Abstract
In response to wounding, a 48-kDa myelin basic protein (MBP) kinase is activated within 2 min, both locally and systemically, in leaves of young tomato plants. The activating signal is able to pass through a steam girdle on the stem, indicating that it moves through the xylem and does not require intact phloem tissue. A 48-kDa MBP kinase is also activated by the 18-amino acid polypeptide systemin, a potent wound signal for the synthesis of systemic wound response proteins (swrps). The kinase activation by systemin is strongly inhibited by a systemin analog having a Thr-17 → Ala-17 substitution, which is a powerful antagonist of systemin activation of swrp genes. A 48-kDa MBP kinase activity also increases in response to polygalacturonic acid and chitosan but not in response to jasmonic acid or phytodienoic acid. In def1, a mutant tomato line having a defective octadecanoid pathway, the 48-kDa MBP kinase is activated by wounding and systemin as in the wild-type plants. This indicates that MBP kinase functions between the perception of primary signals and the DEF1 gene product. In response to wounding, the MBP kinase is phosphorylated on phosphotyrosine residues, indicating a relationship to the mitogen-activated protein kinase family of protein kinases.
Tomato plants respond to insect herbivory and mechanical damage by inducing a localized and systemic synthesis of several defense-related proteins, called systemic wound response proteins (swrps) (1). An 18-amino acid polypeptide, called systemin, has been isolated from tomato leaves that appears to be a primary wound signal and is derived from a 200-amino acid precursor, prosystemin (2, 3). Several lines of evidence support a role for systemin as a primary systemic signal, including its potent activity (fmol range) (2), the suppression of the wound response by the expression of a constitutive antisense CaMV-35S-prosystemin cDNA in tomato leaves (4), and the phloem mobility of systemin that is compatible with the timing of the transport of the wound signal throughout the plants (2, 5, 6).
Wounding of leaves of tomato plants or supplying systemin or oligosaccharide elicitors through their cut stems produces the oxylipin signaling molecule jasmonic acid (JA), which is a potent activator of swrp genes (7). JA is derived from its octadecanoid precursor, linolenic acid, which is released from membranes, presumably via the activation of an intracellular lipase in response to primary wound signals (7). A tomato mutant, def1, that lacks a functional octadecanoid pathway, is severely blocked in the wound induction of defensive genes and is much more susceptible to insect attacks than the wild type (8).
Little is known of the intracellular signal transduction events upstream of the release of linolenic acid from membranes in response to wounding. Recently, several laboratories have demonstrated the wound-activation of myelin basic protein (MBP) kinases (MBPKs) in a variety of plant species, including tomato, that are members of the mitogen-activated protein kinase (MAPK) family (9–11). To date, no direct relationship of these kinases to the activation of wound-response proteins has been demonstrated.
We report here the localized and systemic activation of a 48-kDa MBPK in response to wounding, systemin, and oligosaccharide elicitors. MBPK activation appears to be an early step in the signaling pathway that activates the localized and systemic expression of defensive genes in response to herbivory and pathogen attacks.
MATERIALS AND METHODS
Plant Material.
Wild-type and mutant (def1) (8) tomato plants (Lycopersicon esculentum cv Castlemart) were grown for 14–16 days under a 17 hr/7-hr light-dark cycle (28°C, >300 μE⋅m−2⋅s−1/17°C, darkness). At this stage, they display two expanded leaves and a small apical leaf.
Wounding, Elicitors, and Proteinase Inhibitor Bioassay.
Tomato plants, 14–16 days old, were wounded with a hemostat around the edges of the terminal leaflet and incubated under standard conditions of 300 μE⋅m−2⋅s−1 at 28°C, as reported (8). After the times indicated in the text, the wounded and the unwounded leaves of four plants were excised at the petiole and immediately frozen in liquid nitrogen to assay kinase activities. Six other plants from the same group were further incubated for 24 hr under standard conditions and assayed for proteinase inhibitor accumulation by radial immunodiffusion (12, 13).
All signaling compounds were supplied to the tomato plants through their cut stems as described by Howe et al. (8). The uptake rate was 90 μl/hr on average unless indicated otherwise. At the times indicated in the text, the plants were either frozen in liquid nitrogen for the in-gel MBP kinase assay (four plants at each sampling time) or transferred to 20-ml capacity vials containing water and incubated for 24 hr under standard conditions and assayed for proteinase inhibitor I accumulation. Because of a potential touch activation of MBPKs, an appropriate touch control was carried out for each experiment.
12-oxo-Phytodienoic acid (PDA) was obtained from Cayman Chemical (Ann Arbor, MI). Before use, 90% of the ethanol was evaporated and the compound redissolved in phosphate buffer. Aqueous stock solutions of systemin, the systemin analog Ala-17-systemin, salicylic acid, JA, polygalacturonic acid (degree of polymerisation ≈20), and nitrous acid-treated chitosan were diluted in phosphate buffer. Abscisic acid (ABA) was dissolved in ethanol and diluted in phosphate buffer [in this case the buffer controls contained the same amount of ethanol (0.1%)].
Systemin and Ala-17-systemin were synthesized as described (14).
Insect Feeding Experiments.
Tobacco hornworm (Manduca sexta) eggs and hornworm artificial diet were obtained from Carolina Biological Supply. The eggs were hatched according to the supplier’s manual. When the larvae had reached a size of 7 ± 2 cm, they were prestarved for several hours and then placed close to a leaf of a tomato seedling. The experimental setup allowed the larvae to reach the leaf with its mandibles and chew on it without any other contact. After 3 min, the wounded and the unwounded leaves were cut at the petiole and immediately frozen in liquid nitrogen for the in-gel kinase assay.
Steam Girdling.
Plants were steam girdled similarly as described by Nelson et al. (6). A hot steam jet was directed toward the stem for 1–2 min. One or 2 days after girdling the plants were cut either above or below the girdle and placed with their cut stem into phosphate buffer or a solution containing systemin in the same buffer. To estimate the influence of stress from handling during excision and transfer, plants were severely handled, but not excised. Incubation and sampling for the kinase assay and radial immunodiffusion assay was as described above.
In-Gel Kinase Assay.
The frozen leaf tissue was transferred to a mortar and homogenized in extraction buffer containing 50 mM Hepes⋅KOH (pH 7.6), 2 mM DTT, 1 mM EDTA, 1 mM EGTA, 20 mM β-glycerophosphate, 20% (vol/vol) glycerol, 1 mM Na3VO4, 1 mM NaF, and 1 Complete proteinase inhibitor mixture tablet (Boehringer Mannheim) per 50 ml.
Homogenates were centrifuged for 25 min at 40 000 × g. Protein concentrations in the supernatants were determined with the Pierce BCA protein assay using BSA as the standard. All steps were carried out at 4°C. Equal amounts of proteins in the supernatants (50 μg for 10-well gels and 30 μg for 15-well gels) were separated by SDS/gel electrophoresis on a 10% polyacrylamid minigel. Before polymerization, 0.25 mg/ml MBP (Sigma) was added to the polyacrylamid solution. Denaturing and renaturing of the proteins in the gels and the kinase reaction were performed as described by Usami et al. (9).
The gels were then dried between cellophane sheets and the radioactive signals were analyzed by an InstantImager (Packard) and by autoradiography. To quantify the signals on the InstantImager, the untreated control samples (t = 0) were set as the 1-fold activation in wounding experiments. For elicitor uptake experiments, the kinase activity in the elicitor samples was expressed as the fold activity above the level of the buffer controls which were given a value of 1.0.
Immunoprecipitation.
Immunoprecipitation of tyrosine phosphorylated proteins from a leaf extract (250 μg protein) with the monoclonal antibody against phosphotyrosine, clone PT 66 (Sigma), and a subsequent in-gel kinase assay were performed as described by Zhang and Klessig (15).
RESULTS
A 48-kDa MBPK Is Activated Locally and Systemically upon Wounding.
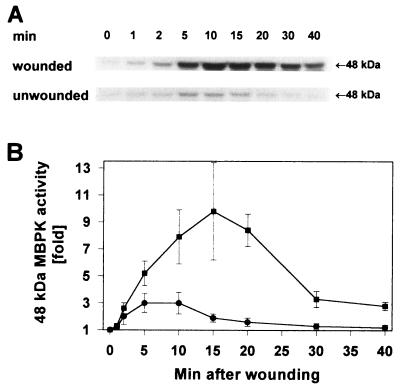
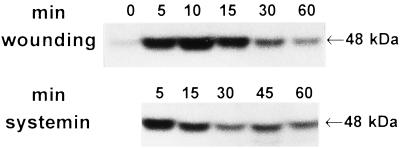
To determine if a MAPK is associated with the signaling of defense genes in young tomato plants, the plants were wounded on the lower leaf with a hemostat and the activation of MBPKs was investigated in both the wounded leaf and the upper, unwounded (systemic) leaf using in-gel kinase assays with MBP and [γ-32P]ATP as substrates. Fig. 1 shows the rapid activation of a kinase with an apparent molecular mass of ≈48 kDa, both in the wounded leaf and in the unwounded leaf. The activity in the wounded leaf was about 3 times higher than that in the unwounded leaf and was sustained for a longer period.
Figure 1.
A 48-kDa MBPK is systemically activated by wounding in both the wounded and in unwounded leaves. The lower leaf of 14- to 15-day-old tomato plants (two expanded leaves) was wounded with a hemostat and incubated under standard conditions as described. At the times indicated, the lower wounded and upper unwounded leaves were excised at the base of the petiole and assayed in an in-gel MBPK assay (A) and quantified using an InstantImager (B) with kinase activities expressed as the fold activity above the level of untreated control leaves, which were assigned a value of 1. ▪, Wounded leaves; •, systemic unwounded leaves.
Systemin Elicits MBPK Activity.
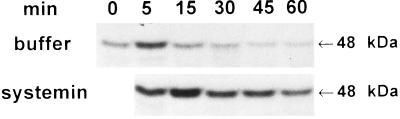
Fig. 2 shows that the polypeptide wound signal, systemin, when supplied to young tomato plants through their cut stems, induces a 48-kDa MBPK activity in leaves. The activity was detected within 5 min, reaching a maximum within 15 min of the application of systemin, and the activity was sustained for at least 60 min. In control plants supplied with buffer or water, a weak, transient kinase activity was recorded that reached a maximum at 5 min and then returned to background levels by 15 min.
Figure 2.
A 48-kDa MBPK is activated by systemin. Young (14 days old) tomato plants were excised at the base of the stem and the cut end placed into a solution containing either 25 nM systemin in 10 mM phosphate buffer (pH 6.5) or buffer alone and incubated under standard conditions. At the times indicated, the leaves were assayed as in Fig. 1A for MBPK activity.
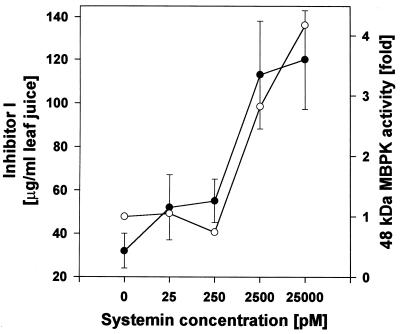
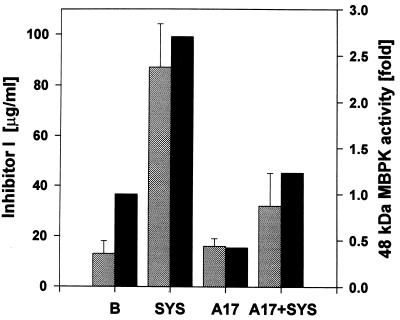
The systemin activation of the MBPK was concentration-dependent and revealed the same dose-dependent response as the systemin induction of proteinase inhibitor I synthesis (Fig. 3). Structure–activity studies of systemin analogs had previously shown that threonine at position 17 in systemin is essential for the induction of proteinase inhibitor proteins (14). Ala-17-systemin is totally inactive, but it is a potent competitive inhibitor of systemin activation of proteinase inhibitor synthesis. Ala-17-systemin also acts as a powerful antagonist of the MBPK activation by systemin (Fig. 4).
Figure 3.
Comparison of the induction of MBPK activity and of inhibitor I protein by systemin in leaves of excised 14-day-old tomato plants. Plants were supplied with systemin in 10 mM phosphate buffer (pH 6.5) or buffer alone. At 30 min, leaves were assayed for MBPK activity (○) and at 24 hr leave juice was assayed for inhibitor I protein accumulation (•). The kinase activities are expressed as the fold activation above or below the level of the buffer control, which was assigned a value of 1.
Figure 4.
Inhibition of the induction of MBPK activity and of inhibitor I protein by the systemin analog, Ala-17-systemin. Young excised tomato plants were supplied with 10 mM phosphate buffer, pH 6.5 (B); 25 nM systemin in buffer (SYS); 2.5 μM Ala-17-systemin in buffer (A17); and 25 nM systemin plus 2.5 μM Ala-17-systemin in buffer (A17 + SYS). The plants were incubated under standard conditions and assayed for MBPK activity (solid bars) after 30 min and for inhibitor I protein accumulation after 24 hr (shaded bars). The kinase activities are expressed as the fold activation above or below the level of the buffer control, which was assigned a value of 1.
MBPK Is Activated in a Wound Signaling-Deficient Mutant Tomato Line, def1.
Defense gene signaling in response to wounding, systemin, or oligosaccharide elicitors has been shown to be mediated through the octadecanoid pathway (8). Linolenic acid, released from membranes, is converted through several oxylipin intermediates to JA, a powerful activator of swrp genes. The def1 mutant of tomato harbors a mutation in the octadecanoid pathway (8) that effectively blocks the activation of defense genes by wounding, systemin, and oligosaccharide elicitors. As shown in Fig. 5, the 48-kDa MBPK was activated by either wounding or systemin in the mutant, indicating that the octadecanoid pathway is not required for its activation.
Figure 5.
The 48-kDa MBPK is activated by wounding and systemin in the octadecanoid signaling mutant def1. Mutant plants (14 days old) were wounded on all leaves with a hemostat across the main veins. A second set of plants were excised at the base of the stem and supplied with a 25 nM systemin solution in 10 mM phosphate buffer (pH 6.5). All plants were incubated under standard conditions, and at the times indicated the leaves were assayed for MBPK activity.
MBPK Is Activated by the Oligosaccharide Elicitors Polygalacturonic Acid and Chitosan.
Polygalacturonic acid and chitosan, primary wound signals during pathogen attacks (16), activated a 48-kDa MBPK in leaves of young tomato plants, when supplied through their cut stems (Table 1). The activity is reported at 15 min after application of the elicitors and represents the maximal activity obtained.
Table 1.
Activation of 48-kDa MBPK by chitosan and polygalacturonic acid, but not by salicylic acid, JA, and ABA
| Signaling compound | MBPK activity |
|---|---|
| Buffer | 1.0 ± 0.0 |
| Chitosan, 125 μg/ml | 2.5 ± 0.8 |
| PGA, 125 μg/ml | 2.5 ± 0.4 |
| JA, 200 μM | 0.9 ± 0.1 |
| ABA, 100 μM | 1.0 ± 0.1 |
| SA, 1 mM | 0.8 ± 0.2 |
Young (14 days old) tomato plants were excised at the base of the stem and the cut end placed into different solutions, containing chitosan, polygalacturonic acid (PGA), JA, ABA, and salicylic acid (SA) in 10 mM phosphate buffer (pH 6.5) or phosphate buffer alone and incubated under standard conditions as described. After 15 min, leaves were assayed for MBPK activity. The kinase activity is expressed as the fold activity above the level of the buffer controls, which were assigned a value of 1.0.
JA and PDA, products of the octadecanoid pathway, did not activate a MBPK (Table 1), confirming that MBPK activity is associated with primary wound signals and does not require a functional octadecanoid pathway. Neither salicylic acid, a potent inhibitor of the octadecanoid pathway (17) and an activator of the tobacco 48-kDa SIP kinase (15), nor ABA, a plant hormone that activates proteinase inhibitor synthesis in ABA-deficient tomato plants (18), activated a MBPK above control levels when supplied to excised plants through their cut stems. Both compounds were inactive even when several concentrations were supplied up to 180 min following excision.
An MBPK Activating Signal Is Transported Through a Steam Girdle.
In response to wounding the leaves of a young tomato plant, and in response to excision of the stem or wounding the roots, an increase in MBPK activity could be detected within minutes throughout the aerial parts of the plant (see Figs. 1 and 2, and data not shown), indicating that the rapid wound signal is propagated both acro- and basipetally.
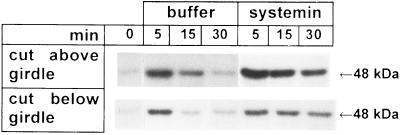
Steam girdling of young tomato plant stems between the roots and the cotyledons was performed with a steam jet, producing a girdle zone of about 1 cm long, comprised only of a thin bundle of intact xylem vessels. On the day following girdling, the leaves above the girdle appeared healthy and had good turgor. Cutting the stems below the girdle and then placing the cut end into buffer generated a rapid signal that was able to pass through the girdle to activate a 48-kDa MBPK (Fig. 6). This response was similar to the effect of cutting the stems above the girdle (Fig. 6) or cutting the stems of ungirdled plants (Fig. 2). Excising the girdled plants either above or below the girdle, and supplying them with systemin, resulted in the activation of the kinase in a sustained manner compared with girdled plants supplied with buffer alone (Fig. 6). However, the rate of uptake of systemin by girdled plants excised below the girdle was about 3-fold lower than that of the girdled plants that were excised above the girdle, therefore resulting in a somewhat weaker response. In girdled plants, MBPK activity increased weakly in response to touch, but the touch-activation was of a much lower magnitude than the activity produced by excision of the stems (data not shown). The cumulative results from these experiments suggest that systemin, as well as the excision-induced MBPK-activating-signal, is propagated through the stem xylem and does not require an intact phloem.
Figure 6.
A wound signal and systemin both pass a steam girdle on the stem. Young plants (14 days old) were girdled with a steam jet as described to produce a 0.5–1.0 cm zone comprised only of a thin bundle of intact xylem vessels. On the following day, the girdled plants were excised either below or above the steam girdle and placed with their cut stems into a solution containing either 10 mM phosphate buffer (pH 6.5) or buffer containing 25 nM systemin and incubated under standard conditions. At the times indicated, leaves were assayed for MBPK activity.
MBPK Activity Is Rapidly Generated by Grazing M. sexta Larvae.
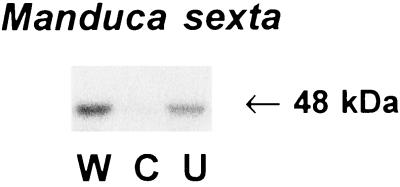
To determine if rapid MBPK activating signals are also generated under more natural conditions, we assayed MBPK activity in response to damage caused by feeding tobacco hornworm larvae (M. sexta). The larvae were allowed to feed for 3 min on the exposed edge of a lower leaf of young plants, with no access to the rest of the leaf. After 3 min of feeding by the larvae a 48-kDa MBPK was activated in both the wounded and unwounded leaves (Fig. 7).
Figure 7.
MBPK is systemically activated by M. sexta larvae attack. A single M. sexta larva (≈7 cm long) was placed near the exposed edge of a terminal leaflet of the lower leaf and allowed to chew continuously for 3 min without other contact with the leaf (see Materials and Methods). The wounded leaf (lane W), the upper unwounded leaf (lane U), and untreated control leaves (lane C) were assayed for MBPK activity.
The 48-kDa Wound-Responsive MBPK Is Phosphorylated on Tyrosine Residues.
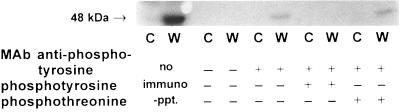
MAPKs are activated by dual-specific MAPK kinases that phosphorylate the MAPK on tyrosine and on serine/threonine residues. We immunoprecipitated proteins from wounded leaves and untreated control leaves with a monoclonal antibody against phosphotyrosine and assayed the activity of the precipitated proteins with the in-gel kinase assay. Fig. 8 shows, that an ≈48-kDa MBPK from wounded leaves was recognized by the antibody, but no MBPK was precipitated from untreated control leaves. Phosphotyrosine, but not phosphothreonine, was a competitor of the immunoprecipitation, demonstrating the specificity of the antibody. These experiments show that the active form of the wound-inducible MBPK, but not the inactive form, is phosphorylated at a tyrosine residue(s), which is typical for members of the MAPK family (9, 15).
Figure 8.
The wound-activated MBPK is phosphorylated on tyrosine residues. Extracts were prepared from wounded 14-day-old tomato plants 10 min following wounding (lanes W) and from unwounded control plants (lanes C) as described. The proteins were immunoprecipitated with a monoclonal antibody specific for phosphotyrosine (mAb anti-phosphotyrosine), and the precipitated proteins were assayed for MBPK activity. The amino acids phosphotyrosine or phosphothreonine were added as competitors to demonstrate the specificity of the antibody.
DISCUSSION
Wound-inducible MBP protein kinases belonging to the MAPK family have been identified and characterized recently in leaves of tobacco and alfalfa (9–11) and were also detected in leaves of tomato (9). We investigated whether a MAPK might be associated with the systemic signaling of defensive genes in tomato plants in response to wounding, as well as to systemin, and oligosaccharide elicitors. We report here that a 48-kDa MBPK is activated by wounding not only in the wounded leaves but also systemically in unwounded leaves of tomato plants (Fig. 1), with the activation being much stronger in the wounded leaves. The systemically induced MBPK activity was about 3-fold higher than the levels found in leaves of unwounded control plants. The properties, particularly the tyrosine phosphorylation in association with its activation, suggest that the 48-kDa MBPK is a MAPK.
A 48-kDa MBPK was also strongly and persistently activated by systemin as well as by the oligosaccharide elicitors polygalacturonic acid and chitosan (Fig. 2 and Table 1). The oxylipins JA and PDA, which are products of the octadecanoid pathway and synthesized in response to wounding, systemin, and oligosaccharide elicitors, did not activate a MBPK in tomato leaves (Table 1), indicating that the MBPK is activated by primary wound signals and independent of PDA or JA synthesis. In accordance with our results, Bögre et al. (11) reported that methyl jasmonate, an active derivative of JA, ABA and electrical activity did not activate the wound-activated MAPK (MMK4) in alfalfa. In leaves of the octadecanoid signaling mutant def1, in which wound and systemin-induced JA synthesis is severely reduced and defensive genes only weakly activated (8), the MBPK was activated by wounding and systemin, similar to the wild type (Fig. 5). The data indicate that the MBPK must function at an early step following the perception of a primary signal, upstream of the DEF1 gene product and may be a key step in the intracellular events that couple the perception of different primary defense signals to the octadecanoid pathway. It is possible that the MBPK activity induced by wounding itself, and by primary chemical signals, resides in the same enzyme. The MBPK activities that were induced by wounding, systemin, and oligosaccharide elicitors migrated identically on long polyacrylamide gels (data not shown). However, whether a single MBPK is responsible for all of the activities or whether multiple kinases are involved remains to be determined. In any case, MBPK activation presently represents the earliest known intracellular event of the signal transduction pathway that activates defensive genes in response to wounding, systemin, and the oligosaccharide signals.
Measurements of the velocity of the systemic signal that induces MBPK activity in 14-day-old tomato plants as well as in 23-day-old plants exhibit a velocity of greater than 0.6 mm/sec, which includes the activation time of the kinase. The rapidity of the response throughout the plants was indicative of responses expected from electrical or hydraulically propagated signals. The increased MBPK activity in the systemic leaf was detected between 1 and 2 min after wounding (Fig. 1), and the kinase activity induced in the leaves after cutting at the base of the stem was also within this time frame. Associations of rapid hydraulic or electric signals with the wound activation of proteinase inhibitors have been reported (19), with electrical signals moving at velocities of 1–20 mm/sec, and hydraulic signals being propagated throughout the plant at velocities of 15–30 mm/sec (19). The movement of the wound-inducible systemic signal that activates defensive genes has been estimated to be about 30 mm/hr (6). Rhodes et al. (20) have recently demonstrated that electrical signals triggered by a heat stimulus are propagated through the sieve-tube/companion cell complex. However, we show here that girdling of the stem, which destroys phloem cell function while maintaining xylem function, does not impair the rapid MBPK activating signal (Fig. 6). This indicates that the signal is propagated through the xylem and is likely the result of hydraulic forces due to pressure changes that are perceived by a very sensitive recognition system. These signals are capable of migrating both basi- and acropetally (19) and must be generated even by the damage caused by the grazing of M. sexta larvae for only a few minutes (Fig. 7). The activation of the kinase appears to be related to a transient up-regulation of the defense response because a low level of proteinase inhibitors accompanies MBPK activation in response to excision alone. However, the high levels of defensive proteins that accumulate in response to wounding require a sustained chemical signal (6). Systemin possesses properties consistent with this signal, being a potent elicitor of both swrp synthesis and MBPK activity in a sustained manner. A transient activation of the signaling pathway by the 48-kDa MBPK may result in an increased ability of the plants to respond to the chemical signals generated by sustained herbivory or pathogen attacks. A MAPK has been shown to be involved in the phosphorylation and activation of cytoplasmic phospholipase A2 (cPLA2) in animal cells in response to polypeptide signals released from macrophages in response to pathogen attacks. The phospholipase releases arachidonic acid from membranes, resulting in the synthesis of prostaglandins as part of the inflammmatory response (21). The wound response in plants exhibits analogies to the animal system (1), with wounding and pathogen-derived polypeptide and oligosaccharide elicitors triggering the release of linolenic acid from membranes and its conversion to JA, a prostaglandin analog that activates plant-defensive genes (1). It is possible that the 48-kDa MBPK reported here may serve an analogous role in plants and may be involved in activating a phospholipase that releases linolenic acid from plant membranes to initiate its conversion to JA.
Acknowledgments
We thank Sue Vogtman and Thom Koehler for growing and maintaining plants. This research was supported in part by the Washington State University College of Agriculture Project 1791, National Science Foundation Grants IBN-9184542 and IBN-9117795 (to C.A.R.) and a Deutsche Forschungsgemeinschaft postdoctoral fellowship (to J.W.S.).
ABBREVIATIONS
- ABA
abscisic acid
- JA
jasmonic acid
- MAPK
mitogen-activated protein kinase
- MBP
myelin basic protein
- MBPK
MBP kinase
- PDA
12-oxo-phytodienoic acid
- swrp
systemic wound response protein
References
- 1.Bergey D R, Howe G, Ryan C A. Proc Natl Acad Sci USA. 1996;93:12053–12058. doi: 10.1073/pnas.93.22.12053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pearce G, Strydom D, Johnson S, Ryan C A. Science. 1991;253:895–898. doi: 10.1126/science.253.5022.895. [DOI] [PubMed] [Google Scholar]
- 3.McGurl B, Pearce G, Orozco-Cardenas M L, Ryan C A. Science. 1992;255:1570–1573. doi: 10.1126/science.1549783. [DOI] [PubMed] [Google Scholar]
- 4.Orozco-Cardenas M L, McGurl B, Ryan C A. Proc Natl Acad Sci USA. 1993;90:8273–8276. doi: 10.1073/pnas.90.17.8273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Narváez-Vásquez J, Pearce G, Orozco-Cardenas M L, Franceschi V R, Ryan C A. Planta. 1995;195:593–600. [Google Scholar]
- 6.Nelson, C. E., Walker-Simmons, M., Makus, D., Zuroske, G., Graham, J. & Ryan, C. A. (1983) in Plant Resistance to Insects, Symposium Series 208, ed. Hedin, P. (Am. Chem. Soc., Washington, DC), pp. 103–122.
- 7.Farmer E E, Ryan C A. Plant Cell. 1992;4:129–134. doi: 10.1105/tpc.4.2.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Howe G A, Lightner J, Browse J, Ryan C A. Plant Cell. 1996;8:2067–2077. doi: 10.1105/tpc.8.11.2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Usami S, Banno H, Ito Y, Nishihama R, Machida Y. Proc Natl Acad Sci USA. 1995;92:8660–8664. doi: 10.1073/pnas.92.19.8660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Seo S, Okamoto M, Seto H, Ishizuka K, Sano H, Ohashi Y. Science. 1995;270:1988–1992. doi: 10.1126/science.270.5244.1988. [DOI] [PubMed] [Google Scholar]
- 11.Bögre L, Ligterink W, Meskiene I, Barker P J, Heberle-Bors E, Huskisson N S, Hirt H. Plant Cell. 1997;9:75–83. doi: 10.1105/tpc.9.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ryan C A. Anal Biochem. 1967;19:434–440. doi: 10.1016/0003-2697(67)90233-3. [DOI] [PubMed] [Google Scholar]
- 13.Trautman R, Cowan K M, Wagner G G. Immunochemistry. 1971;8:901–916. doi: 10.1016/0019-2791(71)90429-0. [DOI] [PubMed] [Google Scholar]
- 14.Pearce G, Johnson S, Ryan C A. J Biol Chem. 1993;268:212–216. [PubMed] [Google Scholar]
- 15.Zhang S, Klessig D F. Plant Cell. 1997;9:809–824. doi: 10.1105/tpc.9.5.809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Walker-Simmons M, Hadwiger L, Ryan C A. Biochem Biophys Res Commun. 1983;110:194–199. doi: 10.1016/0006-291x(83)91279-2. [DOI] [PubMed] [Google Scholar]
- 17.Doares S H, Narváez-Vásquez J, Conconi A, Ryan C A. Plant Physiol. 1995;108:1741–1746. doi: 10.1104/pp.108.4.1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Peña-Cortes H, Sanchez-Serrano J, Mertens R, Willmitzer L, Prat S. Proc Natl Acad Sci USA. 1989;86:9851–9855. doi: 10.1073/pnas.86.24.9851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Malone M. Adv Bot Res. 1996;22:163–228. [Google Scholar]
- 20.Rhodes J D, Thain J F, Wildon C D. Planta. 1996;200:50–57. [Google Scholar]
- 21.Lin L-L, Wartmann M, Lin A Y, Knopf J F, Seth A, Davis R J. Cell. 1993;72:269–278. doi: 10.1016/0092-8674(93)90666-e. [DOI] [PubMed] [Google Scholar]