapy or previous systemic treatment for metastatic disease. The ethics committees at the involved counties approved the present project, and written informed consent was obtained from each patient. Data were collected from the three participating institutions, and all cases were reviewed individually and entered in a central database. The data set was complete with the exception of one missing monocyte count from a single patient.
Treatments
In all, 20 patients received recombinant IL-2 and IFN. Interleukin-2 was administered at a dose of 18 MU m−2 day−1 by a 24-h continuous intravenous (i.v.) infusion. Interferon was administered during the IL-2 infusion periods at a dose of 3 MU m−2 day−1. The treatment plan consisted of two induction cycles and four maintenance cycles with a 3-week rest period following each cycle. Each induction cycle consisted of two IL-2 infusion periods of 120 and 108 h duration, respectively, separated by 6 days rest period. Each maintenance cycle consisted of a 120 h IL-2 infusion period.
In all, 86 patients received IL-2, IFN and cisplatinum (Schmidt et al, 2000). One cycle consisted of cisplatinum i.v. bolus on day 1 at a dose of 100 mg m−2. Recombinant IL-2 was administered subcutaneous (s.c.) at a dose of 9 MU m−2 twice daily on days 5–9 and at a dose of 4.5 MU m−2 daily on days 12, 14 and 16. Interferon was administered s.c. at a dose of 10 MU daily on days 2–4 and thereafter three times weekly.
In total, 83 patients received IL-2, IFN±histamine (Schmidt et al, 2002). One cycle consisted of IL-2 s.c. at a dose of 9 MU twice daily on days 4–8, and daily on days 11–15. Patients received INF at a dose of 5 MU s.c. daily from days 1 to 21. Histamine dihydrochloride was administered s.c. at a dose of 1 mg over 20 min, twice daily. Histamine injections were administered from day 4 and on the same days as IL-2 and INF injections.
A total of 102 patients received IL-2 s.c.±low-dose total body irradiation (LTBI). One cycle consisted of IL-2 s.c. on days 2–5 at a dose of 18 MU twice daily, and at a dose of 9 MU twice daily on days 9–12. A single fraction of LTBI 0.1 Gy were given on days 1 and 8.
In all, 30 patients received intratumoral injections of bleomycin and electroporation (electrochemotherapy) followed by IL-2 s.c. at a dose of 2 MU daily for 21 consecutive days (Gehl et al, 2003).
Statistics
Calculations were performed using SPSS (version 11.5) statistical software. Univariate and multivariate Cox's analyses were performed to investigate the prognostic impact of baseline factors in relation to survival. Factors included were WHO performance status (0–1 vs 2); number of metastatic sites (1–2 vs ⩾3); site of metastases (skin and lymph nodes vs lungs vs other visceral sites); baseline serum LDH (normal vs elevated); baseline blood neutrophil counts (normal vs elevated); and baseline blood monocyte counts (normal vs elevated). Baseline blood measurements (LDH, neutrophils and monocytes) were both analysed as continuous variables (absolute values) and as dichotomous variables using the upper normal reference level from each of laboratories in the participating institutions. Survival was calculated from the day of treatment start to the end point (death or censoring). Patient survival and median duration of response was analysed by the Kaplan–Meier method. The simultaneous relationship of multiple prognostic factors for survival was assessed using Cox's proportional-hazards model. Factors with a P-value <0.10 in the univariate analyses were included in the multivariate analysis to identify factors of independent significance. The multivariate analysis was stratified according to treatment regimens. The multivariate model selection was performed by a stepwise strategy using the likelihood ratio test to create a multiple risk factor model. Hazard ratios were calculated to estimate the magnitude and the direction of the effect. Schoenfeld residuals and log(−log(S)) vs t plots were evaluated to assure the assumption of proportional hazards. Assessment of the model was carried out using crossvalidation techniques (Altman and Royston, 2000), using SAS (version 8.2) statistical software. All survival data were updated on 1 February 2005.
RESULTS
The median survival was 8.1 months (range 1–188), and 19 of 321 patients were currently alive. Of these 19 patients, 17 had a survival length of more than 24 months and were termed long-term survivors. Data were analysed more than 1 year after the last patient had received IL-2-based immunotherapy and the median follow-up time of the 19 patients currently alive was 52 months (range 15–188 months).
Univariate analyses of pretreatment variables
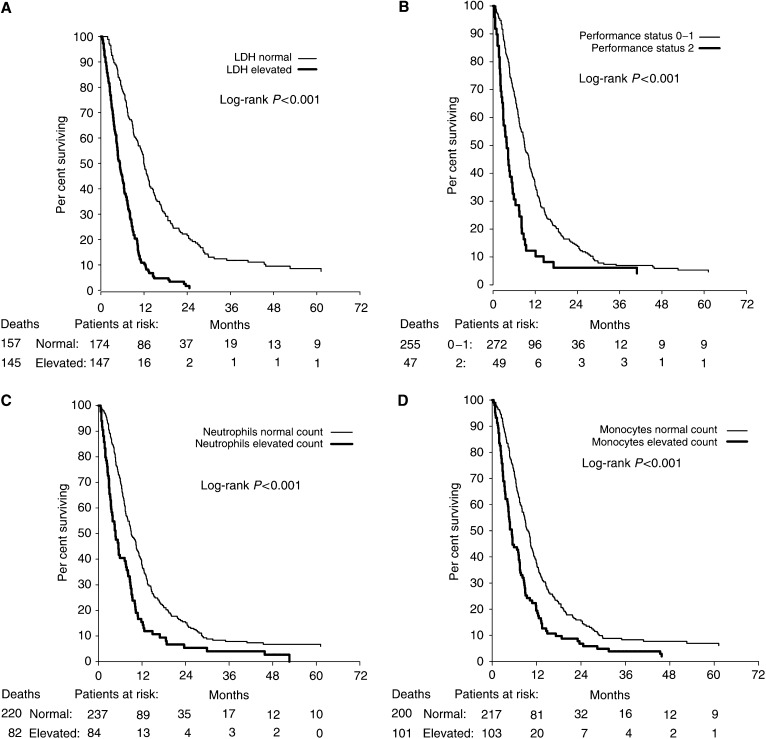
In univariate analyses, the following baseline factors were statistically significantly (P<0.05) associated with poor survival: elevated LDH (P<0.001), a performance status of 2 (P<0.001), elevated neutrophil counts (P<0.001), elevated monocyte counts (P<0.001) (Figure 1A–D), more than two metastatic sites (P<0.001) and site of metastases (P=0.03) (Table 1). Elevated LDH, neutrophil and monocyte counts refer to levels above the upper normal reference levels.
Figure 1.
Kaplan–Meier survival estimates for 321 patients with metastatic melanoma according to baseline: (A) lactate dehydrogenase (LDH), (B) performance status, (C) blood neutrophils and (D) blood monocytes.
Table 1. Univariate Cox's analyses of risk factors and survival in metastatic melanoma.
|
Univariate Cox's analyses
|
|||||
|---|---|---|---|---|---|
| Risk factors | Categories compared | Number of patients | Hazard ratio | 95% CI | P-value |
| Gender | Female vs male | 181 vs 140 | 1.0 | 0.8–1.3 | 0.88 |
| Age (median 51 years) | <51 vs ⩾51 | 157 vs 164 | 1.1 | 0.9–1.3 | 0.59 |
| Performance status | 0–1 vs 2 | 272 vs 49 | 2.1 | 1.5–2.8 | <0.001 |
| No. of metastatic sites | 1–2 vs ⩾3 | 195 vs 126 | 1.6 | 1.3–2.0 | <0.001 |
| Location of metastases | Skin, lymph nodes vs lung vs visceral sites | 82 vs 72 vs 167 | 0.03* | ||
| Skin, lymph nodes vs lung | 82 vs 72 | 1.3 | 0.9–1.8 | 0.11 | |
| Skin, lymph nodes vs visceral sites | 82 vs 167 | 1.5 | 1.1–1.9 | 0.01 | |
| LDH | Normal vs elevated | 174 vs 147 | 2.9 | 2.3–3.7 | <0.001 |
| Neutrophil counts | Normal vs elevated | 237 vs 84 | 1.9 | 1.4–2.4 | <0.001 |
| Monocyte counts | Normal vs elevated | 217 vs 103 | 1.7 | 1.4–2.2 | <0.001 |
CI=confidence interval; LDH=lactate dehydrogenase.
P-value is an overall estimate for all levels.
Multivariate analyses of pretreatment variables
Upon entering the significant variables from the univariate analyses into a multivariate Cox's analysis, three variables turned out as independent factors of poor survival: elevated LDH (P<0.001), elevated neutrophil counts (P=0.02) and a performance status of 2 (P=0.008) (Table 2). Similar results were achieved when entering blood counts as absolute values (data not shown). In patients with a normal LDH, the 5-year survival rate was 9% compared to 0% in patients with an elevated LDH (Figure 1A). Similarly, in patients with a normal neutrophil count, the 5-year survival rate was 7% compared to 0% in patients with an elevated count (Figure 1C). Model assessment using crossvalidation revealed that the model was robust, and there was little evidence of overfitting. The alternative multivariate model including monocytes, instead of neutrophils, yielded a similar result (likelihood ratio χ2 of 87.7 vs 86.6).
Table 2. Multivariate Cox's model of independent prognostic factors for survival in metastatic melanoma.
|
Multivariate Cox's analysisa
|
||||||
|---|---|---|---|---|---|---|
| Risk factors | Categories compared | Hazard ratio | 95% CI | P-value | Regression coefficients | Weight (contribution to cumulative risk score) |
| Performance status | 0–1 vs 2 | 1.6 | 1.1–2.3 | 0.008 | 0.47 | 0 vs 1 |
| LDH | Normal vs elevated | 2.8 | 2.2–3.6 | <0.001 | 1.03 | 0 vs 2 |
| Neutrophils | Normal vs elevated | 1.4 | 1.1–1.8 | 0.02 | 0.32 | 0 vs 1 |
CI=confidence interval; LDH=lactate dehydrogenase. All other variables (location of metastases, number of metastatic sites and blood monocytes) were not significant and therefore excluded from the model.
In all, 320 patients and 301 deaths. Analysis stratified by treatment regimen.
Prognostic model
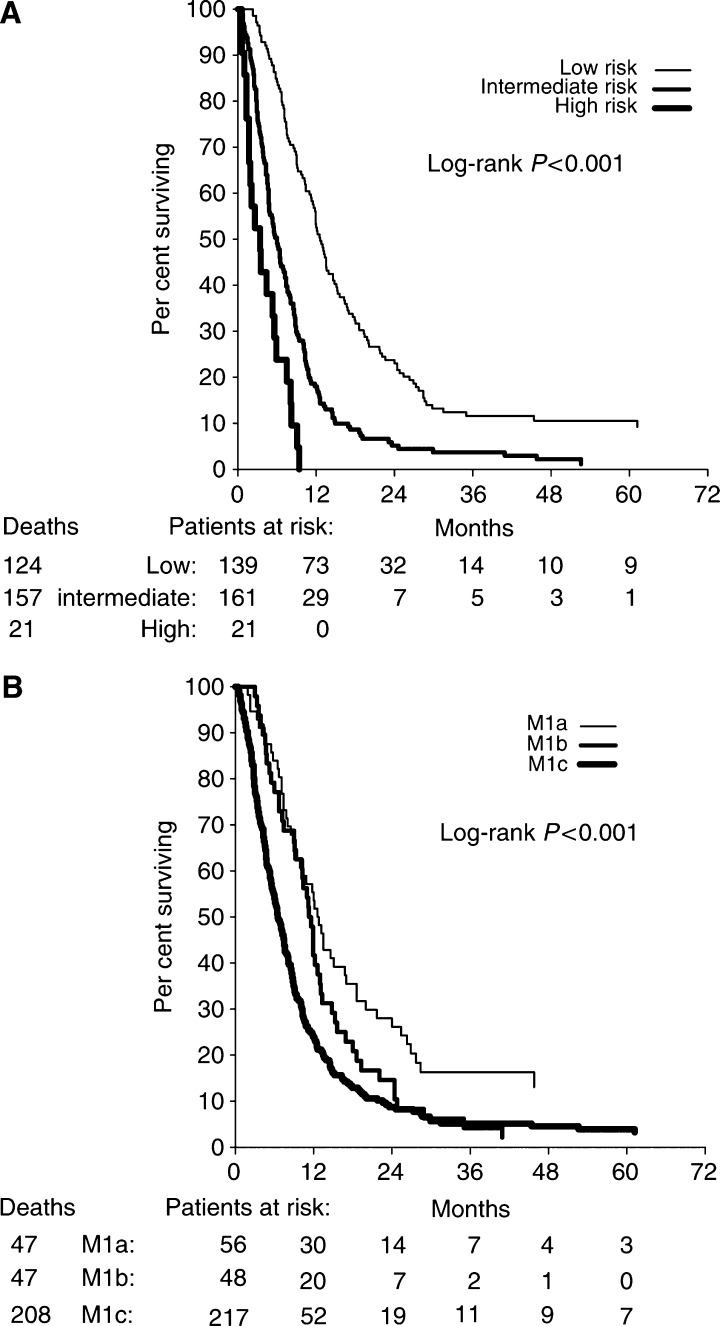
Based on the ratios of regression coefficients (log hazard ratios in the final Cox's model) of variables, we defined the weights of prognostic factors as follows: elevated LDH was assigned weight 2, elevated neutrophil counts weight 1 and performance status of 2 weight 1. A prognostic score of the cumulated weights of these variables was used to assign patients to low-risk (none elevated, score 0), intermediate-risk (any combination of 1–2 elevated variables, score 1–3) and high-risk (all three variables elevated, score 4) groups, respectively. The median survival of low-risk (n=139), intermediate-risk (n=161) and high-risk patients (n=21) was 12.6 months (95% confidence interval (CI) 11.4–13.8), 6.0 months (95% CI, 4.8–7.2) and 3.4 months (95% CI, 1.2–5.6), respectively (Figure 2A). The 5-year survival rates for these three groups were 9, 1 and 0%, respectively. The predicted 12-month survival probabilities were similar to the respective Kaplan–Meier estimates for each risk score. The 12-month survival probabilities predicted by the Cox's model were 48% (low risk), 14% (intermediate risk) and 1% (high risk), and the Kaplan–Meier estimates were 51, 13 and 0%, respectively. Similar differences were observed for the 24-month survival probabilities.
Figure 2.
Kaplan–Meier survival estimates for 321 patients with metastatic melanoma according to combination factors: (A) prognostic model with low, intermediate and high risk. (B) AJCC stage IV classification: M1a, normal lactate dehydrogenase (LDH) and metastases confined to the skin and lymph nodes, M1b including lung metastases and normal LDH and M1c including other visceral organs or elevated serum LDH.
We compared our proposed prognostic model with the current AJCC classification of stage IV disease, which includes LDH, and also the site of metastases. The AJCC model defines stage M1a as skin or lymph node metastases with a normal LDH, M1b as lung metastases with a normal LDH and M1c as other visceral metastases or an elevated LDH. In the univariate analyses, we found a significant difference in survival time between stage M1a and M1c (P<0.001), but not between M1a and M1b (P=0.11) (Figure 2B). When entering the AJCC model together with our proposed model into a multivariate analysis, the prognostic index of LDH, neutrophils and performance status remained highly statistically significant through all levels (P<0.001), while the AJCC classification (P=0.26) had no independent prognostic impact (Table 3).
Table 3. Univariate and multivariate Cox's analyses of the proposed prognostic index and the AJCC stage IV classification in patients with metastatic melanoma.
|
Univariate analyses
|
Multivariate analysisa
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Combination of risk factors | Categories compared | Number of patients | Hazard ratio | 95% CI | P-value | Hazard ratio | 95% CI | P-value |
| LDH, neutrophils and performance status | Low vs intermediate vs high | 139 | <0.001* | <0.001* | ||||
| Low vs intermediate | 161 | 2.4 | 1.9–3.0 | <0.001 | 2.2 | 1.7–2.9 | <0.001 | |
| Low vs high | 21 | 5.9 | 3.7–9.6 | <0.001 | 5.4 | 3.3–9.0 | <0.001 | |
| AJCC stage IV classification | M1a vs M1b vs M1c | 56 | <0.001* | 0.26* | ||||
| M1a vs M1b | 48 | 1.4 | 0.9–2.1 | 0.11 | 1.3 | 0.9–2.0 | 0.21 | |
| M1a vs M1c | 217 | 2.0 | 1.5–2.8 | <0.001 | 1.3 | 0.9–1.8 | 0.11 | |
CI=confidence interval; LDH=lactate dehydrogenase; AJCC=American Joint Committee on Cancer; M1a=skin and lymph node involvement with normal serum LDH; M1b=including lung metastases with normal serum LDH; M1c=other visceral metastases or an elevated serum LDH. Low risk denotes a normal LDH, and a normal neutrophil count, and a performance status of 0–1. Intermediate risk denotes any combination of at least one but not all variables elevated. High risk denotes an elevated LDH, an elevated neutrophil count, and a performance status of 2.
P-values are overall estimates for all levels.
N=321, 302 deaths.
DISCUSSION
Recent studies of IL-2-based immunotherapy in metastatic melanoma have identified mainly LDH, performance status, number of metastatic sites and sites of metastases as independent prognostic factors (Sirott et al, 1993; Eton et al, 1998; Keilholz et al, 1998, 2002; Manola et al, 2000). Our findings of LDH and performance status as independent prognostic factors were therefore expected. However, the prognostic impact of neutrophils and monocytes in peripheral blood was a novel finding. Both elevated neutrophil and monocyte counts were associated with poor survival in the univariate analyses, and if neutrophils were forced out of the multivariate model, monocytes became an independent prognostic factor together with LDH and performance status. The neutrophil and monocyte counts were highly significantly correlated in the patient material (Spearman's rank-correlation coefficient 0.49, P<0.0001), which explains why only one of them was an independent factor in the multivariate analysis. Thus, the neutrophil count could be replaced by the monocyte count resulting in a similar prognostic score.
Our findings in patients with metastatic melanoma correspond with observations in metastatic renal cell carcinoma. Thus, an elevated neutrophil count was an independent risk factor for poor survival in two large studies in metastatic renal cell carcinoma (Negrier et al, 2002; Atzpodien et al, 2003), whereas the monocyte count was not considered in these studies. In a third study, both elevated neutrophil and elevated monocyte counts were statistically significant correlated to poor survival however, in univariate analyses (Donskov et al, 2004). The explanation for the association between high neutrophil or monocyte counts and poor prognosis is not fully clarified. The new paradigm in tumour immunology states that the tumour microenvironment can educate and control invading leucocytes to promote angiogenesis, viability, motility and invasion (Hanahan and Weinberg, 2000; Balkwill and Mantovani, 2001; Coussens and Werb, 2002; Lin and Pollard, 2004). Especially tumour-associated macrophages, which arise from blood monocytes, seem to play a crucial role in this interaction (Pollard, 2004). The chemokines CXCL1 (Gro-α) and CXCL8 (IL-8) are constitutively produced by melanoma cells and the corresponding receptors CXCR1 and CXCR2 are expressed on melanoma cells as well as on macrophages, neutrophils and eosinophils (Moser et al, 1993; Norgauer et al, 1996; Dhawan and Richmond, 2002). The autocrine production of these chemokines by melanoma cells increases their survival, proliferation and dissemination, as well as attracts inflammatory cells, such as monocytes and neutrophils (Haghnegahdar et al, 2000; Schaider et al, 2003; Balkwill, 2004). These tumour-infiltrating neutrophils can produce VEGF, IL-8 and matrix metalloproteinases involved in tumour invasion and angiogenesis (Shamamian et al, 2001; Schaider et al, 2003). In one study, IL-8 was detectable in the serum in 50% of patients with metastatic melanoma, which correlated with tumour load (Scheibenbogen et al, 1995). These findings indicate a link between elevated neutrophils and monocytes in peripheral blood and especially aggressive melanomas leading to a poor prognosis.
The high-risk group in our proposed prognostic index with elevated neutrophil counts, elevated LDH and a performance status of 2, had a very poor prognosis with a median survival of only 3.4 months (95% CI, 1.2–5.6). None of these patients survived for more than 10 months, and this group of patients should probably not be offered IL-2-based immunotherapy. In contrast, the low-risk group with no elevated risk factors had a median survival of 12.6 months (95% CI, 11.4–13.8) and included 15 of the 17 long-term survivors. It should be emphasised that the majority of patients in our study were treated with intermediate s.c. IL-2 regimens and these results cannot directly be applied to patients treated with high-dose i.v. IL-2. However, our hypothesis is that the findings will be similar for such a group of patients.
We applied our data to the AJCC stage IV classification (version 2001), and observed a significant difference in survival between patients with skin/lymph node metastases (M1a) and visceral metastases or an elevated LDH (M1c), but not between M1a and patients with lung metastases (M1b). These results correspond well with the data reported by Balch et al (2001b) demonstrating a significant survival difference between M1a and M1b at 1 year, but not beyond that time frame. When we tested our prognostic model against the AJCC model in a multivariate analysis, the prognostic impact of our model based on neutrophils, LDH and performance status (P<0.001) was superior compared to the AJCC model (P=0.22). Similarly, Keilholz et al (2002) have reported that the prognostic impact of a combination of LDH and performance status was superior to the AJCC model. Our results further supplements that model by adding neutrophil counts. The proposed model has been internally validated (Altman and Royston, 2000), but validation in an independent study is obviously warranted.
In conclusion, the independent prognostic impact of elevated neutrophil or monocyte counts in peripheral blood is a novel finding in patients with metastatic melanoma. As expected, LDH and performance status were also independent prognostic factors. Our proposed prognostic model with neutrophil counts, LDH and performance status was able to identify a low-risk group encompassing the majority of long-term survivors, and a high-risk group with a very poor prognosis, which should probably not be offered IL-2-based immunotherapy. The validation of the prognostic model in an independent study is warranted.
Acknowledgments
We thank the staff members of the departments of Oncology in Aarhus, Odense and Herlev for their careful management of the patients.
References
- Altman DG, Royston P (2000) What do we mean by validating a prognostic model? Stat Med 19: 453–473 [DOI] [PubMed] [Google Scholar]
- Atkins MB, Kunkel L, Sznol M, Rosenberg SA (2000) High-dose recombinant interleukin-2 therapy in patients with metastatic melanoma: long-term survival update. Cancer J Sci Am 6(Suppl 1): S11–S14 [PubMed] [Google Scholar]
- Atzpodien J, Royston P, Wandert T, Reitz M (2003) Metastatic renal carcinoma comprehensive prognostic system. Br J Cancer 88: 348–353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balch CM, Buzaid AC, Soong SJ, Atkins MB, Cascinelli N, Coit DG, Fleming ID, Gershenwald JE, Houghton Jr A, Kirkwood JM, McMasters KM, Mihm MF, Morton DL, Reintgen DS, Ross MI, Sober A, Thompson JA, Thompson JF (2001a) Final version of the American Joint Committee on Cancer staging system for cutaneous melanoma. J Clin Oncol 19: 3635–3648 [DOI] [PubMed] [Google Scholar]
- Balch CM, Soong SJ, Gershenwald JE, Thompson JF, Reintgen DS, Cascinelli N, Urist M, McMasters KM, Ross MI, Kirkwood JM, Atkins MB, Thompson JA, Coit DG, Byrd D, Desmond R, Zhang Y, Liu PY, Lyman GH, Morabito A (2001b) Prognostic factors analysis of 17 600 melanoma patients: validation of the American Joint Committee on Cancer melanoma staging system. J Clin Oncol 19: 3622–3634 [DOI] [PubMed] [Google Scholar]
- Balkwill F (2004) Cancer and the chemokine network. Nat Rev Cancer 4: 540–550 [DOI] [PubMed] [Google Scholar]
- Balkwill F, Mantovani A (2001) Inflammation and cancer: back to Virchow? Lancet 357: 539–545 [DOI] [PubMed] [Google Scholar]
- Barth A, Wanek LA, Morton DL (1995) Prognostic factors in 1521 melanoma patients with distant metastases. J Am Coll Surg 181: 193–201 [PubMed] [Google Scholar]
- Coussens LM, Werb Z (2002) Inflammation and cancer. Nature 420: 860–867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhawan P, Richmond A (2002) Role of CXCL1 in tumorigenesis of melanoma. J Leukocyte Biol 72: 9–18 [PMC free article] [PubMed] [Google Scholar]
- Donskov F, Bennedsgaard KM, Hokland M, Marcussen N, Fisker R, Madsen HH, Fode K, von der Maase H (2004) Leukocyte orchestration in blood and tumour tissue following interleukin-2 based immunotherapy in metastatic renal cell carcinoma. Cancer Immunol Immunother 53: 729–739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eton O, Legha SS, Moon TE, Buzaid AC, Papadopoulos NE, Plager C, Burgess AM, Bedikian AY, Ring S, Dong Q, Glassman AB, Balch CM, Benjamin RS (1998) Prognostic factors for survival of patients treated systemically for disseminated melanoma. J Clin Oncol 16: 1103–1111 [DOI] [PubMed] [Google Scholar]
- Gehl J, Andersen MH, Hastrup H, Straten PT, Geertsen P (2003) Electrochemotherapy with low-dose IL-2 in the treatment of disseminated malignant melanoma: Clinical and paraclinical evidence of systemic immune response. Meet Proc ASCO 2003: 22 (abstract 2906) p 723 [Google Scholar]
- Haghnegahdar H, Du J, Wang D, Strieter RM, Burdick MD, Nanney LB, Cardwell N, Luan J, Shattuck-Brandt R, Richmond A (2000) The tumorigenic and angiogenic effects of MGSA/GRO proteins in melanoma. J Leukocyte Biol 67: 53–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanahan D, Weinberg RA (2000) The hallmarks of cancer. Cell 100: 57–70 [DOI] [PubMed] [Google Scholar]
- Keilholz U, Conradt C, Legha SS, Khayat D, Scheibenbogen C, Thatcher N, Goey SH, Gore M, Dorval T, Hancock B, Punt CJ, Dummer R, Avril MF, Brocker EB, Benhammouda A, Eggermont AM, Pritsch M (1998) Results of interleukin-2-based treatment in advanced melanoma: a case record-based analysis of 631 patients. J Clin Oncol 16: 2921–2929 [DOI] [PubMed] [Google Scholar]
- Keilholz U, Martus P, Punt CJ, Kruit W, Mooser G, Schadendorf D, Lienard D, Dummer R, Koller J, Voit C, Eggermont AM (2002) Prognostic factors for survival and factors associated with long-term remission in patients with advanced melanoma receiving cytokine-based treatments: second analysis of a randomised EORTC Melanoma Group trial comparing interferon-alpha2a (IFNalpha) and interleukin 2 (IL-2) with or without cisplatin. Eur J Cancer 38: 1501–1511 [DOI] [PubMed] [Google Scholar]
- Lin EY, Pollard JW (2004) Role of infiltrated leucocytes in tumour growth and spread. Br J Cancer 90: 2053–2058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manola J, Atkins M, Ibrahim J, Kirkwood J (2000) Prognostic factors in metastatic melanoma: a pooled analysis of Eastern Cooperative Oncology Group trials. J Clin Oncol 18: 3782–3793 [DOI] [PubMed] [Google Scholar]
- Moser B, Barella L, Mattei S, Schumacher C, Boulay F, Colombo MP, Baggiolini M (1993) Expression of transcripts for two interleukin 8 receptors in human phagocytes, lymphocytes and melanoma cells. Biochem J 294(Part 1): 285–292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negrier S, Escudier B, Gomez F, Douillard JY, Ravaud A, Chevreau C, Buclon M, Perol D, Lasset C (2002) Prognostic factors of survival and rapid progression in 782 patients with metastatic renal carcinomas treated by cytokines: a report from the Groupe Francais d’Immunotherapie. Ann Oncol 13: 1460–1468 [DOI] [PubMed] [Google Scholar]
- Norgauer J, Metzner B, Schraufstatter I (1996) Expression and growth-promoting function of the IL-8 receptor beta in human melanoma cells. J Immunol 156: 1132–1137 [PubMed] [Google Scholar]
- Pollard JW (2004) Tumour-educated macrophages promote tumour progression and metastasis. Nat Rev Cancer 4: 71–78 [DOI] [PubMed] [Google Scholar]
- Rosenberg SA, Yang JC, White DE, Steinberg SM (1998) Durability of complete responses in patients with metastatic cancer treated with high-dose interleukin-2: identification of the antigens mediating response. Ann Surg 228: 307–319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaider H, Oka M, Bogenrieder T, Nesbit M, Satyamoorthy K, Berking C, Matsushima K, Herlyn M (2003) Differential response of primary and metastatic melanomas to neutrophils attracted by IL-8. Int J Cancer 103: 335–343 [DOI] [PubMed] [Google Scholar]
- Scheibenbogen C, Mohler T, Haefele J, Hunstein W, Keilholz U (1995) Serum interleukin-8 (IL-8) is elevated in patients with metastatic melanoma and correlates with tumour load. Melanoma Res 5: 179–181 [DOI] [PubMed] [Google Scholar]
- Schmidt H, Geertsen PF, Fode K, Rytter C, Bastholt L, von der Maase H (2000) Subcutaneous interleukin-2 and interferon-alpha plus cisplatin with and without prophylactic cimetidine in patients with metastatic malignant melanoma: a phase II study. Melanoma Res 10: 66–77 [PubMed] [Google Scholar]
- Schmidt H, Larsen S, Bastholt L, Fode K, Rytter C, von der Maase H (2002) A phase II study of outpatient subcutaneous histamine dihydrochloride, interleukin-2 and interferon-alpha in patients with metastatic melanoma. Ann Oncol 13: 1919–1924 [DOI] [PubMed] [Google Scholar]
- Shamamian P, Schwartz JD, Pocock BJ, Monea S, Whiting D, Marcus SG, Mignatti P (2001) Activation of progelatinase A (MMP-2) by neutrophil elastase, cathepsin G, and proteinase-3: a role for inflammatory cells in tumor invasion and angiogenesis. J Cell Physiol 189: 197–206 [DOI] [PubMed] [Google Scholar]
- Sirott MN, Bajorin DF, Wong GY, Tao Y, Chapman PB, Templeton MA, Houghton AN (1993) Prognostic factors in patients with metastatic malignant melanoma. A multivariate analysis. Cancer 72: 3091–3098 [DOI] [PubMed] [Google Scholar]