Abstract
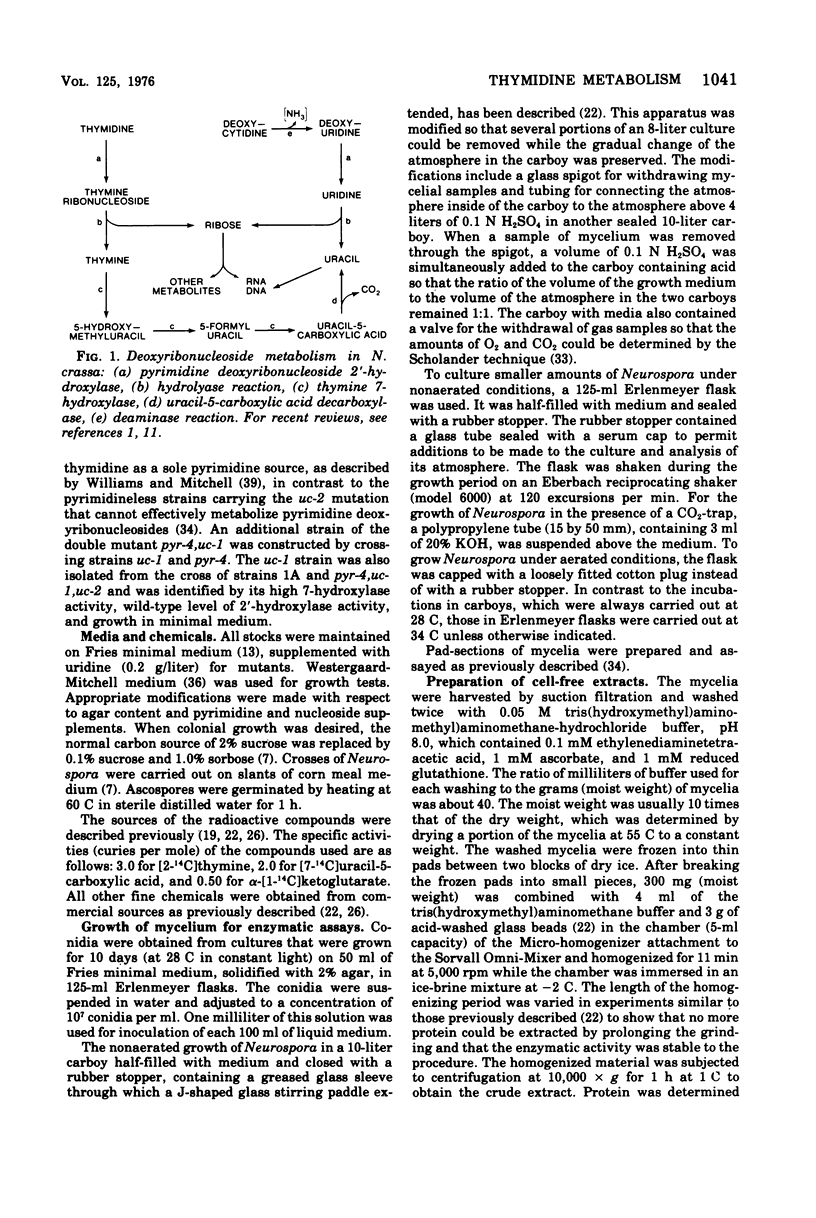
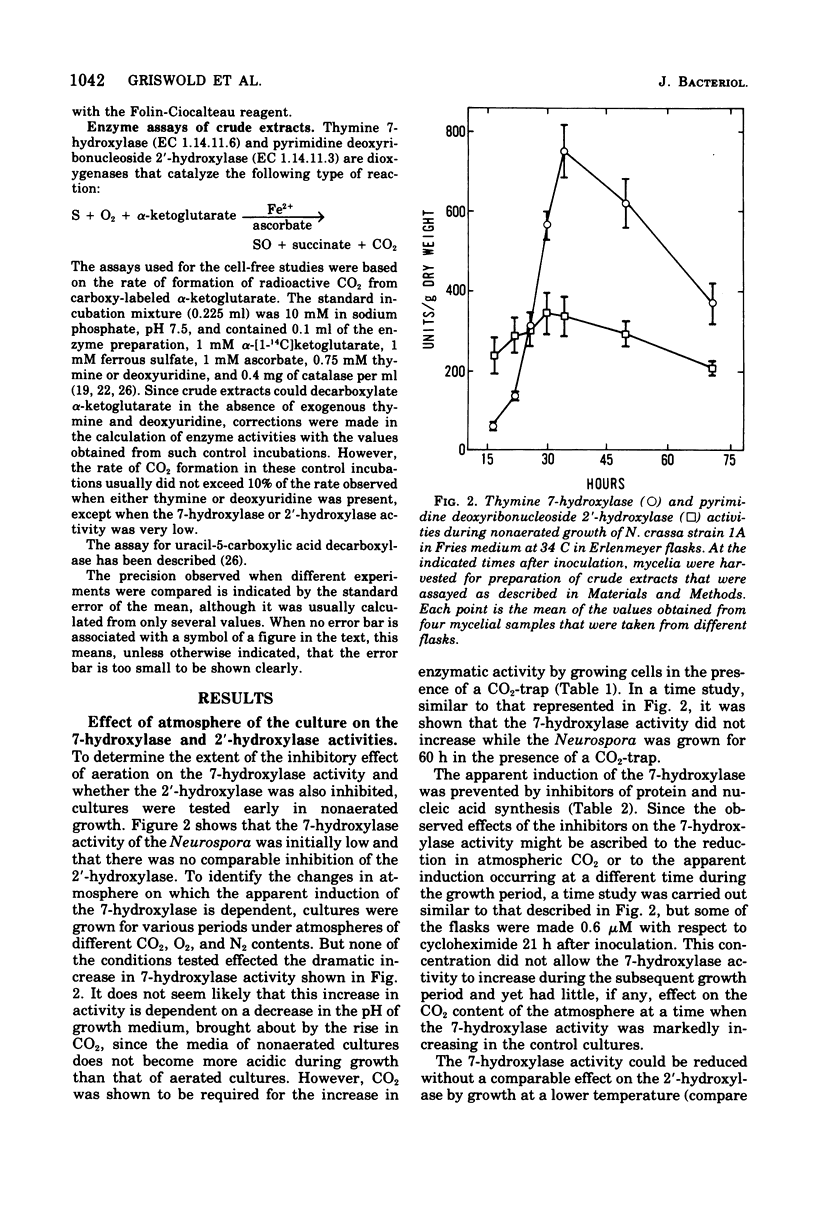
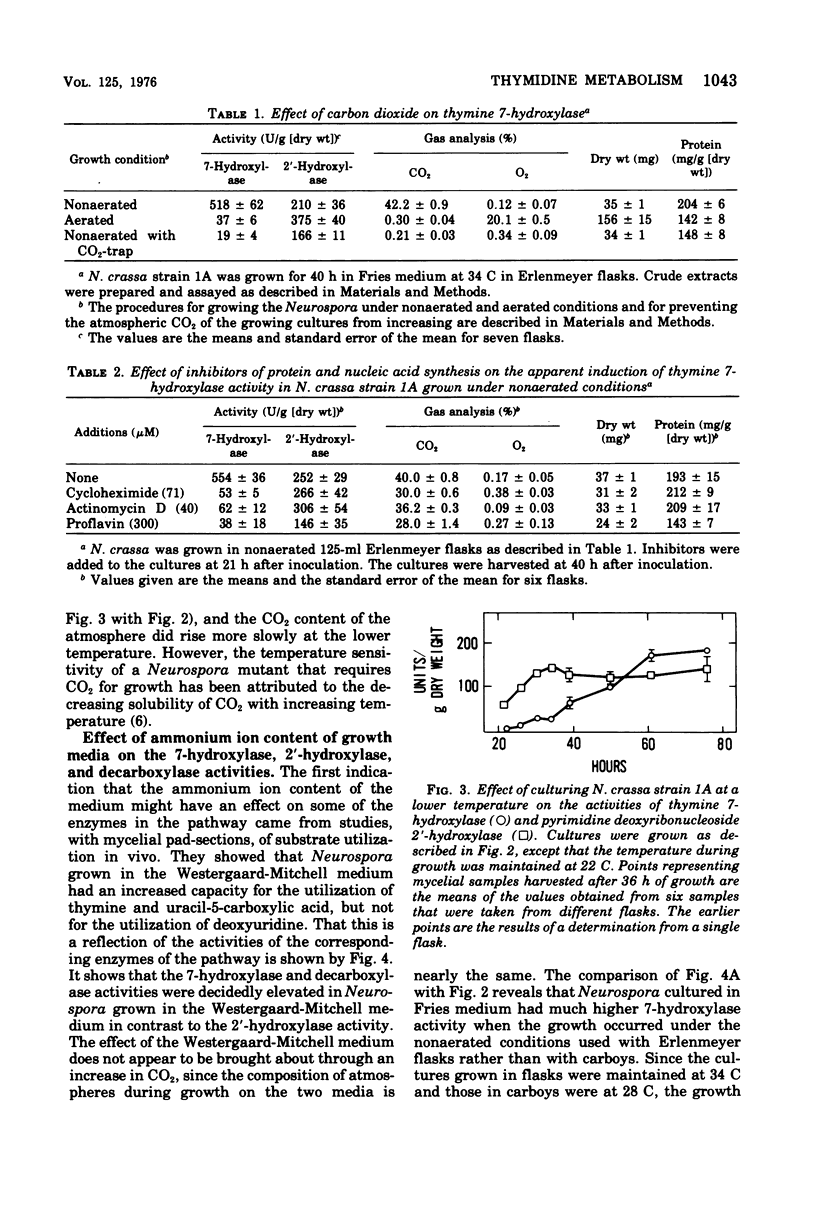
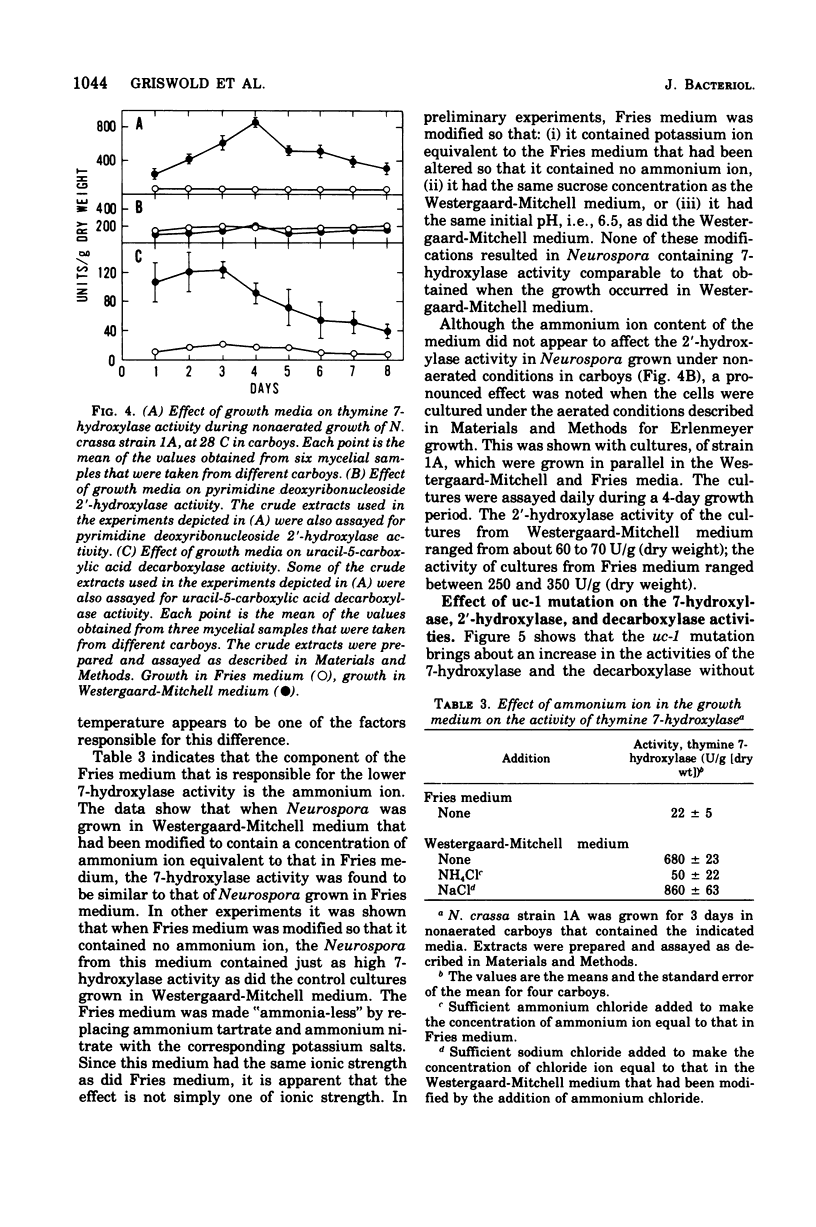
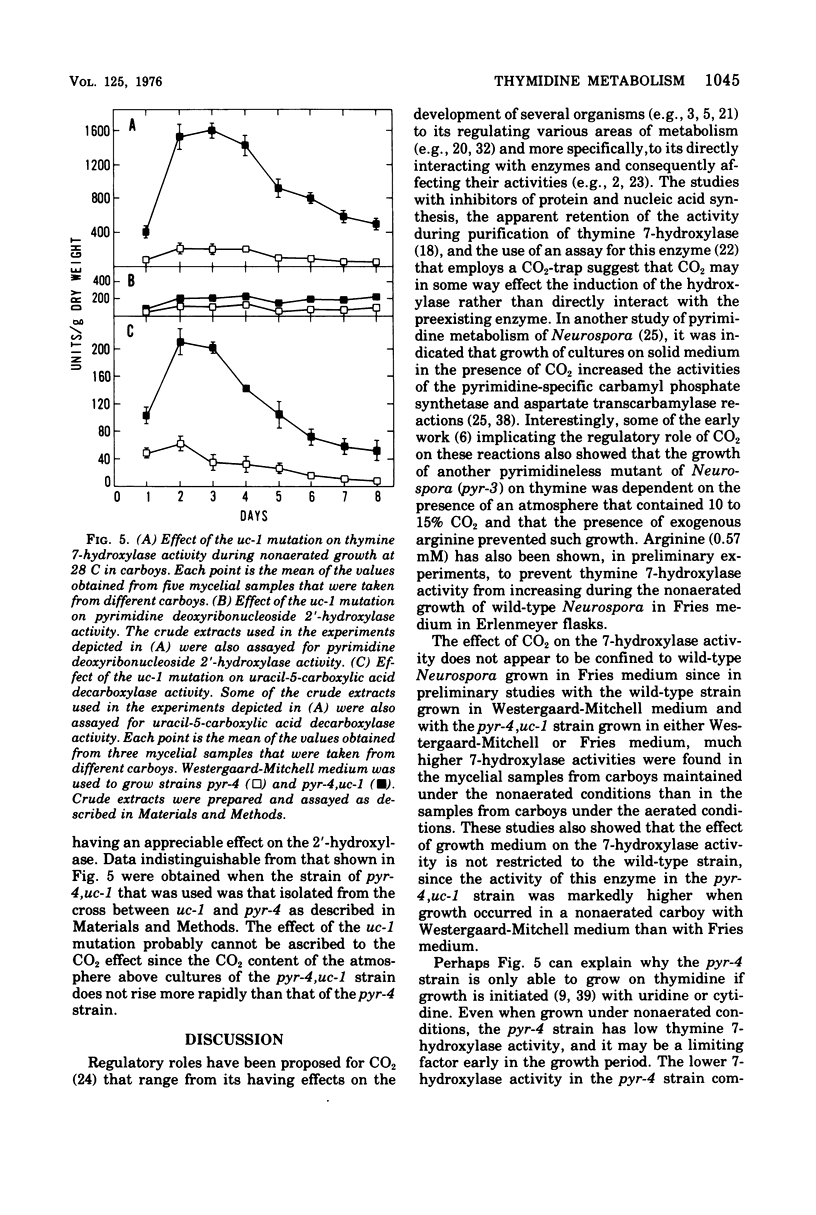
The utilization of thymidine by Neurospora crassa is initiated by the pyrimidine deoxyribonucleoside 2'-hydroxylase reaction and the consequent formation of thymine and ribose. Thymine must then be oxidatively demethylated by the thymine 7-hydroxylase and uracil-5-carboxylic acid decarboxylase reactions. This article shows that the 2'-hydroxylase reaction can be regulated differently than the oxidative demethylation process and suggests that the 2'-hydroxylase has, in addition to the role of salvaging the pyrimidine ring, the role of providing ribose not only for the utilization of the demethylated pyrimidine but also for other metabolic processes. One way that this difference in regulation was observed was with the uc-1 mutation developed by Williams and Mitchell. The present communication shows that this mutation increases the activities of the 7-hydroxylase and the decarboxylase but has no comparable effect on the 2'-hydroxylase. Qualitatively similar effects on these enzymes were bought about by growth of wild-type Neurospora in media lacking ammonium ion, such as the Westergaard-Mitchell medium. The 2'-hydroxylase and 7-hydroxylase are also differently affected by the carbon dioxide content of the atmosphere above the growing culture and the growth temperature. Studies with inhibitors indicated that the carbon dioxide effect is dependent on protein synthesis.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson W. B., Nordlie R. C. Glucose dehydrogenase activity of yeast glucose 6-phosphate dehydrogenase. I. Selective stimulation by bicarbonate, phosphate, and sulfate. Biochemistry. 1968 Apr;7(4):1479–1485. doi: 10.1021/bi00844a034. [DOI] [PubMed] [Google Scholar]
- CHARLES H. P. Response of Neurospora mutants to carbon dioxide. Nature. 1962 Jul 28;195:359–360. doi: 10.1038/195359a0. [DOI] [PubMed] [Google Scholar]
- FINK R. M., FINK K. Biosynthesis of radioactive RNA and DNA pyrimidines from thymidine-2-C-14. Biochem Biophys Res Commun. 1961 Oct 23;6:7–10. doi: 10.1016/0006-291x(61)90174-7. [DOI] [PubMed] [Google Scholar]
- Feldman J. F., Thayer J. P. Cyclic AMP-induced tyrosinase synthesis in Neurospora crassa. Biochem Biophys Res Commun. 1974 Dec 11;61(3):977–982. doi: 10.1016/0006-291x(74)90251-4. [DOI] [PubMed] [Google Scholar]
- HOROWITZ N. H., FLING M., MACLEOD H., WATANABE Y. Structural and regulative genes controlling tyrosinase synthesis in Neurospora. Cold Spring Harb Symp Quant Biol. 1961;26:233–238. doi: 10.1101/sqb.1961.026.01.028. [DOI] [PubMed] [Google Scholar]
- Horowitz N. H., Feldman H. M., Pall M. L. Derepression of tyrosinase synthesis in Neurospora by cycloheximide, actinomycin D, and puromycin. J Biol Chem. 1970 Jun 10;245(11):2784–2788. [PubMed] [Google Scholar]
- Kuttan R., Cardinale G. J., Udenfriend S. An activatable form of prolyl hydroxylase in fibrobalast extracts. Biochem Biophys Res Commun. 1975 Jan 2;64(3):947–954. doi: 10.1016/0006-291x(75)90139-4. [DOI] [PubMed] [Google Scholar]
- LOOMIS W. F. Sexual differentiation in Hydra; control by carbon dioxide tension. Science. 1957 Oct 18;126(3277):735–739. doi: 10.1126/science.126.3277.735. [DOI] [PubMed] [Google Scholar]
- Liu C. K., Hsu C. A., Abbott M. T. Catalysis of three sequential dioxygenase reactions by thymine 7-hydroxylase. Arch Biochem Biophys. 1973 Nov;159(1):180–187. doi: 10.1016/0003-9861(73)90443-8. [DOI] [PubMed] [Google Scholar]
- Liu C. K., Shaffer P. M., Slaughter R. S., McCroskey R. P., Abbott M. T. Stoichiometry of the pyrimidine deoxyribonucleoside 2'-hydroxylase reaction and of the conversions of 5-hydroxymethyluracil to 5-formyluracil and of the latter to uracil-5-carboxylic acid. Biochemistry. 1972 May 23;11(11):2172–2176. doi: 10.1021/bi00761a025. [DOI] [PubMed] [Google Scholar]
- Longmore W. J., Niethe C. M., McDaniel M. L. Effect of CO2 concentration on intracellular pH and on glycogen synthesis from glycerol and glucose in isolated perfused rat liver. J Biol Chem. 1969 Dec 10;244(23):6451–6457. [PubMed] [Google Scholar]
- McCroskey R. P., Griswold W. R., Sokoloff R. L., Sevier E. D., Lin S., Liu K., Shaffer P. M., Palmatier R. D., Parker T. S., Abbott M. T. Studies pertaining to the purification and properties of thymine 7-hydroxylase. Biochim Biophys Acta. 1971 Feb 10;227(2):264–277. doi: 10.1016/0005-2744(71)90059-3. [DOI] [PubMed] [Google Scholar]
- McDaniel M. L., Longmore W. J. Activation and inhibition of the activities of bovine liver acontiase. J Biol Chem. 1971 Aug 10;246(15):4818–4824. [PubMed] [Google Scholar]
- Nazario M., Reissig J. L. Induction of aspartic transcarbamylase by carbon dioxide. Biochem Biophys Res Commun. 1964 May 22;16(1):42–46. doi: 10.1016/0006-291x(64)90208-6. [DOI] [PubMed] [Google Scholar]
- Palmatier R. D., McCroskey R. P., Abbott M. T. The enzymatic conversion of uracil 5-carboxylic acid to uracil and carbon dioxide. J Biol Chem. 1970 Dec 25;245(24):6706–6710. [PubMed] [Google Scholar]
- Prival M. J., Magasanik B. Resistance to catabolite repression of histidase and proline oxidase during nitrogen-limited growth of Klebsiella aerogenes. J Biol Chem. 1971 Oct 25;246(20):6288–6296. [PubMed] [Google Scholar]
- Reinert W. R., Marzluf G. A. Regulation of the purine catabolic enzymes in Neurospora crassa. Arch Biochem Biophys. 1975 Feb;166(2):565–574. doi: 10.1016/0003-9861(75)90421-x. [DOI] [PubMed] [Google Scholar]
- SANWAL B. D., LATA M. Concurrent regulation of glutamic acid dehydrogenases of Neurospora. Arch Biochem Biophys. 1962 Jun;97:582–588. doi: 10.1016/0003-9861(62)90127-3. [DOI] [PubMed] [Google Scholar]
- Scheffler I. E. Conditional lethal mutants of Chinese hamster cells: mutants requiring exogenous carbondioxide for growth. J Cell Physiol. 1974 Apr;83(2):219–230. doi: 10.1002/jcp.1040830208. [DOI] [PubMed] [Google Scholar]
- Shaffer P. M., Hsu C. A., Abbott M. T. Metabolism of pyrimidine deoxyribonucleosides in Neurospora crassa. J Bacteriol. 1975 Feb;121(2):648–655. doi: 10.1128/jb.121.2.648-655.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramanian K. N., Sorger G. J. Regulation of nitrate reductase in Neurospora crassa: regulation of transcription and translation. J Bacteriol. 1972 May;110(2):547–553. doi: 10.1128/jb.110.2.547-553.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitney P. A., Magasanik B. The induction of arginase in Saccharomyces cerevisiae. J Biol Chem. 1973 Sep 10;248(17):6197–6202. [PubMed] [Google Scholar]
- Williams L. G., Bernhardt S., Davis R. H. Copurification of pyrimidine-specific carbamyl phosphate synthetase and aspartate transcarbamylase of Neurospora crassa. Biochemistry. 1970 Oct 27;9(22):4329–4335. doi: 10.1021/bi00824a013. [DOI] [PubMed] [Google Scholar]
- Williams L. G., Mitchell H. K. Mutants affecting thymidine metabolism in Neurospora crassa. J Bacteriol. 1969 Oct;100(1):383–389. doi: 10.1128/jb.100.1.383-389.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]



