Abstract
Integrin β1 is both overexpressed and in an ‘active’ conformation in vulval squamous cell carcinomas (VSCCs) compared to matched normal skin. To investigate the significance of integrin β1 deregulation we stably knocked-down integrin β1 expression in the VSCC cell line A431. In vitro analysis revealed that integrin β1 is required for cell adhesion, cell spreading and invasion. However, integrin β1 is not required for cell growth or activation of FAK and ERK signalling in vitro or in vivo. Strikingly, while control tumours were able to invade the dermis, integrin β1 knockdown tumours were significantly more encapsulated and less invasive.
Keywords: integrin β1, vulval squamous cell carcinoma, invasion
Interactions between keratinocytes and the extracellular matrix (ECM) are crucial for epidermal tissue architecture, cell proliferation and cell survival (Hynes, 2002). The integrin family ECM receptors are key mediators of these interactions and are frequently deregulated in skin disorders (Watt, 2002) and neoplasias (Parise et al, 2000). Integrin-mediated cell adhesion is required for cell motility and also affects cell proliferation and survival in many systems (Schwartz and Assoian, 2001). Integrins function as membrane spanning heterodimers consisting of an α subunit and a β subunit. To date, 18 α and eight β subunits have been identified and these can associate in numerous different combinations with different ligand specificities (Hynes, 2002). Integrin β1 can bind to various ECM components depending on its α subunit partner: the heterodimers α2β1, α3β1 and α5β1 bind preferentially to collagen, laminin and fibronectin, respectively. The intracellular tail of integrin β1 is linked to the actin cytoskeleton via association with proteins such as talin, α-actinin and vinculin (Brakebusch and Fassler, 2003). Integrin β1 is crucial for cell motility in a number of contexts; it is required for efficient keratinocyte wound healing in vivo (Grose et al, 2002) and for the collective movement of tumour cells (Hegerfeldt et al, 2002). Integrins also indirectly recruit a range of signalling molecules including EGFR, FAK and Src-family kinases (Giancotti and Ruoslahti, 1999; Yamada and Even-Ram, 2002). Activation of these signalling molecules following integrin engagement can promote the activation of the small GTPase Ras leading to increased and sustained ERK/Map kinase signalling, which may in turn affect cell proliferation (Renshaw et al, 1996; Roovers et al, 1999).
In normal skin integrin β1 expression is restricted to the basal layer of stem and transit amplifying cells. Loss of integrin β1 in the skin leads to blistering, failure in basement membrane organisation and hemidesmosome instability (Raghavan et al, 2000). In contrast, forced expression of integrin β1 in the suprabasal layer of the epidermis antagonises cell differentiation and cell cycle exit (Carroll et al, 1995), possibly by promoting ERK/MAP kinase activity (Zhu et al, 1999). Consistent with a role in promoting proliferation, integrin β1 is mutated in an oral squamous cell carcinoma cell line (Evans et al, 2003) and its expression is increased in upper aerodigestive tract (Van Waes et al, 1995) and cervical SCC (Hughes et al, 1994). Contrastingly, other studies have reported reduced expression in oral SCC (Jones et al, 1993).
Vulval squamous cell carcinoma (VSCC) has an incidence of 2/100 000 per year in industrialised countries. Five-year survival drops from 90% for those with FIGO Stage I disease at presentation to 18% for women with Stage IV. Death from VSCC is caused by tumour invasion into local tissues resulting either directly in haemorhage, sepsis or uraemia from bilateral ureteric obstruction or indirectly in inanition. There are two distinct patterns of VSCC invasion into surrounding tissue: a ‘pushing’ pattern displays distinct tumour–stroma boundaries, whereas a ‘spray’ pattern of invasion is characterised by ill-defined tumour–stroma boundaries with small clusters of invading cells and correlates with poor prognosis (Heaps et al, 1990; Qureshi et al, 1999). Understanding the molecular mechanisms of VSCC invasion will aid developing strategies to combat this life-threatening aspect of the disease. In this study, we have analysed the expression of integrin β1 and its major α subunit partners in matched VSCC and normal skin samples. Integrin β1 is more highly expressed in VSCC; to address the functional significance of this change we have abrogated integrin β1 expression in the VSCC cell line A431. This approach has allowed the role of integrin β1 in tumour to be analysed in vivo. We find that integrin β1 is not required for proliferation of VSCC cells in vivo but is important for invasive tumour margins.
MATERIALS AND METHODS
Cell culture, transfection and tumour experiments
A431 cells were obtained from the ATCC and routinely maintained in 10% FCS DMEM. Cells were transfected using Lipofectamine 2000 in accordance with the manufacturer's instructions: briefly, subconfluent cells were transferred into Optimem-1 for transfection and incubated with a mixture containing 0.7 μg of DNA and 4 μl of Lipofectamine 2000 for 5 h. The plasmid to reduce integrin β1 expression contained the sequence GGAACAGCAGAGAAGCTCATTCAAGAGATGAGCTTCTCTG CTGTTCCTTTTT subcloned immediately downstream of the human RNA polymerase III H1 promoter bases −245 to −1; this sequence was subcloned into the EGFP-C1 (Clontech) backbone cut with AseI and HpaI to remove the entire CMV-GFP expression cassette. An equivalent empty vector was used as a control. Stably transfected clones were selected with 600 μg ml−1 of G418. One million cells were injected subcutaneously into the flank of female MF1 nude mice. Tumour size was recorded at regular intervals and the tumours were analysed when they had reached an average diameter of 1 cm.
Analysis of tumour samples
Following approval by the Committee for Clinical Research and the Research Ethics Committee of the Royal Marsden NHS Trust tumour samples were collected from 14 consenting women.
Preparation of frozen sections
Specimens were mounted onto cork discs with tissue-tek OCT compound (Sakura; 4583), and frozen in either liquid nitrogen or dry ice. 7 or 8 μm sections were cut and mounted onto electrostatic slides (Surgipath Superfrost ‘Plus’ white; 08143G). The slides were allowed to air dry and then fixed in 4% formaldehyde/PBS for 10 min followed by permeabilisation in 0.2% Triton X-100/PBS for 5 min. Samples were incubated overnight at 4C with primary antibodies diluted 1 : 100 in PBS/3% BSA/0.1% Tween before washing in PBS and incubation for 1 h at 37C with secondary antibodies diluted 1 : 100 in PBS/3% BSA/0.1% Tween. The antibodies used were as follows: anti-Pan integrin β1 (P5D2 – Santa Cruz sc13590), antiactive β1-integrin: (9EG7 – BD pharmingen 550531) and (HUTS-21 – BD pharmingen 556048), blocking β1-integrin (AIIB2) was a gift from F Watt, anti-integrin α2 (Sigma I6403), anti-integrin α3 (Santa Cruz sc-13545), anti-integrin α5 (Santa Cruz sc-13547), anti-integrin α6 (Santa Cruz sc-13542), anti-integrin αV (Santa Cruz sc-9969), antikeratin 14 (Covance PRB-155P), antilaminin B2 (Upstate 05-206), antiphosphoY118 paxillin (Biosource 44-722), antivinculin (Sigma V-9131). Images were captured using a Biorad MRC1024 confocal microscope; to enable comparison of tumour and normal tissue images were acquired using the same settings. Quantification of the fluorescence was performed using Image J, the mean pixel intensity was compared between the normal epidermis (basal and differentiating cells were analysed together) and the tumour sample from each patient. Haematoxylin and eosin staining was carried out using standard procedures.
Preparation of lysates
Specimens were snap frozen in liquid nitrogen then crushed with a pestle and mortar surrounded by liquid nitrogen to prevent defrosting. The powdered sample was transferred into an eppendorf tube and 1 ml of lysate buffer added (20 mM TrisCl, 40 mM Na pyrophosphate, 50 mM NaF, 5 mM MgCl2, 100 μM Na vanadate, 10 mM EGTA, 1% Triton X-100, 0.5% NaDeoxycholate and protease inhibitors). The sample was repeatedly passed through a 21G needle and centrifuged at 16 000 g for 5 min. The supernatant was analysed by Western blotting. The antibodies used were as follows: phospho-ERK1/2 (Sigma M8159), ERK1/2 (Transduction labs 554093), phospho-FAK (Affinity BioReagents OPA1-03071), FAK (Upstate Biotech 05-537).
Flow cytometry
Subconfluent cells from a 6-cm dish were detached using cell dissociation buffer (Gibco/BRL 13150-016) before centrifugation at 250 g. They were then resuspended in 200 μl of PBS+1 μg ml−1 P5D2. After incubation on ice for 1 h, cells were washed repeatedly by centrifugation at 250 g followed by resuspension in PBS. Cells were then incubated for 30 min on ice in 200 μl of PBS+FITC anti-mouse antibodies (Jackson Stratech) before washing with PBS and analysis using a Becton Dickinson flow cytometer.
Immune-fluorescence
Cells were fixed using 4% formaldehyde diluted in PBS for 15 min before permeabilisation with 0.2% Triton X-100 diluted in PBS. Samples were subsequently treated as described above for frozen tissue sections.
Cell adhesion, spreading and invasion assays
Adhesion assays were performed using the cytomatrix screen kit (Chemicon International) according to their protocol. Cells that had been detached using Cell Dissociation Buffer (Gibco/BRL 13150-016) were allowed to attach to either collagen I, collagen IV, or fibronectin for 10 min before washing to remove nonadherent cells followed by fixation of adherent cells. The adherent cells were stained with crystal violet and quantified using a plate reader. AIIB2 was incubated with cells at 0.05 mg ml−1 for 10 min prior to the start of the adhesion assay. Cell spreading was determined by re-plating cells that had been detached using Cell Dissociation Buffer on either Collagen I or Fibronectin coated dishes in 1% FCS (BD BioCoat Variety Pack 354431) and recording their behaviour by time-lapse microscopy. Images were captured every 2 min and scored manually for the proportion of cells with a spread morphology. For Western blot analysis, cells were handled as described for the cell spreading assay; cells were lysed directly by the addition of SDS–PAGE loading buffer to the dish. The lysate was then transferred to an eppendorf tube and sonicated to disrupt DNA.
Inverse cell invasion assays were performed as previously described (Hennigan et al, 1994) using Transwell dishes with 8 μm pores (Costar 3422) and Matrigel (BD Bioscience 354234) diluted 1 : 1 with DMEM. Cells were fixed and stained with phalloidin and propidium iodide. A confocal microscope was used to identify cells that had migrated through the 8 μm pores and at least 10 μm into the Matrigel: the percentage of invasive cells (calculated relative to the total number of cells in the z stack) was determined for at least three fields per experiment.
RESULTS
Increased integrin β1 expression in VSCC
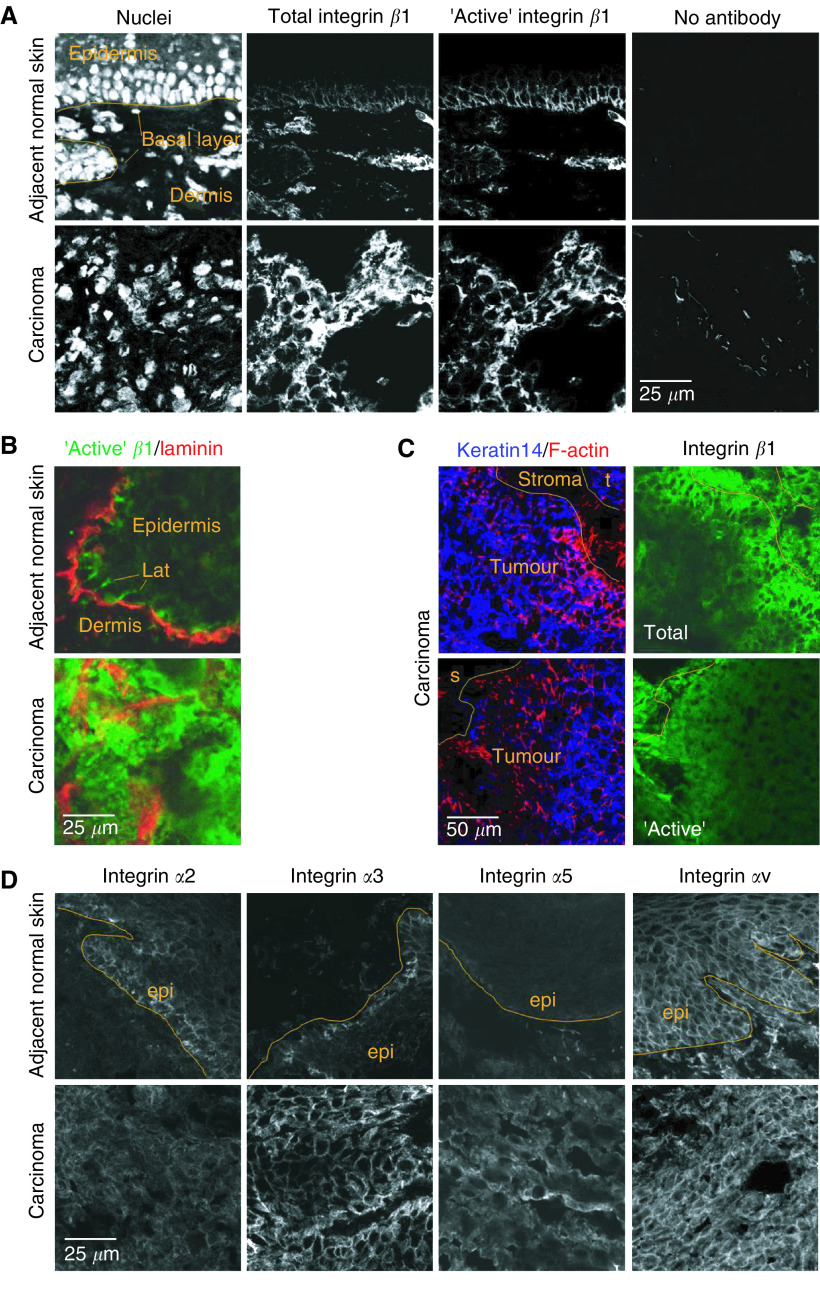
VSCC and normal vulval skin tissue pairs were obtained from 14 women. Multichannel immunofluorescence on cryostat sections was used to compare integrin β1 expression between VSCC and normal vulval skin from the same patient. Three different monoclonal antibodies were used to detect integrin β1: P5D2 recognises integrin β1 regardless of its conformation, whereas 9EG7 and HUTS21 only recognise integrin β1 that is either competent to bind ligand or bound to ligand – this is often considered to reflect integrin β1 in its active conformation (Lenter et al, 1993; Bazzoni et al, 1995; Luque et al, 1996). Staining with P5D2 revealed that in normal vulval epidermis integrin β1 is expressed in the basal layer of cells in contact with the basement membrane and a small number of cells in the suprabasal layer (Figure 1A). Furthermore, staining with 9EG7 revealed that integrin β1 is in its ligand-bound conformation in these cells (Figure 1A). Integrin β1 staining with P5D2 and 9EG7 antibodies increased in the tumours of 11 out of 12 and nine out of 12 cases, respectively (Table 1). This reflected both an increased proportion of cells staining and increased intensity of staining (Figure 1A). Furthermore, the localization of integrin β1 was no longer restricted to basal and lateral membranes. Staining with a second conformation-specific antibody, HUTS21, and gave similar results to 9EG7 confirming that there are increased levels of ligand-bound or ‘active’ integrin β1 in VSCC compared to matched normal skin (Figure 1B). Co-staining for laminin revealed that HUTS21 recognises integrin β1 at both basal and lateral membranes (Figure 1B – also similar pattern in Figure 1A). These data suggest that integrin β1 is binding to nonbasement membrane ligands along the lateral membranes in vulval epithelia. Integrin β1 expression was also detected in the dermis. Control analysis confirmed that the staining observed was dependent upon the primary antibody and that secondary antibodies did not crossreact (Figure 1A and data not shown).
Figure 1.
Altered integrin expression in vulval squamous cell carcinoma. (A) Panels show three channel fluorescence images of DNA (left), total integrin β1-P5D2 (left-mid), and ‘active’ integrin β1-9EG7 (right-mid) staining in normal and tumour tissue from the same patient. Orange line indicates the boundary between the epidermis and dermis in normal tissue. Normal and tumour tissue stained with no primary antibody (right). (B) Panels show fluorescence images of ‘active’ integrin β1-HUTS21 staining (green) and laminin B2 (red) in normal and tumour tissue from the same patient. ‘lat’ indicates lateral membrane staining. (C) Panels show three channel fluorescence images of F-actin (red in left panels), keratin 14 (blue in left panels), integrin β1-P5D2 (upper right panel) staining, and ‘active’ integrin β1-HUTS21 (lower right panel). Orange line indicates boundary between the tumour and stroma. (D) Panels show fluorescence images of integrin α2 (left panels), integrin α3 (left-mid panels), integrin α5 (right-mid panels) and integrin αV (right panels) in normal and tumour tissue (pairs of samples are from the same patient). Orange line indicates the boundary between the epidermis and dermis in normal tissue.
Table 1. Changes in integrin expression in VSCC.
|
Change in staining (tumour vs normal tissue)
|
|||||
|---|---|---|---|---|---|
| Integrin | <0.66 | 0.66–1.5 | 1.5–5 | 5–20 | >20 |
| β1 (P5D2) | — | 1 | 3 | 3 | 5 |
| β1 (9EG7) | — | 3 | 1 | 3 | 5 |
| β1 (HUTS21)a | — | 1 | 1 | 3 | — |
| α2 | 1 | 2 | 5 | 3 | 1 |
| α3 | — | — | 6 | 4 | 2 |
| α4b | 2 | 2 | 3 | 1 | 2 |
| α5 | — | 2 | 7 | — | 3 |
| αV | 1 | 2 | 6 | 3 | — |
| α6 | 1 | 1 | 4 | 3 | 1 |
Paired normal vulval skin and VSCC samples were analysed for integrin expression by immunohistochemical methods. The amount of fluorescence was quantified from images taken using a confocal microscope. The table shows the fold change in integrin staining in the tumour relative to the normal epidermis from the same patient. Changes of 0.66–1.5 are regarded as no change.
Only five samples were stained with HUTS21.
Two samples had no detectable integrin α4 staining.
All three integrin β1 antibodies stained the tumour sections somewhat heterogeneously; detailed examination of the staining pattern suggested that integrin β1 staining was greatest in regions close to stromal cells. To confirm this we performed three-channel immunofluorescence to simultaneously stain for integrin β1 and markers that would help identify the tumour boundary. We used a combination of keratin 14 staining, which is commonly expressed in keratinocyte-derived neoplasias, and phalloidin, which strongly stains cortical F-actin in tumour cells, to delineate the tumour boundary. This was compared with either P5D2 or HUTS21 staining in the same section. The tumour/stroma interface is highlighted in Figure 1C, both total and active integrin β1 staining is increased in the cells at the tumour/stroma boundary.
Integrin β1 can associate with many different α subunits. Previous studies have suggested that the main α subunits expressed in epidermal tissue are α2, α3, α5, α6 and αV (Hertle et al, 1991; Hodivala et al, 1994); we therefore examined if the expression of these α subunits changed between the normal and tumour tissue. In normal skin, integrin α2 and α3 were expressed in the basal layer of cells and localised to the lateral membranes. Integrin α2 and α3 staining was increased in nine out of 12 and 12 out of 12 tumour samples, respectively (Figure 1D and Table 1). Integrin αV was expressed throughout the normal epidermis and its expression increased modestly in the tumour tissue (Figure 1D and Table 1); this differs from cervical epidermis where integrin αV distribution is restricted to the basal layer of cells (Hughes et al, 1994) and may be unique to vulval epidermis. The expression of integrin α4 was somewhat variable between samples. Integrin α5 and α6 staining was present in the basal layer of normal epidermis and was increased in most tumour samples, although not as dramatically as integrin β1 (Figure 1D and Table 1).
Generation of integrin β1 knockdown VSCC cell lines
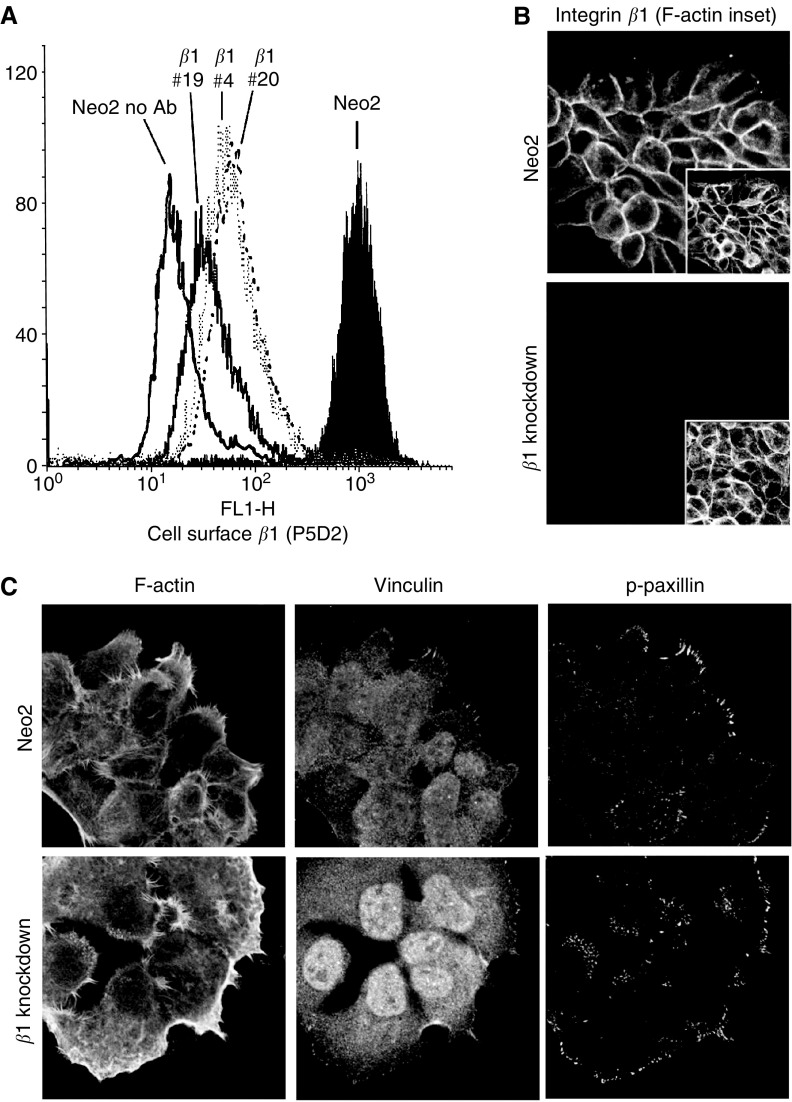
To investigate the functional significance of integrin β1 overexpression in VSCC we sought to knockdown integrin β1 expression in an appropriate cell line. A431 cells are derived from a VSCC and are amenable to standard cell culture techniques. To reduce integrin β1 levels we cloned an inverted repeat of an siRNA sequence that is capable of reducing the expression of all integrin β1 isoforms (Vial et al, 2003) downstream of the RNA polymerase III H1 promoter (Brummelkamp et al, 2002; Hannon, 2002). This construct or an empty vector control was transfected into A431 cells and G418-resistant clones were selected and screened for integrin β1 expression by using immunofluorescence. Six of the 28 G418 clones screened had no detectable integrin β1 expression in immunofluorescence assays. These clones were further screened by flow cytometry for a more quantitative analysis of integrin β1 expression. Figure 2A shows the integrin β1 expression in three clones that were chosen for further analysis compared to a control pool of cells stably transfected with the empty vector. All three clones had homogeneous profiles of integrin β1 expression; clones 4 and 20 had a roughly 18-fold reduction in expression and clone 19 a 50-fold reduction. Flow cytometry analyses the levels of integrin β1 at the cell surface, to exclude the possibility that these clones expressed intracellular integrin β1 we performed confocal microscopy analysis. In control cells integrin β1 was localised at the cell periphery, while in clones 4, 19 and 20 no staining was detected either at the cell surface or in the cytoplasm (Figure 2B and data not shown). These results demonstrate the generation of three-independent A431 cell lines with dramatically reduced integrin β1 expression.
Figure 2.
Analysis of integrin β1 knockdown cell lines. (A) Flow cytometry analysis of integrin β1 expression. Control and integrin β1 knockdown clones were stained using P5D2 anti-integrin β1 antibody or no antibody and 10 000 cells analysed by flow cytometry. (B) Control and integrin β1 knockdown cells (clone 20 shown) were stained with P5D2 antiintegrin β1 antibody (green) and phalloidin to visualise F-actin (red). (C) Control and integrin β1 knockdown cells (clone 4 shown) were stained with phalloidin to visualise F-actin (left) and vinculin (middle) and phospho-Y118 paxillin (right) to visualise focal adhesions.
Previous studies have shown that knocking out integrin β1 affects the expression of other integrin subunits (Brakebusch et al, 1999; Guan et al, 2001); we therefore tested if expression of other integrin subunits was altered in the integrin β1 knockdown clones we generated. Quantitative immune-fluorescence was used to determine the relative levels of integrin expression in control and integrin β1 knockdown cells. No significant difference in the expression of integrins β3, β4, α2 and α6 was seen between control and the knockdown cell lines (Table 2 and data not shown). However, we noted dramatic reductions in the expression of integrins α3 and α5 in all three integrin β1 knockdown clones and a modest increase in integrin αV expression. Therefore, loss of integrin β1 expression affects the levels of other integrin subunits within the cell.
Table 2. Changes in integrin expression in integrin β1 knockdown cell lines.
| Integrin | Change in expression (knockdown cells relative to control) |
|---|---|
| α2 | 0.80 (±0.61) |
| α3 | 0.01 (±0.0) |
| α5 | 0.12 (±0.14) |
| αV | 3.80 (±1.97) |
| α6 | 1.12 (±0.46) |
The expression of integrin α-subunits relative to control cells was analysed in integrin β1 knockdown cell lines by quantitative immune-fluorescence. The results for the three knockdown cell lines (#4, #19 & #20) were averaged to produce the numbers shown above – standard deviation is shown in parentheses.
Integrin β1 is required for cell adhesion, spreading and invasion
Since integrin β1 is a mediator of cell attachment to the extracellular matrix at focal adhesions we investigated if cells lacking integrin β1 retained focal adhesions. Figure 2C shows that both control and integrin β1 knockdown cells had peripheral vinculin and phospho-paxillin staining characteristic of focal adhesions. Interestingly, these often extended perpendicular to the cell border in control cells but were less extended in the integrin β1 knockdown cells.
Previous work has shown that integrin β1 is required for cell adhesion and migration; to determine if reduced integrin β1 expression affects the behaviour of VSCC cells we investigated the ability of these cells to adhere to different matrices. Cells were allowed to adhere for 10 min before washing and quantification of the number of attached cells. Figure 3A shows that reduced integrin β1 expression prevents efficient cell adhesion to collagen I and collagen IV, but not fibronectin (at longer timepoints the differences between the control and knockdown cell lines became much less pronounced, data not shown). In contrast, interference with integrin β1 function using the blocking antibody AIIB2 prevented adhesion to all three substrates (Werb et al, 1989). While adhesion to fibroncetin did not appear to be compromised by β1 knockdown, spreading was compromised (see below).
Figure 3.
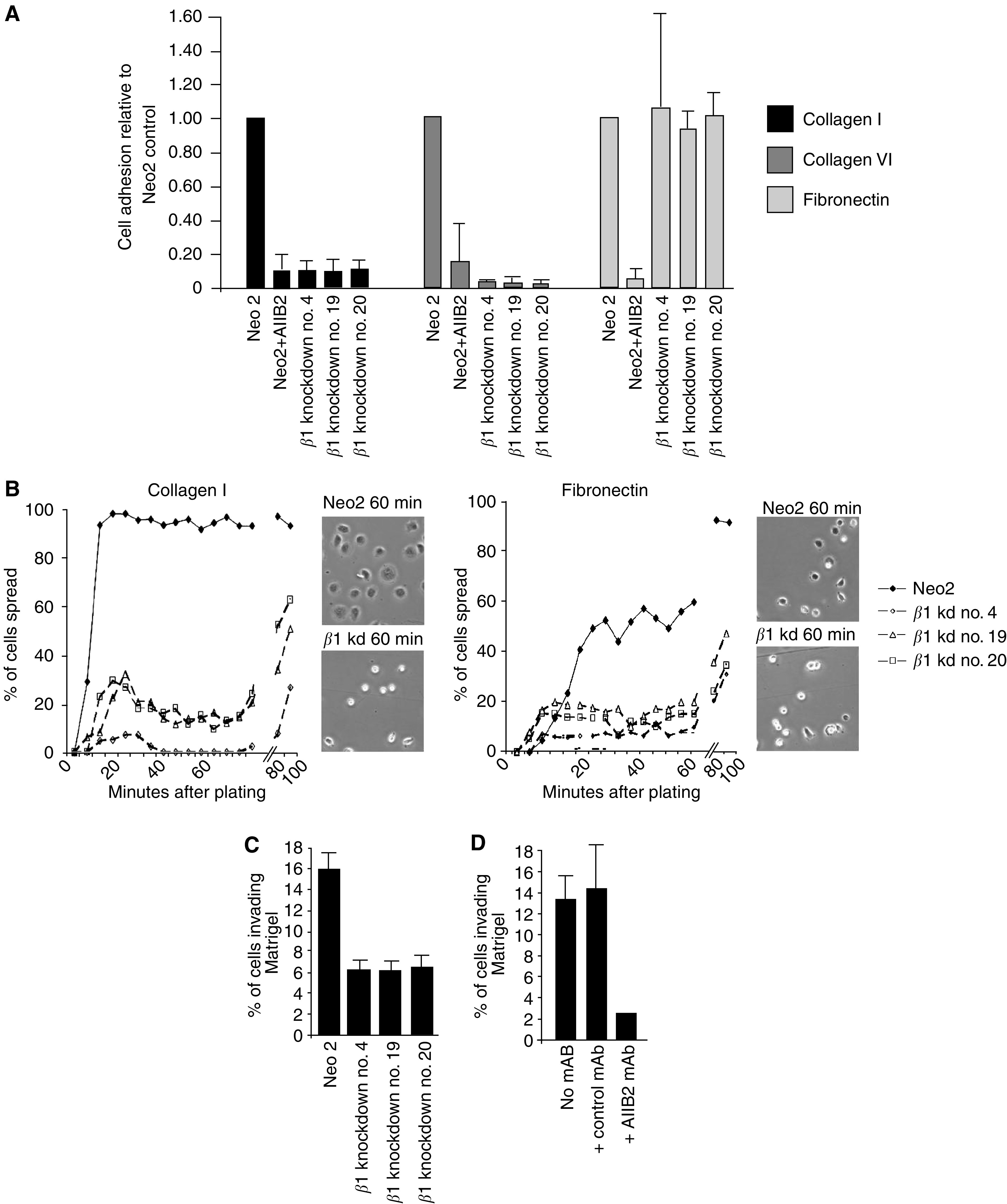
Integrin β1 is required for cell adhesion, spreading and invasion. (A) Control A431 cells with or without integrin β1 blocking antibody AIIB2 and integrin β1 knockdown cells were assayed for their ability to adhere to collagen I, IV or fibronectin using Chemicon cytomatrix screen kits. Results are average of two experiments, error bars indicate the standard deviation. (B) Control A431 cells and integrin β1 knockdown cells were replated on either collagen I or fibronectin and monitored by time-lapse microscopy. The proportion of cells with a spread morphology (an adherent area of at least 400 μm2) was scored at 4-min intervals by analysing the time-lapse videos, one representative experiment of three is shown. Inset panels show phase contrast images of the cells at the indicated times. (C) Control A431 cells and integrin β1 knockdown cells were assayed for their ability to invade Matrigel. The proportion of cells invading at least 10 μm was scored in three 20 × fields and averaged: one representative experiment of three is shown.
Following the initial event of cell adhesion, adherent cells flatten and adopt a ‘spread’ morphology. It is believed that integrin-dependent activation of signalling molecules is required for the spreading process (Defilippi et al, 1999). We next investigated whether integrin β1 is required for cell spreading. Cells in suspension were added to plates coated with either collagen I or fibronectin and their morphology was monitored using time-lapse videomicroscopy. Control A431 cells changed from a rounded morphology to a flat spread morphology within 12 min of plating on collagen I coated dishes (Figure 3B, left). In contrast, in all three integrin β1 knockdown clones, the majority of cells were not spread at this time and even after 100 min only between 25 and 60% of the cells were spread. Both control and integrin β1 knockdown cells began to spread at a similar rate on fibronectin coated plates (Figure 3B right); these data are consistent their similar abilities to adhere to fibronectin (Figure 3A). However, integrin β1 knockdown cells failed to continue spreading 12–16 min after plating while the proportion of spread control cells increased further (Figure 3B, right). Similar results were obtained using the integrin β1 function blocking antibody AIIB2 (data not shown). These data demonstrate that integrin β1 is required for the ability of A431 cells to adhere and spread on collagen I and to spread efficiently on fibronectin.
We next tested if integrin β1 is required for cells to invade into a three-dimensional matrix. For this study we used Matrigel, which consists of laminin and collagen IV. This in vitro assay aims to recapitulate the process of tumour cells traversing basement membranes (Hennigan et al, 1994). Cells were seeded on a filter that separated them from a thick layer of Matrigel containing growth factors, after 3 days the number of cells that had crossed the filter and invaded 10 μm or more into the Matrigel were scored. In total, 16% of control vector expressing cells invaded the Matrigel while approximately 6% of integrin β1 knockdown cells were invasive (Figure 3C). To confirm that integrin β1 is required for A431 cells to efficiently invade Matrigel we used the antibody AIIB2 to block integrin β1 function. Figure 3D shows that treatment of A431 cells with AIIB2 inhibited the number of A431 cells invading into Matrigel, while a control antibody had no effect. These data demonstrate the importance of integrin β1 for VSCC cells to effectively invade a three-dimensional matrix.
Integrin β1 is not required for cell signalling or proliferation in adherent cells
Integrin-dependent cell attachment and spreading is accompanied by the activation of many signal transduction pathways; in particular focal adhesion kinase (FAK) and ERK/MAP kinase are activated. Given the defects in adhesion and spreading observed in the integrin β1 knockdown cells (described above), we tested if activation of FAK and ERK is compromised in these cells. To promote integrin-dependent signalling we detached cells and replated them onto collagen I; cell lysates were made from cells either immediately prior to replating or 20, 60 or 120 min after replating (we were unable to analyse samples at earlier timepoints because the integrin β1 knockdown cells were not sufficiently adherent to collect). Phoshorylation of FAK on tyrosine 397 correlates with its activity (Hanks et al, 2003); we therefore used an antibody that recognises phosho-Y397 to determine the activity of FAK. Figure 4A shows that FAK activity was slightly higher in control cells compared to integrin β1 knockdown cells both prior to and after replating; similar results were obtained using different integrin β1 knockdown clones (data not shown). We also investigated if ERK1/2 activity was altered by monitoring the phosphorylation of sites that are required for and correlate with ERK1/2 activity (Her et al, 1993; Marshall, 1994). In contrast to FAK activity, ERK1&2 activity was increased to similar levels after replating in integrin β1 knockdown cells. However, before replating ERK1/2 activity was consistently higher in control cells (compare 0 timepoints in Figure 4B). Similar results were obtained when cells were replated onto fibronectin-coated dishes (data not shown). These data demonstrate that although integrin β1 is required for cell spreading it is not required for ERK1/2 activation and makes only a small contribution to FAK activation after replating cells on collagen I.
Figure 4.
Integrin β1 is not required for proliferation or activation of FAK or ERK. (A) Control A431 cells and integrin β1 knockdown cells were detached and replated onto collagen I. Cell lysates were made at the times indicated (0 is detached cells immediately prior to plating) and analysed for FAK activity by Western blotting. Upper panel shows phospho-FAK Y397 immunoblot, and lower panel shows total FAK immunoblot – numbers are quantification of phospho-FAK divided by quantification of total FAK normalised to control cells at time 0. (B) As for ‘(A)’ except lysates were analysed for ERK activity by Western blotting. Upper panel shows phospho-ERK1/2 T283 and Y285 immunoblot, and lower panel shows total ERK2 immunoblot – numbers are quantification of phospho-ERK divided by quantification of total ERK normalised to control cells at time 0. (C) 40 000 control A431 cells or integrin β1 knockdown cells were seeded in six-well plates. The number of cells in three 20 × fields was counted after 24, 48 and 72 h. Data shown are the average of six 20 × fields from two experiments with the cell numbers normalised to day 1. The data for the integrin β1 knockdown cells are the average obtained from the different knockdown cell lines.
Forced expression of integrin β1 in suprabasal keratinocytes has been suggested to antagonise cell cycle exit (Carroll et al, 1995; Zhu et al, 1999), to investigate whether reduced integrin β1 expression affects proliferation we analysed the ability of the knockdown cell lines to grow in 5% serum. Control and integrin β1 knockdown cells were plated at low density and the number of cells counted after 24, 48 and 72 h. To eliminate clone to clone variation we averaged the growth curves for the three integrin b1 knockdown cell lines. Integrin β1 knockdown cells grew at the same rate as control cells (Figure 4C). Apoptosis was not increased in integrin β1 knockdown cells compared to control cells in either adherent or nonadherent culture conditions (data not shown).
In vivo analysis of integrin β1 function
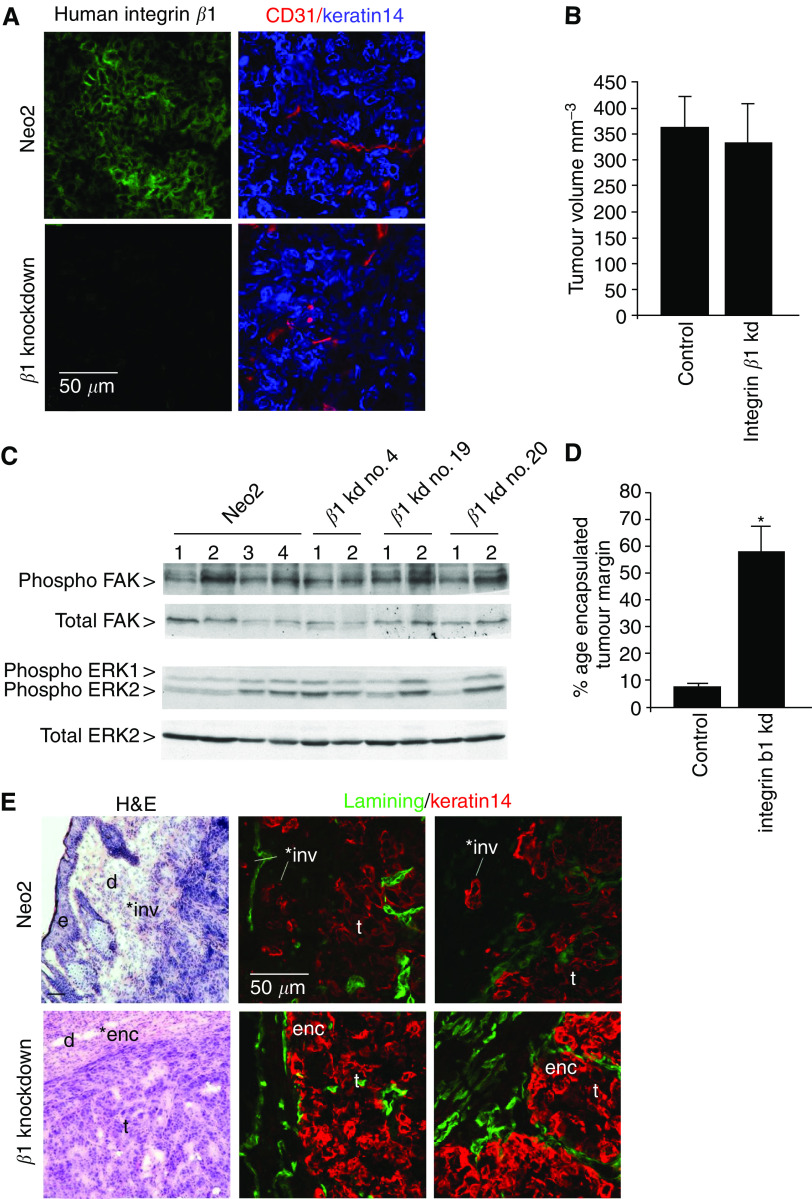
The results described above implicate integrin β1 in the motility, signalling and proliferation of VSCC cells. To investigate the role of integrin β1 in vivo we injected cells subcutaneously into nude mice. Eight mice were injected with A431 cells stably transfected with the empty vector and a total of 15 mice were injected with three different integrin β1 knockdown clones. To confirm that integrin β1 remained stably knocked down in vivo we stained frozen sections of the tumours for integrin β1, keratin 14 to identify tumour cells and CD31 to identify endothelial cells. Integrin β1 was clearly expressed in control tumours but not in integrin β1 knockdown tumours (Figure 5A). No integrin β1 staining was observed from host tissue because the P5D2 antibody we used does not recognise murine integrin β1. These results demonstrate that the integrin β1 levels remained knocked-down in vivo. No difference was observed between the size of control and integrin β1 knockdown tumours after 11 and 15 days (Figure 5B and data not shown), indicating that integrin β1 is not required for the proliferation of VSCC cells in vivo. We next tested if there were any differences in FAK and ERK/MAP kinase activity in vivo. Phospho-FAK and phospho-ERK1/2 levels were compared between tumour lysates by Western blotting. Figure 5C shows that there was no consistent difference in either FAK or ERK1/2 activity between control and integrin β1 knockdown tumours.
Figure 5.
Analysis of integrin β1 function in tumours in vivo. (A) Frozen sections of tumours formed by control A431 cells or integrin β1 knockdown cells were stained for integrin β1 (left panels, P5D2 antibody), keratin 14 (right panels, blue) and CD31 (right panels, red). (B) 106 control A431 cells or integrin β1 knockdown cells were injected subcutaneously into nude mice. The average tumour volume after 15 days is shown (the data for the three integrin β1 knockdown clones were pooled, no statistically significant differences were observed between the three integrin β1 knockdown clones). (C) Lysates were made from tumours formed by control A431 cells or integrin β1 knockdown cells and analysed by Western blotting for ERK1/2 and FAK activity. Upper panel shows phospho-FAK Y397 immunoblot, upper-mid panel shows total FAK immunoblot, lower-mid panel shows phospho-ERK1/2 T283 and Y285 immunoblot, and lower panel shows total ERK2 immunoblot. (D) The proportion of encapsulated or invasive tumour boundary (scored blind) is shown for control and integrin β1 knockdown tumours. *Indicates a statistically significant difference from control (P<0.05). (E) Frozen sections of tumour margins from control A431 cells (upper panels) or integrin β1 knockdown clone 19 cells (lower panels) were stained with haematoxylin and eosin (left) or for laminin and keratin 14 (middle and right). ‘*inv’ indicates invasive margin, ‘*enc’ indicates encapsualted margin, ‘d’ – dermis, ‘e’ – host epidermis, ‘t’ – tumour.
We have shown that cells lacking integrin β1 have defects in cell attachment and spreading; in vivo such difference could be reflected by altered morphological characteristics of the tumours. We therefore investigated if the histology of the integrin β1 knockdown tumours was different from the control tumours. When stained with haematoxylin and eosin the internal areas of the knockdown and control tumours were indistinguishable (data not shown). However, there was a difference in the appearance of the tumour margins; the boundaries between the control tumours and the surrounding dermal tissue were poorly defined with clusters of carcinoma cells invading the dermis (Figure 5E upper panels). This pattern of invasion resembles the ‘spray’ pattern of invasion observed in VSCC (Heaps et al, 1990; Qureshi et al, 1999). In contrast, the boundaries of integrin β1 knockdown tumours were better-defined and were often encapsulated by fibroblasts aligned parallel to the tumour edge (Figure 5E, lower panels). To further investigate this difference we stained the tumour margins for keratin 14 to unambiguously identify the tumour cells and laminin to stain any structures resembling basement membranes. Control tumours clearly had tumour cells breaking away from the tumour and there was no organisation of laminin relative to the tumour boundary. Integrin b1 knockdown tumours had significantly fewer cells invading the surrounding dermal tissue. Furthermore, in some places, tumour cells remained partly bounded by laminin; however, the laminin was not continuous or well organised as in an intact basement membrane (compare Figure 1B and 5E). To quantify this difference, the percentage of encapsulated tumour boundary was measured in control and integrin β1 knockdown tumours. Figure 5D shows the relative proportions of the different morphologies of the tumour boundaries for the control and integrin β1 knockdown tumours. In all three integrin β1 knockdown cell lines the proportion of invasive tumour boundary was reduced and the encapsulated tumour boundary increased compared to controls (data not shown). We were unable to determine if integrin β1 is required for metastasis as we did not observe metastases in any of the mice (data not shown). These data demonstrate that integrin β1 is required for invasive tumour margins in vivo.
DISCUSSION
In this paper we have documented the change in integrin expression in VSCC, with particular focus on the expression and conformation of integrin β1. In normal epidermis, integrin β1 is localised to lateral and basal membranes in the basal layer of cells. The lateral localisation of integrin β1 is somewhat unusual but has been previously reported by Surico et al (1995). In VSCC the proportion of cells expressing integrins α2, α3, α5, α6 and β1 increases and their localisation becomes disorganised. In addition, the levels of integrins α3 and β1 expressed also increase. To analyse the functional significance of these changes we generated VSCC cell lines with knocked-down levels of integrin β1. We found that reducing the levels of integrin β1 in VSCC cells also caused a reduction in the levels of integrin α3 and α5. Both integrin α3 and α5 heterodimerise with integrin β1; it is possible than these integrins are not stable if they are unable to bind to the appropriate β-subunit. Brakebusch et al (1999) found that reexpression of integrin β1 in fibroblasts in which the gene had been deleted led to increased integrin α3 and α5 levels. It is likely that the increased integrin β1 expression may be needed to allow the increased levels of integrins α3 and α5 observed in VSCC. Integrins α3 and α5 only heterodimerise with integrin β1; therefore, their reduced expression would not be predicted to have additional consequences in integrin β1 knockdown cells.
Integrin β1 is required for both efficient cell adhesion and spreading on collagen I. Intriguingly, integrin β1 was not required for efficient cell adhesion to fibronectin but is necessary for cell spreading. It is possible that the very low levels of integrin β1 expression in the knockdown clones are sufficient to allow adhesion to fibronectin but not cell spreading. Alternatively, other integrins may be able to mediate adhesion to fibronectin, but integrin β1 may be required to activate intracellular molecules that mediate cell spreading. In support of this hypothesis, integrin β1 null fibroblasts only show defects in adhering to fibronectin when excess RGD peptide is added demonstrating that other integrins can mediate adhesion to fibronectin in the complete absence of integrin β1 (Brakebusch et al, 1999). We observed altered organisation of mature focal adhesions and reduced FAK activation in integrin β1 knockdown cells. These effects may result from the failure to activate integrin β1-dependent signals that modulate the remodelling of adhesion complexes. Indeed, reduced FAK activity may directly contribute to reduced cell spreading as FAK has been shown to promote cell spreading (Owen et al, 1999). No defects in cell adhesion to laminin or vitronectin were observed in integrin β1 knockdown cells (data not shown), indicating that adhesion to these substrates is mediated by other integrins, possibly α6β4 and αVβ3. Consistent with this, we found that expression of integrins β3, β4 and α6 was not affected by integrin β1 knockdown and integrin αV expression was slightly increased.
Integrin β1 expression is particularly high at the margins of tumours, suggesting that it may be involved in tumour invasion into the dermis. We find that loss of integrin β1 leads to better-defined tumour borders; in particular, the amount of tumour boundary that exhibits a ‘spray-type’ pattern of invasion is reduced. The requirement for integrin β1 to form effective cell adhesions to collagen IV probably explains the reduced ability of cells lacking integrin β1 to invade into a Matrigel matrix since collagen IV is a major component of Matrigel. Similarly, it is likely that the reduction in tumour cell invasion in vivo is caused by the inability of tumour cells lacking integrin β1 to interact with the ECM at the tumour margin. Consistent with this hypothesis, ablation of integrin β1 in the skin prevents hair follicle keratinocytes from re-modelling the basement membrane and invaginating into the dermis (Raghavan et al, 2000). An additional possibility is that these cells also fail to interact with host cells at the tumour edge; teratomas derived from integrin β1 deficient cells recruit lower numbers of host cells in tumours (Bloch et al, 1997). These results show that loss of integrin β1 affects the invasive behaviour of VSCC cells in vivo, while integrin β1 has previously been implicated in the ability of experimentally transformed fibroblasts and lymphoma cells and to metastasise, it is not clear in these studies if this is linked to defects in cell invasion or other tumour cell properties (Stroeken et al, 1998; Brakebusch et al, 1999). In this study we demonstrate that loss of integrin β1 does not affect the growth of VSCC in vivo or the activation of FAK and ERK signalling but does affects the invasive behaviour of VSCC cells in vivo.
Our data suggest that integrin β1 promotes the invasion of VSCC and this may in turn lead to a worse prognosis. Unfortunately, the limited number of samples in this study prevent making correlations between integrin expression levels and clinical outcome. Agents that block integrin β1 function may be able to block VSCC invasion in vivo. Intravenous delivery of antiintegrin β1 antibodies can inhibit neutrophil migration into sites of pulmonary inflammation (Ridger et al, 2001) and peptides mimicking integrin ligands can interfere with integrin function in vivo (Tucker, 2003). Other tumour types may also be effectively targeted by strategies that block integrin β1 function; mammary carcinoma cells revert to a normal phenotype when integrin β1 is inhibited (Weaver et al, 1997).
Loss of integrin β1 did not affect ERK signalling or proliferation in adherent cells, suggesting that there is redundancy between cell adhesion molecules in promoting the activation of mitogenic pathways; although it is possible that very low levels of integrin β1 remaining in the knockdown cell lines are sufficient to mediate signalling in adherent cells. Both ERK and FAK activities were reduced in nonadherent cells with reduced levels of integrin β1. These results suggest that integrin β1 promotes the formation of certain signalling complexes even when it is not bound to ECM in the conventional way. It is possible that even in nonadherent cells, integrin β1 can engage the fibronectin present in serum or that unbound integrin β1 has a role in the formation of signalling complexes. Interestingly, reduced levels of integrin β1 in vivo did not affect the overall levels of ERK and FAK signalling or tumour growth; this suggests that for VSCC cells in vitro adherent culture more closely resembles the tumour situation than nonadherent cell culture. However, it is possible that there are particular microenvironments within the tumour where integrin β1 is required for mitogenic signalling. Taken together these results demonstrate that in vivo the main function of integrin β1 in VSCC is to promote cell motility and invasion and that it is not required for activation of FAK, ERK or cell proliferation.
Acknowledgments
EB was funded by The Joint Research Committee of The Chelsea and Westminster NHS Trust Charity and The Westminster Medical School Research Trust, and CJM and ES were funded by Cancer Research UK. We thank Demelza Bird and Susan Clinton for technical help.
References
- Bazzoni G, Shih DT, Buck CA, Hemler ME (1995) Monoclonal antibody 9EG7 defines a novel beta 1 integrin epitope induced by soluble ligand and manganese, but inhibited by calcium. J Biol Chem 270: 25570–25577 [DOI] [PubMed] [Google Scholar]
- Bloch W, Forsberg E, Lentini S, Brakebusch C, Martin K, Krell HW, Weidle UH, Addicks K, Fassler R (1997) Beta 1 integrin is essential for teratoma growth and angiogenesis. J Cell Biol 139: 265–278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brakebusch C, Fassler R (2003) The integrin-actin connection, an eternal love affair. EMBO J 22: 2324–2333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brakebusch C, Wennerberg K, Krell HW, Weidle UH, Sallmyr A, Johansson S, Fassler R (1999) Beta1 integrin promotes but is not essential for metastasis of ras-myc transformed fibroblasts. Oncogene 18: 3852–3861 [DOI] [PubMed] [Google Scholar]
- Brummelkamp TR, Bernards R, Agami R (2002) A system for stable expression of short interfering RNAs in mammalian cells. Science 296: 550–553 [DOI] [PubMed] [Google Scholar]
- Carroll JM, Romero MR, Watt FM (1995) Suprabasal integrin expression in the epidermis of transgenic mice results in developmental defects and a phenotype resembling psoriasis. Cell 83: 957–968 [DOI] [PubMed] [Google Scholar]
- Defilippi P, Olivo C, Venturino M, Dolce L, Silengo L, Tarone G (1999) Actin cytoskeleton organization in response to integrin-mediated adhesion. Microsc Res Technol 47: 67–78 [DOI] [PubMed] [Google Scholar]
- Evans RD, Perkins VC, Henry A, Stephens PE, Robinson MK, Watt FM (2003) A tumor-associated beta 1 integrin mutation that abrogates epithelial differentiation control. J Cell Biol 160: 589–596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giancotti FG, Ruoslahti E (1999) Integrin signaling. Science 285: 1028–1032 [DOI] [PubMed] [Google Scholar]
- Grose R, Hutter C, Bloch W, Thorey I, Watt FM, Fassler R, Brakebusch C, Werner S (2002) A crucial role of beta 1 integrins for keratinocyte migration in vitro and during cutaneous wound repair. Development 129: 2303–2315 [DOI] [PubMed] [Google Scholar]
- Guan K, Czyz J, Furst DO, Wobus AM (2001) Expression and cellular distribution of alpha(v)integrins in beta(1)integrin-deficient embryonic stem cell-derived cardiac cells. J Mol Cell Cardiol 33: 521–532 [DOI] [PubMed] [Google Scholar]
- Hanks SK, Ryzhova L, Shin NY, Brabek J (2003) Focal adhesion kinase signaling activities and their implications in the control of cell survival and motility. Front Biosci 8: d982–d996 [DOI] [PubMed] [Google Scholar]
- Hannon GJ (2002) RNA interference. Nature 418: 244–251 [DOI] [PubMed] [Google Scholar]
- Heaps JM, Fu YS, Montz FJ, Hacker NF, Berek JS (1990) Surgical-pathologic variables predictive of local recurrence in squamous cell carcinoma of the vulva. Gynecol Oncol 38: 309–314 [DOI] [PubMed] [Google Scholar]
- Hegerfeldt Y, Tusch M, Brocker EB, Friedl P (2002) Collective cell movement in primary melanoma explants: plasticity of cell–cell interaction, beta1-integrin function, and migration strategies. Cancer Res 62: 2125–2130 [PubMed] [Google Scholar]
- Hennigan RF, Hawker KL, Ozanne BW (1994) Fos-transformation activates genes associated with invasion. Oncogene 9: 3591–3600 [PubMed] [Google Scholar]
- Her JH, Lakhani S, Zu K, Vila J, Dent P, Sturgill TW, Weber MJ (1993) Dual phosphorylation and autophosphorylation in mitogen-activated protein (MAP) kinase activation. Biochem J 296(Part 1): 25–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hertle MD, Adams JC, Watt FM (1991) Integrin expression during human epidermal development in vivo and in vitro. Development 112: 193–206 [DOI] [PubMed] [Google Scholar]
- Hodivala KJ, Pei XF, Liu QY, Jones PH, Rytina ER, Gilbert C, Singer A, Watt FM (1994) Integrin expression and function in HPV 16-immortalised human keratinocytes in the presence or absence of v-Ha-ras. Comparison with cervical intraepithelial neoplasia. Oncogene 9: 943–948 [PubMed] [Google Scholar]
- Hughes DE, Rebello G, al-Nafussi A (1994) Integrin expression in squamous neoplasia of the cervix. J Pathol 173: 97–104 [DOI] [PubMed] [Google Scholar]
- Hynes RO (2002) Integrins: bidirectional, allosteric signaling machines. Cell 110: 673–687 [DOI] [PubMed] [Google Scholar]
- Jones J, Sugiyama M, Watt FM, Speight PM (1993) Integrin expression in normal, hyperplastic, dysplastic, and malignant oral epithelium. J Pathol 169: 235–243 [DOI] [PubMed] [Google Scholar]
- Lenter M, Uhlig H, Hamann A, Jeno P, Imhof B, Vestweber D (1993) A monoclonal antibody against an activation epitope on mouse integrin chain beta 1 blocks adhesion of lymphocytes to the endothelial integrin alpha 6 beta 1. Proc Natl Acad Sci USA 90: 9051–9055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luque A, Gomez M, Puzon W, Takada Y, Sanchez-Madrid F, Cabanas C (1996) Activated conformations of very late activation integrins detected by a group of antibodies (HUTS) specific for a novel regulatory region (355–425) of the common beta 1 chain. J Biol Chem 271: 11067–11075 [DOI] [PubMed] [Google Scholar]
- Marshall CJ (1994) MAP kinase kinase kinase, MAP kinase kinase and MAP kinase. Curr Opin Genet Dev 4: 82–89 [DOI] [PubMed] [Google Scholar]
- Owen J, Ruest PJ, Fry DW, Hanks SK (1999) Induced focal adhesion kinase (FAK) expression in FAK-null cells enhances cell spreading and migration requiring both auto- and activation loop phosphorylation sites and inhibits adhesion-dependent tyrosine phosphorylation of Pyk2. Mol Cell Biol 19: 4806–4818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parise LV, Lee J, Juliano RL (2000) New aspects of integrin signaling in cancer. Semin Cancer Biol 10: 407–414 [DOI] [PubMed] [Google Scholar]
- Qureshi F, Munkarah A, Banerjee M, Jacques SM (1999) Tumor angiogenesis in vulvar squamous cell carcinoma. Gynecol Oncol 72: 65–70 [DOI] [PubMed] [Google Scholar]
- Raghavan S, Bauer C, Mundschau G, Li Q, Fuchs E (2000) Conditional ablation of beta1 integrin in skin. Severe defects in epidermal proliferation, basement membrane formation, and hair follicle invagination. J Cell Biol 150: 1149–1160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renshaw MW, Toksoz D, Schwartz MA (1996) Involvement of the small GTPase rho in integrin-mediated activation of mitogen-activated protein kinase. J Biol Chem 271: 21691–21694 [DOI] [PubMed] [Google Scholar]
- Ridger VC, Wagner BE, Wallace WA, Hellewell PG (2001) Differential effects of CD18, CD29, and CD49 integrin subunit inhibition on neutrophil migration in pulmonary inflammation. J Immunol 166: 3484–3490 [DOI] [PubMed] [Google Scholar]
- Roovers K, Davey G, Zhu X, Bottazzi ME, Assoian RK (1999) Alpha5beta1 integrin controls cyclin D1 expression by sustaining mitogen-activated protein kinase activity in growth factor-treated cells. Mol Biol Cell 10: 3197–3204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz MA, Assoian RK (2001) Integrins and cell proliferation: regulation of cyclin-dependent kinases via cytoplasmic signaling pathways. J Cell Sci 114: 2553–2560 [DOI] [PubMed] [Google Scholar]
- Stroeken PJ, van Rijthoven EA, van der Valk MA, Roos E (1998) Targeted disruption of the beta1 integrin gene in a lymphoma cell line greatly reduces metastatic capacity. Cancer Res 58: 1569–1577 [PubMed] [Google Scholar]
- Surico N, Priori L, Savoia P, Cremona O, Marchisio PC (1995) Distribution of integrins and extracellular matrix proteins in vulvar squamous cell carcinomas. Eur J Gynaecol Oncol 16: 147–154 [PubMed] [Google Scholar]
- Tucker GC (2003) alpha v integrin inhibitors and cancer therapy. Curr Opin Invest Drugs 4: 722–731 [PubMed] [Google Scholar]
- Van Waes C, Surh DM, Chen Z, Kirby M, Rhim JS, Brager R, Sessions RB, Poore J, Wolf GT, Carey TE (1995) Increase in suprabasilar integrin adhesion molecule expression in human epidermal neoplasms accompanies increased proliferation occurring with immortalization and tumor progression. Cancer Res 55: 5434–5444 [PubMed] [Google Scholar]
- Vial E, Sahai E, Marshall CJ (2003) ERK-MAPK signaling coordinately regulates activity of Rac1 and RhoA for tumor cell motility. Cancer Cell 4: 67–79 [DOI] [PubMed] [Google Scholar]
- Watt FM (2002) Role of integrins in regulating epidermal adhesion, growth and differentiation. EMBO J 21: 3919–3926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver VM, Petersen OW, Wang F, Larabell CA, Briand P, Damsky C, Bissell MJ (1997) Reversion of the malignant phenotype of human breast cells in three-dimensional culture and in vivo by integrin blocking antibodies. J Cell Biol 137: 231–245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werb Z, Tremble PM, Behrendtsen O, Crowley E, Damsky CH (1989) Signal transduction through the fibronectin receptor induces collagenase and stromelysin gene expression. J Cell Biol 109: 877–889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada KM, Even-Ram S (2002) Integrin regulation of growth factor receptors. Nat Cell Biol 4: E75–E76 [DOI] [PubMed] [Google Scholar]
- Zhu AJ, Haase I, Watt FM (1999) Signaling via beta1 integrins and mitogen-activated protein kinase determines human epidermal stem cell fate in vitro. Proc Natl Acad Sci USA 96: 6728–6733 [DOI] [PMC free article] [PubMed] [Google Scholar]