Abstract
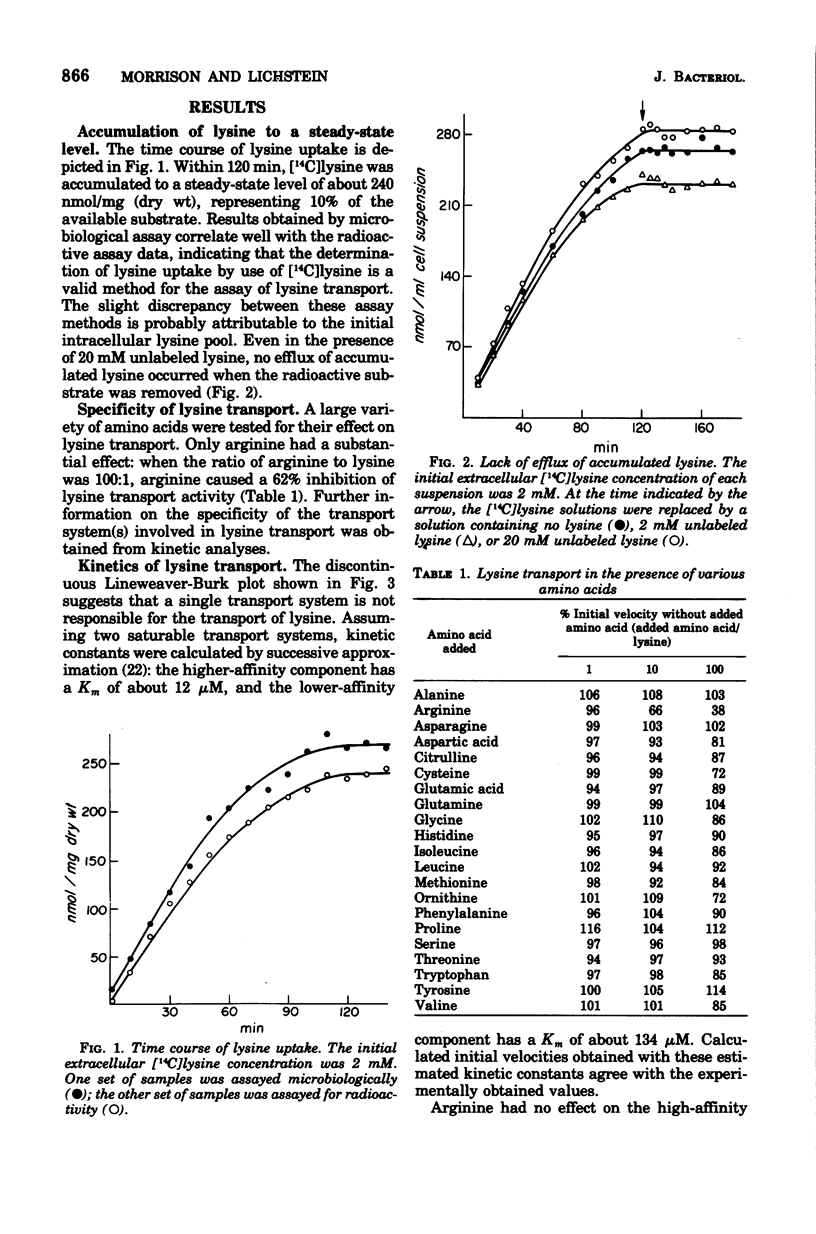
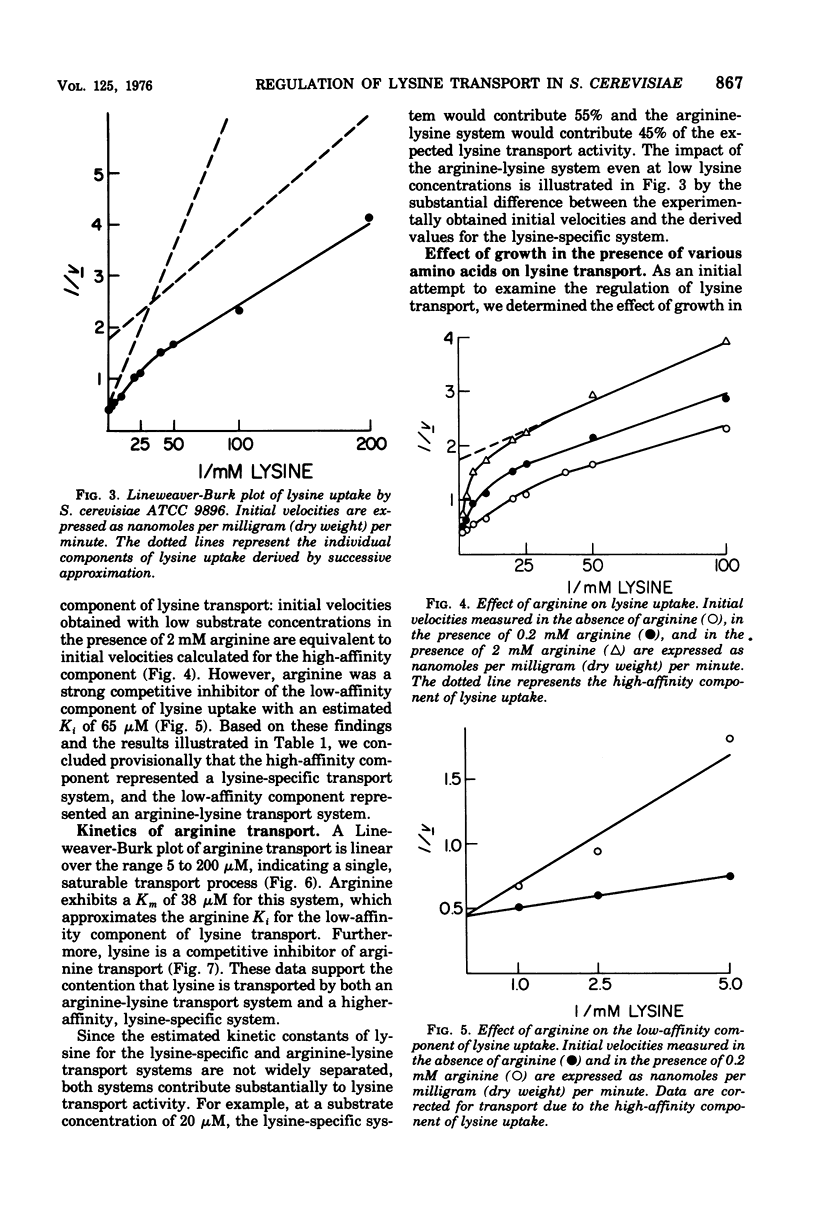
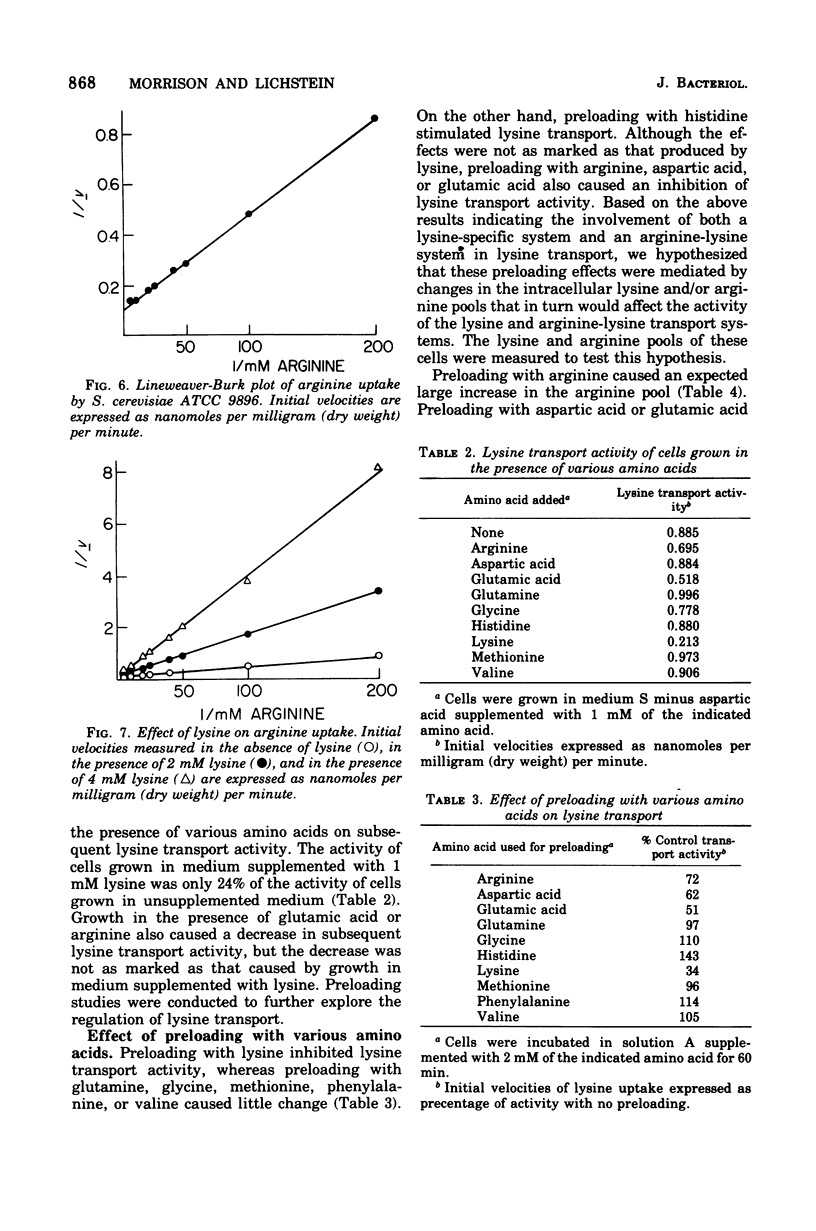
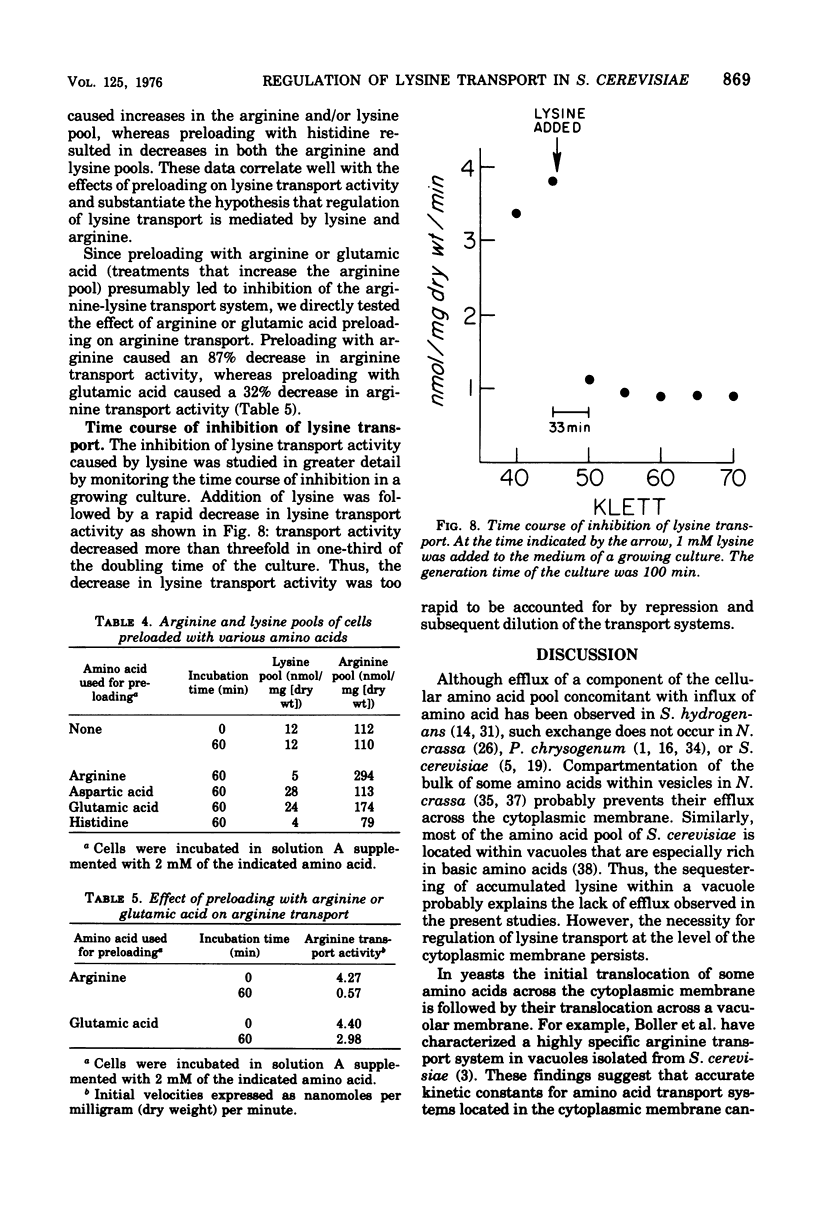
A steady-state level of about 240 nmol/mg (dry wt) occurs during lysine transport in Saccharomyces cerevisiae. No subsequent efflux of the accumulated amino acid was detected. Two transport systems mediate lysine transport, a high-affinity, lysine-specific system and an arginine-lysine system for which lysine exhibits a lower affinity. Preloading with lysine, arginine, glutamic acid, or aspartic acid inhibited lysine transport activity; preloading with glutamine, glycine, methionine, phenylalanine, or valine had little effect; however, preloading with histidine stimulated lysine transport activity. These preloading effects correlated with fluctuations in the intracellular lysine and/or arginine pool: lysine transport activity was inhibited when increases in the lysine and/or arginine pool occurred and was stimulated when decreases in the lysine and/or arginine pool occurred. After addition of lysine to a growing culture, lysine transport activity was inhibited more than threefold in one-third of the doubling time of the culture. These results indicate that the lysine-specific and arginine-lysine transport systems are regulated by feedback inhibition that may be mediated by intracellular lysine and arginine.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benko P. V., Wood T. C., Segel I. H. Multiplicity and regulation of amino acid transport in Penicillium chrysogenum. Arch Biochem Biophys. 1969 Feb;129(2):498–508. doi: 10.1016/0003-9861(69)90207-0. [DOI] [PubMed] [Google Scholar]
- Bhattacharjee J. K., Sinha A. K. Relationship among the genes, enzymes, and intermediates of the biosynthetic pathway of lysine in Saccharomyces. Mol Gen Genet. 1972;115(1):26–30. doi: 10.1007/BF00272214. [DOI] [PubMed] [Google Scholar]
- Boller T., Dürr M., Wiemken A. Characterization of a specific transport system for arginine in isolated yeast vacuoles. Eur J Biochem. 1975 May;54(1):81–91. doi: 10.1111/j.1432-1033.1975.tb04116.x. [DOI] [PubMed] [Google Scholar]
- Crabeel M., Grenson M. Regulation of histidine uptake by specific feedback inhibition of two histidine permeases in Saccharomyces cerevisiae. Eur J Biochem. 1970 May 1;14(1):197–204. doi: 10.1111/j.1432-1033.1970.tb00278.x. [DOI] [PubMed] [Google Scholar]
- Dubois E. L., Grenson M. Absence of involvement of glutamine synthetase and of NAD-linked glutamate dehydrogenase in the nitrogen catabolite repression of arginase and other enzymes in Saccharomyces cerevisiae. Biochem Biophys Res Commun. 1974 Sep 9;60(1):150–157. doi: 10.1016/0006-291x(74)90185-5. [DOI] [PubMed] [Google Scholar]
- Dubois E., Grenson M., Wiame J. M. Release of the "ammonia effect" on three catabolic enzymes by NADP-specific glutamate dehydrogenaseless mutations in Saccharomyces cerevisiae. Biochem Biophys Res Commun. 1973 Feb 20;50(4):967–972. doi: 10.1016/0006-291x(73)91500-3. [DOI] [PubMed] [Google Scholar]
- Dubois E., Grenson M., Wiame J. M. The participation of the anabolic glutamate dehydrogenase in the nitrogen catabolite repression of arginase in Saccharomyces cerevisiae. Eur J Biochem. 1974 Oct 2;48(2):603–616. doi: 10.1111/j.1432-1033.1974.tb03803.x. [DOI] [PubMed] [Google Scholar]
- Gits J. J., Grenson M. Multiplicity of the amino acid permeases in Saccharomyces cerevisiae. 3. Evidence for a specific methionine-transporting system. Biochim Biophys Acta. 1967 Jul 3;135(3):507–516. doi: 10.1016/0005-2736(67)90040-5. [DOI] [PubMed] [Google Scholar]
- Grenson M., Hou C. Ammonia inhibition of the general amino acid permease and its suppression in NADPH-specific glutamate dehydrogenaseless mutants of saccharomyces cerevisiae. Biochem Biophys Res Commun. 1972 Aug 21;48(4):749–756. doi: 10.1016/0006-291x(72)90670-5. [DOI] [PubMed] [Google Scholar]
- Grenson M., Hou C., Crabeel M. Multiplicity of the amino acid permeases in Saccharomyces cerevisiae. IV. Evidence for a general amino acid permease. J Bacteriol. 1970 Sep;103(3):770–777. doi: 10.1128/jb.103.3.770-777.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grenson M., Mousset M., Wiame J. M., Bechet J. Multiplicity of the amino acid permeases in Saccharomyces cerevisiae. I. Evidence for a specific arginine-transporting system. Biochim Biophys Acta. 1966 Oct 31;127(2):325–338. doi: 10.1016/0304-4165(66)90387-4. [DOI] [PubMed] [Google Scholar]
- Grenson M. Multiplicity of the amino acid permeases in Saccharomyces cerevisiae. II. Evidence for a specific lysine-transporting system. Biochim Biophys Acta. 1966 Oct 31;127(2):339–346. doi: 10.1016/0304-4165(66)90388-6. [DOI] [PubMed] [Google Scholar]
- Gross W., Burkhardt K. L. Multiple transport systems for basic amino acid transport in Streptomyces hydrogenans. Biochim Biophys Acta. 1973 Mar 16;298(2):437–445. doi: 10.1016/0005-2736(73)90371-4. [DOI] [PubMed] [Google Scholar]
- Hunter D. R., Segel I. H. Acidic and basic amino acid transport systems of Penicillium chrysogenum. Arch Biochem Biophys. 1971 May;144(1):168–183. doi: 10.1016/0003-9861(71)90466-8. [DOI] [PubMed] [Google Scholar]
- Hunter D. R., Segel I. H. Control of the general amino acid permease of Penicillium chrysogenum by transinhibition and turnover. Arch Biochem Biophys. 1973 Jan;154(1):387–399. doi: 10.1016/0003-9861(73)90071-4. [DOI] [PubMed] [Google Scholar]
- Hynes M. J. Alterations in the control of glutamate uptake in mutants of Aspergillus nidulans. Biochem Biophys Res Commun. 1973 Sep 18;54(2):685–689. doi: 10.1016/0006-291x(73)91477-0. [DOI] [PubMed] [Google Scholar]
- Jensen D. E., Neidhardt F. C. Effect of growth rate on histidine catabolism and histidase synthesis in Aerobacter aerogenes. J Bacteriol. 1969 Apr;98(1):131–142. doi: 10.1128/jb.98.1.131-142.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotyk A., Ríhová L. Transport of -aminoisobutyric acid in Saccharomyces cerevisiae. Biochim Biophys Acta. 1972 Nov 2;288(2):380–389. doi: 10.1016/0005-2736(72)90259-3. [DOI] [PubMed] [Google Scholar]
- Kuznar J., Schwencke J., Magaña-Schwencke N. Sarcosine and imino acid uptake in Saccharomyces chevalieri. Derepression by nitrogen starvation. Biochim Biophys Acta. 1973 Aug 22;318(2):273–280. doi: 10.1016/0005-2736(73)90120-x. [DOI] [PubMed] [Google Scholar]
- Murphy J. T., Spence K. D. Transport of S-adenosylmethionine in Saccharomyces cerevisiae. J Bacteriol. 1972 Feb;109(2):499–504. doi: 10.1128/jb.109.2.499-504.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pall M. L. Amino acid transport in Neurospora crassa. 3. Acidic amino acid transport. Biochim Biophys Acta. 1970 Sep 15;211(3):513–520. doi: 10.1016/0005-2736(70)90256-7. [DOI] [PubMed] [Google Scholar]
- Pall M. L. Amino acid transport in Neurospora crassa. I. Properties of two amino acid transport systems. Biochim Biophys Acta. 1969 Jan 28;173(1):113–127. doi: 10.1016/0005-2736(69)90042-x. [DOI] [PubMed] [Google Scholar]
- Pall M. L. Amino acid transport in Neurospora crassa. IV. Properties and regulation of a methionine transport system. Biochim Biophys Acta. 1971 Mar 9;233(1):201–214. doi: 10.1016/0005-2736(71)90372-5. [DOI] [PubMed] [Google Scholar]
- Pall M. L., Kelly K. A. Specificity of transinhibition of amino acid transport in neurospora. Biochem Biophys Res Commun. 1971 Mar 5;42(5):940–947. doi: 10.1016/0006-291x(71)90521-3. [DOI] [PubMed] [Google Scholar]
- Pateman J. A., Kinghorn J. R., Dunn E., Forbes E. Ammonium regulation in Aspergillus nidulans. J Bacteriol. 1973 Jun;114(3):943–950. doi: 10.1128/jb.114.3.943-950.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pateman J. A., Kinghorn J. R., Dunn E. Regulatory aspects of L-glutamate transport in Aspergillus nidulans. J Bacteriol. 1974 Aug;119(2):534–542. doi: 10.1128/jb.119.2.534-542.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos F., Thuriaux P., Wiame J. M., Bechet J. The participation of ornithine and citrulline in the regulation of arginine metabolism in Saccharomyces cerevisiae. Eur J Biochem. 1970 Jan;12(1):40–47. doi: 10.1111/j.1432-1033.1970.tb00818.x. [DOI] [PubMed] [Google Scholar]
- Ring K., Gross W., Heinz E. Negative feedback regulation of amino acid transport in Streptomyces hydrogenans. Arch Biochem Biophys. 1970 Mar;137(1):243–252. doi: 10.1016/0003-9861(70)90431-5. [DOI] [PubMed] [Google Scholar]
- Robinson J. H., Anthony C., Drabble W. T. Regulation of the acidic amino-acid permease of Aspergillus nidulans. J Gen Microbiol. 1973 Nov;79(1):65–80. doi: 10.1099/00221287-79-1-65. [DOI] [PubMed] [Google Scholar]
- Schwencke J., Magaña-Schwencke N. Derepression of a proline transport system in Saccharomyces chevalieri by nitrogen starvation. Biochim Biophys Acta. 1969 Mar 11;173(2):302–312. doi: 10.1016/0005-2736(69)90113-8. [DOI] [PubMed] [Google Scholar]
- Skye G. E., Segel I. H. Independent regulation of cysteine and cystine transport in Penicillium chrysogenum. Arch Biochem Biophys. 1970 May;138(1):306–318. doi: 10.1016/0003-9861(70)90311-5. [DOI] [PubMed] [Google Scholar]
- Subramanian K. N., Weiss R. L., Davis R. H. Use of external, biosynthetic, and organellar arginine by Neurospora. J Bacteriol. 1973 Jul;115(1):284–290. doi: 10.1128/jb.115.1.284-290.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VOGEL H. J., BONNER D. M. Acetylornithinase of Escherichia coli: partial purification and some properties. J Biol Chem. 1956 Jan;218(1):97–106. [PubMed] [Google Scholar]
- Weiss R. L. Intracellular localization of ornithine and arginine pools in Neurospora. J Biol Chem. 1973 Aug 10;248(15):5409–5413. [PubMed] [Google Scholar]
- Wiemken A., Dürr M. Characterization of amino acid pools in the vacuolar compartment of Saccharomyces cerevisiae. Arch Microbiol. 1974;101(1):45–57. doi: 10.1007/BF00455924. [DOI] [PubMed] [Google Scholar]


