Abstract
Graft-versus-host disease (GVHD) is a principal cause of morbidity following allogeneic hematopoietic cell transplantation (HCT). Standard therapy for GVHD, high-dose steroids, results in complete responses (CRs) in 35% of patients. Because tumor necrosis factor-α (TNFα) is an important effector of experimental GVHD, we treated patients with new-onset GVHD with steroids plus the TNFα inhibitor etanercept on a previously reported pilot trial (n = 20) and a phase 2 trial (n = 41). We compared their outcomes with those of contemporaneous patients with GVHD (n = 99) whose initial therapy was steroids alone. Groups were similar with respect to age, conditioning, donor, degree of HLA match, and severity of GVHD at onset. Patients treated with etanercept were more likely to achieve CR than were patients treated with steroids alone (69% vs 33%; P < .001). This difference was observed in HCT recipients of both related donors (79% vs 39%; P = .001) and unrelated donors (53% vs 26%; P < .001). Plasma TNFR1 levels, a biomarker for GVHD activity, were elevated at GVHD onset and decreased significantly only in patients with CR. We conclude that etanercept plus steroids as initial therapy for acute GVHD results in a substantial majority of CRs. This trial was referenced at www.clinicaltrials.gov as NCT00141713.
Introduction
Allogeneic hematopoietic cell transplantation (HCT) is a curative treatment for a number of hematologic malignancies and genetic disorders. Despite the routine use of immunosuppressive agents that target T cells, such as calcineurin inhibitors, up to 50% of HCT recipients still experience significant graft-versus-host disease (GVHD) that requires treatment with high-dose systemic steroids, which have been the primary therapy of GVHD for more than 25 years.1 The risk of mortality in patients who do not respond completely to initial therapy climbs exponentially,2,3 but complete response (CR) rates have remained approximately 35%.4–6
Animal models have established that the pathophysiology of GVHD involves complex immunologic interactions between cellular effectors, such as cytotoxic T lymphocytes and soluble effectors, such as inflammatory cytokines.7 One of the most important inflammatory cytokines involved in this process is tumor necrosis factor-α (TNFα), which mediates GVHD both through the amplification of donor immune response to host tissues as well as direct toxicity to target organs.8,9 These preclinical data served as the rationale to use an anti-TNFα agent, either infliximab or etanercept, to treat steroid-refractory GVHD, with complete remissions achieved in 18% to 62% of patients.10–13 Infliximab, a monoclonal antibody directed at TNFα, binds to both soluble and membrane-bound TNFα, resulting in clearance of both circulating TNFα and T cells.14 Etanercept, a soluble dimeric TNFα receptor 2, competes for TNFα binding and renders it inactive.14,15 This mechanism of action combined with its relative ease of administration by subcutaneous injection and generally minor side effects,15 make etanercept attractive as primary GVHD therapy. We therefore tested the hypothesis that etanercept plus systemic corticosteriods would result in CR rates of at least 50% when used as primary therapy for GVHD.
Methods
Study cohort
A total of 738 patients who underwent allogeneic HCT transplantation at the University of Michigan between April 1, 2000, and September 30, 2006, provided informed consent for the collection of blood and plasma samples during their HCT course. Blood samples were obtained at onset of GVHD and prior to initiation of systemic therapy and 4 weeks later in 160 (55%) of 291 patients who developed acute GVHD requiring treatment during this time period. Between September 2001 and September 2006, 61 of these patients provided additional informed consent and were enrolled in one of 2 prospective consecutive clinical trials to test the combination of etanercept (supplied by Amgen, Thousand Oaks, CA) and methylprednisolone as the initial treatment of GVHD during this time period. The first trial enrolled 20 patients to establish the safety of etanercept administration as part of primary GVHD therapy.16 A follow-up phase 2 clinical trial with identical eligibility criteria and identical treatment design enrolled an additional 41 patients. Based on the observed CR rate of 75% in the pilot trial,16 the follow-up study was designed to provide 80% power that the true CR rate was at least 50% when results from both trials were combined. The clinical trials were approved and reviewed by the University of Michigan Institutional Review Board (IRB) and the University of Michigan Cancer Center Data and Safety Monitoring Board. Eligibility criteria included diagnosis of new onset acute GVHD, clinical grades 2 to 4, that was confirmed on biopsy and treated for less than 72 hours with 2 mg/kg/day methylprednisolone prior to first dose of etanercept; an absolute neutrophil count greater than 500/mm3 for at least 3 days; absence of uncontrolled infection; and absence of inotropic agents for blood pressure support. The group of contemporaneous patients (99 of 160) who had blood samples obtained at onset of GVHD and 4 weeks later, whose initial GVHD therapy was steroids alone and who did not participate in an etanercept trial, served as a control group for the study subjects. Patients who did not have blood samples obtained prior to initiation of steroids for treatment of GVHD were not considered as control patients. To be a control patient, the patient must also have met the eligibility critieria for participation on an etanercept treatment trial. The reasons for lack of participation in 99 patients include the absence of an IRB-approved trial at the time of onset of GVHD (n = 49), patient/physician refusal (n = 23), and more than 72 hours of steroid therapy for GVHD (n = 22) and simultaneous infection (n = 5). An additional 191 patients who underwent transplantation between April 2000 and September 2006 who never developed GVHD and who had blood samples drawn at the appropriate time after HCT form a non-GVHD control population.
GVHD treatment
GVHD prophylaxis for all recipients included tacrolimus (titrated to a goal level of 8 to 12 ng/mL). After myeloablative HCT, patients also received minidose methotrexate (5 mg/m2 per dose intravenously on days 1, 3, 6, and 11). After reduced-intensity HCT, patients also received mycophenolate mofetil (500 mg/m2 per dose every 8 hours) from days 0 to 28. All patients who were treated for GVHD received methylprednisolone 2 mg/kg per day and continued their GVHD prophylaxis agents at therapeutic dosing. Generally, steroids continued at the starting dose for the first 7 days and were then tapered as tolerated in patients with CRs. A CR was defined as the resolution of all manifestations of GVHD (all organs stage 0). Second-line agents were added for progression of GVHD, for lack of response after 5 to 7 days, or for slow responses as deemed necessary by the treating physician. A flare of GVHD was defined as achievement of CR with later worsening of GVHD by at least one clinical grade that required additional systemic therapy. Acute and chronic GVHD was staged and graded according to published GVHD scales.17
The primary endpoint of the 2 etanercept trials was the complete response rate at 4 weeks. Patients who required additional therapy for GVHD in the first 4 weeks were considered treatment failures for the primary endpoint. None of these patients ever achieved a CR. Patients who died before day 28 regardless of GVHD status at the time of death were also regarded as treatment failures. A total of 20 patients (4 patients treated with etanercept plus steroids and 16 patients treated with steroids alone) had improvement in their GVHD scores by day 28 but not a CR (and therefore primary endpoint failures), but went on to a CR after day 28. In 5 of 20 patients (all in the steroids-alone group), additional therapy was begun after day 28, and these CRs were included in analyses other than the primary endpoint analysis. No other patient receiving additional GVHD therapy achieved a CR.
Etanercept was given subcutaneously twice weekly for 8 weeks at a dose of 0.4 mg/kg per dose (maximum dose, 25 mg) based on historic data from our center that CRs can occur up to 8 weeks following initiation of systemic treatment. Doses were held and not replaced until resolution of bacteremia, hemodynamic instability, or fever above 100.5°F for more than 5 days. A total of 15 patients had one or 2 doses held for fever or for a positive blood culture. Etanercept was permanently discontinued in patients whose GVHD required additional systemic therapy in the first 28 days of treatment; these patients were categorized as nonresponders. Etanercept was also permanently discontinued in 5 patients who missed more than 2 doses of etanercept. A total of 4 patients, all evaluable for the primary endpoint, were removed from the study after day 28 because of disease relapse or progression. All patients who enrolled in the study were included in the analyses even if they did not complete study treatment.
Infection prophylaxis consisted of norfloxacin 400 mg twice daily and fluconazole 100 mg daily. Infection prophylaxis changed in 2005. The last 36 patients treated with etanercept plus steroids and the last 48 patients treated with steroids alone received levofloxacin 500 mg daily instead of norfloxacin and voriconazole 200 mg twice daily instead of fluconazole. IgG levels were monitored monthly beginning on day 100 and intravenous immunoglobulin 400 mg/kg replacement therapy was given for IgG levels lower than 4.0 g/L (400 mg/dL). Patients received sulfamethoxazole/trimethoprim or pentamadine for PCP prophylaxis starting on day 30. Acyclovir 400 mg twice daily was given for herpes simplex and varicella-zoster prophylaxis in seropositive patients. Cytomegalovirus DNA was monitored weekly by quantitative polymerase chain reaction (PCR), and preemptive therapy with antiviral agents were begun in the event of a positive assay.
Cytokine analysis
Blood obtained at the time of onset of GVHD and 4 weeks later was separated into cellular and plasma components and frozen for later batch processing. The plasma component of each blood sample was analyzed for soluble TNF receptor 1 (sTNFR1) levels by the Immunologic Monitoring Core Laboratory of the University of Michigan Cancer Center using cytokine enzyme-linked immunosorbent assay (R&D Systems, Minneapolis, MN). The assays were performed according to the manufacturer's protocol, and all samples and standards were run in duplicate.
Statistical analysis
Comparisons of patient characteristics between treatment groups were based upon a 2-sample t test for continuous variables and a chi-squared test of association for categoric variables. Comparison of infection and 4-week CR rates between treatment groups were based upon a chi-squared test of association. Mean and median steroid dose at 4 weeks were compared by 2-sample t test and nonparametric tests, respectively. Time to complete response was estimated using standard competing risks methodology.18 The significance of differences in mean TNFR1 levels was based upon a paired t test. Effects of potential factors such as patient age, transplantation conditioning regimen (myeloablative vs reduced intensity), degree of HLA match, donor source (related vs unrelated), and post-HCT day of onset of GVHD on CR at 4 weeks were examined in a Cox proportional-hazards model. All reported P values are 2-sided.
Results
Patient characteristics
The characteristics of patients without GVHD (n = 191), patients with GVHD treated with steroids alone (n = 99), and patients with GVHD who were treated with etanercept plus steroids (n = 61) are provided in Table 1. Patients in the 2 treatment groups were similar with respect to age, transplantation conditioning intensity (myeloablative vs reduced intensity), donor type (related vs unrelated), degree of match (matched vs mismatched), and severity of organ involvement of GVHD at onset. There were no statistically significant differences between patients treated on the pilot trial and the follow-up phase 2 trial (Table S1, available on the Blood website; see the Supplemental Materials link at the top of the online article).
Table 1.
Patient characteristics according to GVHD treatment group and for non-GVHD controls
| Non-GVHD controls n = 191 | Steroids alone n = 99 | Etanercept plus steroids n = 61 | P for difference between 2 treatment groups | |
|---|---|---|---|---|
| Median age, y (range) | 44 (1-69) | 49 (1-71) | 51 (7-65) | .77 |
| Patients < 18 y, % | 15 | 9 | 10 | .88 |
| Myeloablative conditioning, % | 77 | 68 | 61 | .37 |
| Unrelated donor, % | 32 | 46 | 31 | .06 |
| Mismatched, % | 6 | 16 | 11 | .60 |
| Advanced malignancy, %* | 22 | 25 | 31 | .42 |
| Nonmalignant disease, % | 6 | 1 | 0 | .43 |
| GVHD at start of treatment, % | ||||
| Grade 2 | — | 69 | 67 | .84 |
| Grades 3-4 | — | 31 | 33 | |
| Skin | — | 69 | 61 | .60 |
| Skin only target organ | 49 | 34 | .08 | |
| Liver | — | 15 | 15 | .47 |
| GI tract | — | 44 | 61 | .16 |
| Upper GI only target organ | — | 5 | 3 | .60 |
GI indicates gastrointestinal; and —, not applicable.
Chronic myelogenous leukemia in chronic phase, myelodysplastic syndrome, multiple myeloma, acute leukemia in CR, and lymphoma in partial response (PR) or CR were considered nonadvanced disease for the purposes of analysis. Leukemia or lymphoma in untreated relapse or refractory to therapy were considered advanced disease for the purposes of analysis.
Clinical responses
The primary endpoint of the clinical trial was CR (resolution of all GVHD manifestations) at 4 weeks after the initiation of treatment. Patients treated with etanercept plus steroids were significantly more likely to achieve CR 4 weeks later than were patients treated with steroids alone (69% vs 33%; P < .001; Figure 1A). The benefit of etanercept persisted so that by 12 weeks after initiation of GVHD treatment, 77% of patients treated with etanercept plus steroids had achieved CR compared with 50% of patients treated with steroids alone (P < .001). Differences in steroid dosing did not account for differences in the response rate at day 28 because the mean steroid dose on day 28 in patients treated with etanercept plus steroids was 0.9 mg/kg (median, 0.7 mg/kg; range, 0.05-2 mg/kg per day), similar to the 1 mg/kg (median, 0.9 mg/kg; range, 0-2 mg/kg per day) in patients initially treated with steroids alone. We performed a univariate analysis comparing CR rates according to conditioning regimen (myeloablative vs reduced intensity), a factor known to influence GVHD.19 Conditioning regimen did not affect CR rates in patients treated with etanercept plus steroids (Table S3). In multivariate analyses, the only 2 variables associated with increased likelihood of CR were the use of etanercept and a related donor stem-cell source. Patient age, conditioning regimen, degree of HLA match, and the day of onset of GVHD all had no statistically significant association with response. The CR rates in the 9 patients who received less than 8 doses of etanercept by day 28 (primary endpoint) were not statistically different from the remaining patients (P = .33).
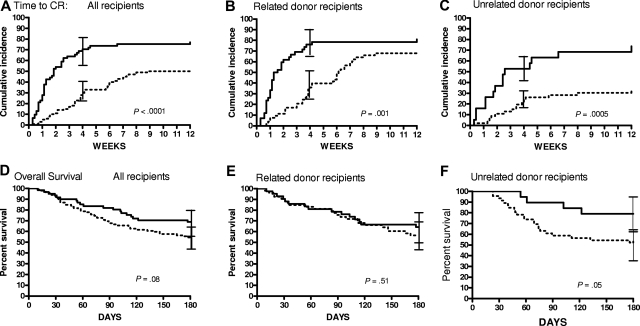
Figure 1.
Time to CR for patients with GVHD treated with steroids alone or etanercept plus steroids. (A) Time to CR for all patients with GVHD treated with steroids alone (n = 99; dotted line) or etanercept plus steroids (n = 61; solid line). (B) Time to CR for patients who underwent related-donor HCT treated with steroids alone (n = 53) or with etanercept plus steroids (n = 42). (C) Time to CR for patients who underwent unrelated-donor HCT treated with steroids alone (n = 46) or with etanercept plus steroids (n = 19). The 95% confidence intervals for CR rate at 4 weeks are shown as error bars in panels A to C. Overall survival curves through 6 months from initiation of GVHD treatment by treatment group for all patients (D), patients who underwent related-donor HCT (E), and patients who underwent unrelated-donor HCT (F). The 95% confidence intervals for survival at 6 months are shown as error bars in panels D to F.
All patients without CR to initial treatment were treated with additional agents, most commonly mycophenolate, at a median of 18 days (range, 3-47 days) from initiation of treatment. None of these patients achieved a CR by day 28. The higher response rate seen in patients treated with etanercept plus steroids translated into improved survival at 100 days from initiation of GVHD treatment (etanercept plus steroids, 82% vs steroids alone, 66%; P = .04). At 6 months from initiation of treatment, a higher proportion of patients treated with etanercept plus steroids were still alive 69%, compared with 55% of patients treated with steroids alone, but this difference did not meet the criteria for statistical significance (P = .08; Figure 1D).
Because of the trend toward an increased proportion of unrelated donor transplantations in the group treated with steroids alone, we analyzed the results by stem cell source. The superiority of etanercept was evident in transplant recipients from both related donors (79% vs 39%; P = .001; Figure 1B) and unrelated donors (53% vs 26%; P < .001; Figure 1C). This latter difference is particularly noteworthy, because studies have shown that acute GVHD in recipients of unrelated donor transplants is more difficult to treat than GVHD in recipients of related donor transplants.4 As can be seen in the time to CR curves, different response patterns were observed between recipients of related and unrelated donors. The time to CR was significantly faster in recipients of related donor transplants who were treated with etanercept plus steroids, but by 12 weeks, nearly equivalent proportions of patients in both groups achieved a CR (etanercept plus steroids, 80%; steroids alone, 70%). It is therefore not surprising that survival 6 months from initiation of GVHD treatment was similar in both treatment groups (Figure 1E). Recipients of unrelated donor transplants who failed to achieve a CR by day 28 were likely to never achieve CR (Figure 1C). The superior efficacy of etanercept plus steroids in this group thus translated into a survival advantage 6 months later (P = .05; Figure 1F).
Superior CR rates for each GVHD target organ (skin, liver, gastrointestinal tract) were observed in patients 4 weeks following treatment with etanercept plus steroids (Table 2). Furthermore, patients treated with etanercept plus steroids were twice as likely to achieve CR whether the grade of GVHD at presentation was grade 2 (75% vs 37%) or grades 3 to 4 (50% vs 26%). There were no statistically significant differences in CR rates between patients treated on the pilot trial and the follow-up phase 2 trial (Table S2). Importantly, most patients who achieved a CR remained in remission: Only 10% (4 of 41) of responding patients treated with etanercept plus steroids experienced a flare of GVHD. This rate of recurrence is similar to that in patients treated with steroids alone (12%; 4 of 33 patients). Chronic GVHD developed in 57% of patients treated with etanercept plus steroids (extensive: 53%), which was not statistically different from the 56% incidence of chronic GVHD (extensive: 53%) observed in patients treated with steroids alone (Figure S1).
Table 2.
CR rates at 4 weeks according to treatment group
| Steroids alone | Etanercept plus steroids | P | |
|---|---|---|---|
| Overall, no. (%) | 33/99 (33) | 42/61 (69) | < .001 |
| 95% CI | 24, 42 | 57, 80 | |
| Skin | 32/68 (47) | 30/37 (81) | < .001 |
| 95% CI | 35, 59 | 68, 94 | |
| Liver | 3/15 (20) | 6/9 (67) | .03 |
| 95% CI | 0, 40 | 36, 98 | |
| GI | 21/44 (48) | 29/37 (78) | .005 |
| 95% CI | 33, 63 | 65, 92 |
We also examined relapse rates by the overall incidence of relapse in patients who underwent transplantation for malignancy without adjustment or censoring. Although the rate of relapse in patients treated with etanercept plus steroids (10 of 61; 16%) was lower than that observed in patients treated with steroids alone (21 of 98; 21%), the difference was not statistically significant (P = .44).
Infections
The infection rates in the first 100 days from initiation of GVHD treatment were not different between patients treated with etanercept plus steroids or treated with steroids alone for bacterial, invasive fungal or viral infections (Table 3). There were no mycobacterial infections observed in any patients.
Table 3.
Infection rates according to treatment group
| Steroids alone (n = 99) | Etanercept plus steroids (n = 61) | P | |
|---|---|---|---|
| Bacterial, no. (%) | |||
| Gram-positive | 84 (85) | 49 (80) | .46 |
| Gram-negative | 22 (22) | 13 (21) | .89 |
| Anaerobic | 4 (4) | 2 (3) | .79 |
| Fungal, no. (%) | 19 (19) | 17 (28) | .20 |
| Viral, no. (%) | 33 (33) | 19 (31) | .78 |
Plasma levels of TNFR1 as a biomarker for GVHD response to treatment
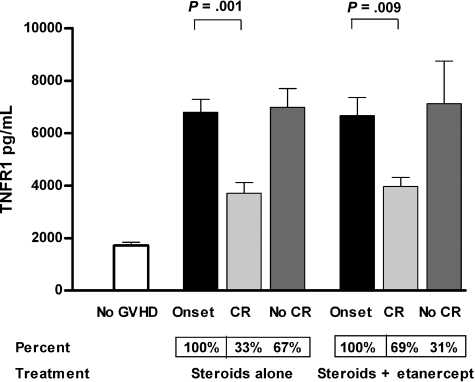
TNFR1 levels in the plasma are reproducibly measured, correlate with levels of TNFα,20 and are elevated in patients who develop GVHD.21 Plasma TNFR1 levels in patients at the onset of GVHD were more than 3 times higher than in patients without GVHD (Figure 2; P < .001). As expected, TNFR1 levels at the onset of GVHD were equivalent in both treatment groups of patients. In the group who received steroids alone, TNFR1 levels fell by more than 50% toward baseline after 4 weeks of treatment in the 33% of patients who achieved a CR, but did not change in the remaining 67% of patients (P = .001). Results were strikingly similar in the group who received etanercept plus steroids: TNFR1 levels fell significantly in the 69% of patients achieving a CR after 4 weeks of therapy, but did not change in the 31% who still had active disease (P = .009).
Figure 2.
Mean TNFR1 plasma levels at initiation of GVHD treatment and 4 weeks later. Mean TNFR1 plasma levels were significantly lower in patients without GVHD (□; n = 190) at time points similar to the onset of GVHD (■) compared with patients treated with steroids alone (n = 99) and etanercept plus steroids (n = 61; P < .001). At 4 weeks after initiation of treatment, mean TNFR1 plasma levels in patients in CR ( ) were significantly lower than at initiation of treatment both in patients receiving steroids alone (33 of 99; 33%) and patients receiving etanercept plus steroids (42 of 61; 69%). Mean TNFR1 plasma levels (
) were significantly lower than at initiation of treatment both in patients receiving steroids alone (33 of 99; 33%) and patients receiving etanercept plus steroids (42 of 61; 69%). Mean TNFR1 plasma levels ( ) were unchanged in patients not in CR in both patients treated with steroids alone (66 of 99; 67%) and patients treated with etanercept plus steroids (19 of 61; 31%). Error bars are mean plus or minus SEM.
) were unchanged in patients not in CR in both patients treated with steroids alone (66 of 99; 67%) and patients treated with etanercept plus steroids (19 of 61; 31%). Error bars are mean plus or minus SEM.
Discussion
Current standards for the treatment of acute GVHD rely primarily on steroids alone as initial therapy, and reserve additional agents for steroid refractory disease. Previous studies in steroid refractory GVHD have shown that anti-TNFα agents have significant efficacy, but most patients still die from GVHD or its complications.10–13 Our results show that the combination of etanercept plus steroids as initial treatment for GVHD results in significantly better CR rates 4 weeks later compared with steroids alone. These CRs were durable and could not be explained by differences in the steroid dose at 4 weeks. Of note, etanercept plus steroids improved the outcome for recipients of both related donor and unrelated donor transplants, which translated into significantly improved survival at 6 months for unrelated donor transplant recipients. The improvements in outcome were realized without an increased incidence of serious infections, chronic GVHD, or relapse. These comparisons are based on nonrandomized patient populations, and factors other than treatment with etanercept may have contributed to the observed results. Although the similarity in outcomes for the patients treated with steroids alone are very similar to published results from other centers,4–6 a prospective multicenter randomized study will be necessary to confirm these encouraging results.
Animal studies have demonstrated that TNFα plays a critical role in both the gastrointestinal tract22,23 and the skin,24 but its role in the liver is more controversial.8,22 In our study, the addition of etanercept to steroids resulted in high response rates in all 3 target organs, suggesting that TNFα is involved in the clinical pathophysiology of all 3. Moreover, our data confirm mechanistic studies of GVHD pathophysiology in animal models that have delineated both TNFα-dependent and TNFα-independent pathways of disease,8 because TNFα inhibition increases response rates but does not completely eliminate GVHD. All patients who received etanercept also received steroids, and therefore the relative importance of these pathways in clinical GVHD remains to be determined in future studies.
One potential concern regarding the use of TNF inhibitors to treat GVHD is an increased risk of infections, particularly mycobacterial and fungal infections.25 Invasive Aspergillus infections have been associated with use of the anti-TNFα monoclonal antibody infliximab for treatment of GVHD in 2 retrospective studies involving a total of 32 patients.11,26 Although the overall incidence of infection was significant, as would be expected in patients with GVHD receiving high-dose steroids, we did not observe any significant difference in infection rates, including fungal infections, in patients also treated with etanercept. The discrepancy between the 2 studies may be due to potential differences in the mechanism of action of the 2 drugs: infliximab can induce systemic elimination and clearance of monocytes and macrophages that express membrane-bound TNFα, whereas etanercept does not.27 In addition, the recent availability of more effective prophylactic agents against a broad range of fungal species may have contained the overall infectious risk. We also did not observe increased morbidity and mortality from etanercept in patients who developed Gram-positive infections, which differs from prior reports in other disease settings.28
Elevated TNFR1 plasma levels correlated with active GVHD both before and after treatment. TNFR1 levels in patients achieving CR was the same in both treatment groups, although the proportion of patients achieving CR in the etanercept group was significantly higher (Figures 1,2). These data raise the intriguing possibility that clinical GVHD manifestations occur above a certain critical threshold of TNFR1 levels. Reduction below that threshold, by any means, induces a CR; etanercept plus steroids was able to achieve that reduction twice as often as steroids alone. Interestingly, TNFR1 levels remained high in patients whose GVHD did not remit despite TNF inhibition. It is possible the TNFα in these patients was not completely neutralized, and that higher doses of etanercept than used in our study might be beneficial for some of these patients. We do not favor this explanation because analysis of a limited number of patients treated with etanercept demonstrated circulating TNFR2 levels at least 10-fold higher than physiologic levels (data not shown). An alternative explanation for the persistently high TNFR1 levels in patients with persistent GVHD is that some cellular effectors continue to express membrane-bound TNFα and shed TNFR1 despite the quenching of soluble TNFα by etanercept. This explanation is supported by a recent animal study demonstrating that although soluble TNFα is responsible for a major portion of GVHD morbidity and mortality, donor cells that express membrane-bound TNFα still cause significant clinical GVHD.29
The number of unrelated donor transplantations continues to increase annually, and GVHD following unrelated donor HCT is more prevalent, more severe, and less responsive to treatment compared with related donor HCT. In this study, the post-GVHD survival for unrelated donor hematopoietic cell transplant recipients treated with etanercept and steroids was similar to that of related donor HCT recipients, suggesting that treatment with etanercept and steroids may be particularly useful in the unrelated donor HCT setting.
Supplementary Material
Acknowledgments
We thank the patients, their families, and the clinical personnel who participated in this study; Jennifer Lay-Luskin and the BMT Program Team at the Clinical Trials Office at the University of Michigan for outstanding data collection and management; and the BMT Program research nurses for research support, without which this study would not have been possible.
This work was supported by grants from the Food and Drug Administration (5R01FD002397-03-2), the National Institutes of Health (2P01CA039542-18 and 5P30CA046592), the Doris Duke Charitable Foundation, and Amgen. J.L.M.F. is the recipient of a Doris Duke Distinguished Clinical Scientist Award.
Footnotes
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: J.E.L., T.B., R.J.H., and J.L.M.F. designed and planned the study. T.B. was the study statistician. S.P. performed the cytokine assays. S.P., S.W.C., D.J., and C.K. were in charge of data collection and quality assurance. All authors participated in writing the report.
Conflict-of-interest disclosure: J.E.L. reports research grant support from Amgen. J.L.M.F. reports research grant and symposium support from Amgen. The other authors declare no competing financial interests.
Correspondence: John E. Levine, 1500 E Medical Center Dr, 5303 Cancer Center, Ann Arbor, MI, 48109-0941; e-mail: jelevine@umich.edu.
References
- 1.Chao NJ, Chen BJ. Prophylaxis and treatment of acute graft-versus-host disease. Semin Hematol. 2006;43:32–41. doi: 10.1053/j.seminhematol.2005.09.007. [DOI] [PubMed] [Google Scholar]
- 2.Leisenring WM, Martin PJ, Petersdorf EW, et al. An acute graft-versus-host disease activity index to predict survival after hematopoietic cell transplantation with myeloablative conditioning regimens. Blood. 2006;108:749–755. doi: 10.1182/blood-2006-01-0254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Van Lint MT, Milone G, Leotta S, et al. Treatment of acute graft-versus-host disease with prednisolone: significant survival advantage for day +5 responders and no advantage for nonresponders receiving anti-thymocyte globulin. Blood. 2006;107:4177–4181. doi: 10.1182/blood-2005-12-4851. [DOI] [PubMed] [Google Scholar]
- 4.MacMillan ML, Weisdorf DJ, Wagner JE, et al. Response of 443 patients to steroids as primary therapy for acute graft-versus-host disease: comparison of grading systems. Biol Blood Marrow Transplant. 2002;8:387–394. doi: 10.1053/bbmt.2002.v8.pm12171485. [DOI] [PubMed] [Google Scholar]
- 5.Martin PJ, Schoch G, Fisher L, et al. A retrospective analysis of therapy for acute graft-versus-host disease: initial treatment. Blood. 1990;76:1464–1472. [PubMed] [Google Scholar]
- 6.Weisdorf D, Haake R, Blazar B, et al. Treatment of moderate/severe acute graft-versus-host disease after allogeneic bone marrow transplantation: an analysis of clinical risk features and outcome. Blood. 1990;75:1024–1030. [PubMed] [Google Scholar]
- 7.Reddy P, Ferrara JL. Immunobiology of acute graft-versus-host disease. Blood Rev. 2003;17:187–194. doi: 10.1016/s0268-960x(03)00009-2. [DOI] [PubMed] [Google Scholar]
- 8.Teshima T, Ordemann R, Reddy P, et al. Acute graft-versus-host disease does not require alloantigen expression on host epithelium. Nat Med. 2002;8:575–581. doi: 10.1038/nm0602-575. [DOI] [PubMed] [Google Scholar]
- 9.Korngold R, Marini JC, de Baca ME, Murphy GF, Giles-Komar J. Role of tumor necrosis factor-alpha in graft-versus-host disease and graft-versus-leukemia responses. Biol Blood Marrow Transplant. 2003;9:292–303. doi: 10.1016/s1083-8791(03)00087-9. [DOI] [PubMed] [Google Scholar]
- 10.Jacobsohn DA, Hallick J, Anders V, McMillan S, Morris L, Vogelsang GB. Infliximab for steroid-refractory acute GVHD: a case series. Am J Hematol. 2003;74:119–124. doi: 10.1002/ajh.10392. [DOI] [PubMed] [Google Scholar]
- 11.Couriel D, Saliba R, Hicks K, et al. Tumor necrosis factor-alpha blockade for the treatment of acute GVHD. Blood. 2004;104:649–654. doi: 10.1182/blood-2003-12-4241. [DOI] [PubMed] [Google Scholar]
- 12.Busca A, Locatelli F, Marmont F, Ceretto C, Falda M. Recombinant human soluble tumor necrosis factor receptor fusion protein as treatment for steroid refractory graft-versus-host disease following allogeneic hematopoietic stem cell transplantation. Am J Hematol. 2007;82:45–52. doi: 10.1002/ajh.20752. [DOI] [PubMed] [Google Scholar]
- 13.Patriarca F, Sperotto A, Damiani D, et al. Infliximab treatment for steroid-refractory acute graft-versus-host disease. Haematologica. 2004;89:1352–1359. [PubMed] [Google Scholar]
- 14.Sieper J, Van Den Brande J. Diverse effects of infliximab and etanercept on T lymphocytes. Semin Arthritis Rheum. 2005;34:23–27. doi: 10.1016/j.semarthrit.2005.01.006. [DOI] [PubMed] [Google Scholar]
- 15.Dhillon S, Lyseng-Williamson KA, Scott LJ. Etanercept: a review of its use in the management of rheumatoid arthritis. Drugs. 2007;67:1211–1241. doi: 10.2165/00003495-200767080-00011. [DOI] [PubMed] [Google Scholar]
- 16.Uberti JP, Ayash L, Ratanatharathorn V, et al. Pilot trial on the use of etanercept and methylprednisolone as primary treatment for acute graft-versus-host disease. Biol Blood Marrow Transplant. 2005;11:680–687. doi: 10.1016/j.bbmt.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 17.Sullivan KM. Graft-vs.-host disease. In: Blume KG, Forman SJ, Appelbaum FR, editors. Thomas' Hematopoietic Cell Transplantation. 3rd ed. Malden, MA: Blackwell; 2004. pp. 635–664. [Google Scholar]
- 18.Gooley TA, Leisenring W, Crowley J, Storer BE. Estimation of failure probabilities in the presence of competing risks: new representations of old estimators. Stat Med. 1999;18:695–706. doi: 10.1002/(sici)1097-0258(19990330)18:6<695::aid-sim60>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 19.Levine JE, Uberti JP, Ayash L, et al. Lowered-intensity preparative regimen for allogeneic stem cell transplantation delays acute graft-versus-host disease but does not improve outcome for advanced hematologic malignancy. Biol Blood Marrow Transplant. 2003;9:189–197. doi: 10.1053/bbmt.2003.50012. [DOI] [PubMed] [Google Scholar]
- 20.Hintermeier-Knabe R, Brockhaus M, Holler E, et al. Sequential release of tumor necrosis factor alpha and tumor necrosis factor receptors in complications of human bone marrow transplantation. In: Freund M, Link H, Schmidt RE, Welte K, editors. Cytokines in Hemopoiesis, Oncology, and AIDS, II. Berlin: Springer-Verlag; 1992. pp. 443–448. [Google Scholar]
- 21.Or R, Kalinkovich A, Nagler A, et al. Soluble tumor necrosis factor (sTNF) receptors: a possible prognostic marker for bone marrow transplantation-related complications. Cytokines Mol Ther. 1996;2:243–250. [PubMed] [Google Scholar]
- 22.Hattori K, Hirano T, Miyajima H, et al. Differential effects of anti-Fas ligand and anti-tumor necrosis factor alpha antibodies on acute graft-versus-host disease pathologies. Blood. 1998;91:4051–4055. [PubMed] [Google Scholar]
- 23.Brown GR, Lee EL, Thiele DL. TNF enhances CD4+ T cell alloproliferation, IFN-gamma responses, and intestinal graft-versus-host disease by IL-12-independent mechanisms. J Immunol. 2003;170:5082–5088. doi: 10.4049/jimmunol.170.10.5082. [DOI] [PubMed] [Google Scholar]
- 24.Piguet PF, Grau GE, Allet B, Vassalli P. Tumor necrosis factor/cachectin is an effector of skin and gut lesions of the acute phase of graft-vs.-host disease. J Exp Med. 1987;166:1280–1289. doi: 10.1084/jem.166.5.1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Giles JT, Bathon JM. Serious infections associated with anticytokine therapies in the rheumatic diseases. J Intensive Care Med. 2004;19:320–334. doi: 10.1177/0885066604267854. [DOI] [PubMed] [Google Scholar]
- 26.Marty FM, Lee SJ, Fahey MM, et al. Infliximab use in patients with severe graft-versus-host disease and other emerging risk factors of non-Candida invasive fungal infections in allogeneic hematopoietic stem cell transplant recipients: a cohort study. Blood. 2003;102:2768–2776. doi: 10.1182/blood-2003-01-0267. [DOI] [PubMed] [Google Scholar]
- 27.Ehlers S. Tumor necrosis factor and its blockade in granulomatous infections: differential modes of action of infliximab and etanercept? Clin Infect Dis. 2005;41:S199–S203. doi: 10.1086/429998. [DOI] [PubMed] [Google Scholar]
- 28.Fisher CJ, Jr, Agosti JM, Opal SM, et al. Treatment of septic shock with the tumor necrosis factor receptor:Fc fusion protein: the Soluble TNF Receptor Sepsis Study Group. N Engl J Med. 1996;334:1697–1702. doi: 10.1056/NEJM199606273342603. [DOI] [PubMed] [Google Scholar]
- 29.Borsotti C, Franklin ARK, Lu SX, et al. Absence of donor T-cell–derived soluble TNF decreases graft-versus-host-disease without impairing graft-versus-tumor activity. Blood. 2007;110:783–786. doi: 10.1182/blood-2006-10-054510. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.