Abstract
Patients with mucinous colorectal cancer generally have worse prognoses than those with the nonmucinous variety. The reason for this disparity is unclear, but may result from a differential response to adjuvant chemotherapy. We examined known molecular markers for response to common chemotherapy in these two histological subtypes. In all, 21 patients with mucinous and 30 with nonmucinous Dukes C colorectal cancer were reviewed for demographic data and outcome. Total RNA from the tumours and adjacent normal mucosa was isolated and reverse transcribed. Quantitative expression levels of drug pathway genes were determined using TaqMan RT–PCR (5-fluorouracil (5-FU): TYMS, DPYD, ECGF1; oxaliplatin: GSTP1 (glutathione S-transferase pi), ERCC1 and 2; irinotecan: ABCB1, ABCG2, CYP3A4, UGT1A1, CES2, TOP1). Mucinous tumours significantly overexpressed both TYMS and GSTP1 relative to nonmucinous tumours and patient-matched normal mucosa. No significant differences in expression of the remaining markers were found. Mean follow-up was 20 months; 17 patients had recurrent disease. Among patients receiving 5-FU, those with mucinous tumours experienced shorter disease-free survival (DFS) than those with nonmucinous tumours (median DFS 13.8 vs 46.5 months, P=0.053). Mucinous colorectal cancer overexpresses markers of resistance to 5-FU and oxaliplatin. Likewise, DFS may be decreased in patients with mucinous tumours who receive 5-FU. The presence of mucin should be carefully evaluated in developmental trials of new agents for treating colorectal cancer.
Keywords: mucinous adenocarcinoma, colorectal neoplasms, pharmacogenomics, messenger ribonucleic acid, 5-fluorouracil, irinotecan, oxaliplatin, adjuvant chemotherapy
Most evidence suggests that colorectal adenocarcinomas that produce an abundance of the glycoprotein mucin portend a worse clinical prognosis (Umpleby et al, 1985; Green et al, 1993; Nozoe et al, 2000), although this finding has not been consistently established (Sasaki et al, 1987; Halvorsen and Seim, 1988). Comprising between 10 and 15% of all colorectal adenocarcinomas, these tumours have a mucin content of at least 60% of tumour volume, typically at the advancing edge. The reasons for the worse prognosis with mucinous tumours are not clear, but possibly include difficulty obtaining complete resections (Umpleby et al, 1985), propensity for early spread to regional lymph nodes (Nozoe et al, 2000), and detection at more advanced stages of disease (Halvorsen and Seim, 1988). There also may be a higher proportion of mucinous tumours in familial cancer syndromes; approximately 30% of mucinous tumours demonstrate microsatellite instability, a defining trait of hereditary nonpolyposis colorectal cancer (Kakar et al, 2004). The responsiveness to adjuvant chemotherapy of mucinous adenocarcinoma specifically has not been examined.
Various genes are known to influence tumour response or patient survival following chemotherapy (McLeod and Murray, 1999). Their products consist of intracellular drug targets, cell-membrane transporters, components that detoxify or metabolise, and DNA repair enzymes. TYMS encodes thymidylate synthase, a key enzyme in pyrimidine metabolism. Numerous studies have demonstrated that overexpression of TYMS correlates with poor response to 5-fluorouracil (5-FU) (Johnston et al, 1995; Leichman et al, 1997; Salonga et al, 2000; Iacopetta et al, 2001; Kornmann et al, 2002). Likewise, elevated levels of other enzymes in the 5-FU pathway (namely, DPYD (dihydropyrimidine dehydrogenase) and ECGF1 (thymidine phosphorylase)) have been shown to affect epithelial tumour sensitivity (Etienne et al, 1995; Nita et al, 1998), clinical response (Metzger et al, 1998a), and time to recurrence (Salonga et al, 2000) following treatment with 5-FU. Additionally, expression levels of DPYD and ECGF1 appear to be coregulated, particularly in metastatic colorectal cancer (Collie-Duguid et al, 2001).
Platinum agents such as oxaliplatin rely on the inability of nucleotide excision repair genes within the tumour to successfully remove bulky DNA adducts. Polymorphisms in ERCC1 (excision repair crosscomplementing isoform 1) have been related to decreased survival in patients with colorectal cancer (Park et al, 2001). Similarly, expression of ERCC2 (excision repair crosscomplementing isoform 2) in gastric cancers predicts response and survival (Metzger et al, 1998b). GSTP1 (glutathione S-transferase pi) is a major route of detoxification of platinum agents. It is highly overexpressed in colon cancer, and drug-resistant tumours have elevated levels (Stoehlmacher et al, 2002).
Irinotecan is bioactivated to a more potent form via CES2 (carboxylesterase 2), and colorectal adenocarcinomas overexpressing CES2 are more sensitive to the drug (Wu et al, 2002). Irinotecan causes double-strand DNA breaks by binding to topoisomerase-I (TOP1) (Xu and Villalona-Calero, 2002), making it a key intracellular target. Members of the ATP-binding cassette superfamily of proteins (ABCB1 (Patel and Mitra, 2001; Gottesman et al, 2002) and ABCG2 (Wierdl et al, 2003)) function to export irinotecan from tumour cells, while drug metabolism via the cytochrome P450 system (CYP3A4 (Kehrer et al, 2002; Xu and Villalona-Calero, 2002)) or glucuronidation (UGT1A1 (Iyer et al, 1999, 2002)) can also occur.
In the current study, we examined expression of the above 12 genes in colorectal cancer in order to characterise possible differences in response between the mucinous and nonmucinous subtypes of colorectal cancer. These genes were selected as likely candidates affecting clinical response to common adjuvant chemotherapy (i.e. 5-FU, irinotecan, and oxaliplatin) administered following resection of locally advanced tumours. A secondary goal was to correlate gene expression with disease-free survival (DFS), in the context of histological subtype.
MATERIALS AND METHODS
In all, 51 Dukes stage C colorectal cancer specimens were selected from the Tissue Procurement Core of the Siteman Cancer Center, Washington University, St Louis, MO, USA for genetic evaluation. No tumours from patients with known inflammatory bowel disease or familial cancer syndromes (familial adenomatous polyposis or hereditary nonpolyposis colon cancer) were included. In total, 21 mucinous adenocarcinomas were selected, histologically defined by a mucin content exceeding 60% of the total tumour volume. The remaining 30 nonmucinous tumours were randomly chosen from a contemporary selection of 145 available Dukes' C colorectal cancer specimens. Adjacent normal mucosa was available for all tumours for comparison with the malignant epithelial component. Tissue was snap-frozen in liquid nitrogen immediately following surgical resection and stored at −80°C. Macrodissection was performed on all tumour specimens to ensure high tumour cellularity (median 86.3%, 65–95%). Clinical data were extracted from patients' medical records and clinic charts. The median clinical follow-up was 17.2 months (mean 20, range 2–59 months). Six patients with rectal cancer received neoadjuvant external beam radiation (total dose range 2000–4500 cGy). Patients gave informed consent for tumour banking, collection of clinical information, and subsequent decoded genetic analysis, as approved by the Washington University Human Studies Committee and the Siteman Cancer Center Protocol Review and Monitoring Committee.
Total RNA was extracted from the specimens using the Stratagene MicroRNA Isolation kit (Stratagene, La Jolla, CA, USA), and RNA was quantified with spectrophotometry. Reverse transcription to cDNA was performed using StrataScript reverse transcriptase with RNase Block Ribonuclease Inhibitor (Stratagene) and Oligo(dT) primers. TaqMan real-time PCR was performed on the ABI Prism 7900 Sequence Detector System (ABI, Foster City, CA, USA) with gene-specific dual-fluorescence-labelled oligonucleotide probes (Table 1). Each reaction well contained 10 ng cDNA, JumpStart Taq ReadyMix 2 × (5 μl, Sigma-Aldrich, St Louis, MO, USA), forward and reverse primers 10 μM (0.5 μl each), fluorescence-labelled oligonucleotide 5 μM (0.4 μl), ROX passive reference dye 100 × (0.1 μl), and molecular-grade water for a total reaction volume of 10 μl. RT–PCR was performed with the following parameters: 50°C for 2 min to activate UNG enzyme, 95°C for 10 min to denature UNG and activate DNA polymerase, 40 cycles at 95°C for 20 s and 60°C for 1 min. All specimen reactions were performed in triplicate with appropriate nontemplate controls, and the coefficient of variation (CV) was less than 5% for all replicates.
Table 1. TaqMan real-time PCR primers and probes.
| Gene (NCBI LocusLink ID) | Sequence | Reporter dye |
|---|---|---|
| ABCB1 (5243) | GCTGGCACAGAAAGGCATCT | |
| CAGAGTTCACTGGCGCTTTG | ||
| TCCAGCCTGGACACTGACCATTGAAA | TET | |
| ABCG2 (9429) | CAGGTCTGTTGGTCAATCTCACA | |
| CATATCGTGGAATGCTGAAGTACTG | ||
| CCATTGCATCTTGGCTGTCATGGC | TET | |
| CES2 (8824) | AATCCCAGCTATTGGGAAGGA | |
| CTGGCTGGTCGGTCTCAAAC | ||
| TGGCCTCAAGCCATCCTCCCATCT | TET | |
| CYP3A4 (1576) | TCTCCTTTCATATTTCTGGGAGACA | |
| GCATCGAGACAGTTGGGTGTT | ||
| TGTTTCCCTACACCTCTTGCATTCCATCCT | TET | |
| DPYD (1806) | ATCTTGAATCGTTGGATCTAGATCGA | |
| GAGTAGTAGCTAGTCGCTACTGAT | ||
| ACCGTACTCATCGTAGCTACCATGAA | TET | |
| ECGF1 (1890) | GGTTCCTGCGGACGGAAT | |
| GAGTAGTAGCTAGTCGCTACTGAT | ||
| CAGCCAGAGATGTGACAGCCACCG | TET | |
| ERCC1 (2067) | TACCCCTCGACGAGGATGAG | |
| CAGTGGGAAGGCTCTGTGTAGA | ||
| CCTGGAGTGGCCAAGCCCTTATTCC | TET | |
| ERCC2 (2068) | TTGGCGTCCCCTACGTCTAC | |
| CTGGTCCCGCAGGTATTCC | ||
| CACAGAGCCGCATTCTCAAGGCG | FAM | |
| GSTP1 (2950) | CCTCACCCTGTACCAGTCCAATA | |
| TCCTGCTGGTCCTTCCCATA | ||
| TCACCTTGGGCCGCACCCTTG | TET | |
| TOP1 (7150) | GGCGAGTGAATCTAAGGATAATGAA | |
| TGGATATCTTAAAGGGTACAGCGAA | ||
| ACCATTTTCCCATCATCCTTTGTTCTGAGC | TET | |
| TYMS (7298) | GCCTCGGTGTGCCTTTCA | |
| CGTGATGTGCGCAATCATG | ||
| CATCGCCAGCTACGCCCTGCTC | TET | |
| UGT1A1 (7361) | TTGGGAGTGCGGGATTCA | |
| AGATAAGATTAAAACTGCCATTTGCA | ||
| TGGTCCCACCGCTGCCCCTA | TET |
Sequences are listed as forward, reverse, and probe oligonucleotides, in the 5′ to 3′ direction.
Relative gene expression was calculated using a modified comparative threshold cycle (CT) method, as described previously (Pfaffl, 2001; Yu et al, 2003). This method accounts for varying RT–PCR amplification efficiencies between different primer/probe sets, rather than assuming all genes of interest amplify at an equal rate. Amplification efficiency (E) was determined from standard dilution curves for each primer/probe set. β-actin was used as the internal reference gene, as it had no significant variation between tumour and normal samples, and less than three-fold variation among all samples. Expression is given as a unit-less measure relative to the calibrator specimen that had the highest CT (i.e. the 1 × sample), using the following equation:
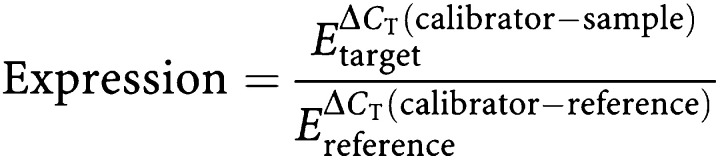 |
All statistical analyses were performed with GraphPad InStat (GraphPad Software, San Diego, CA, USA). The Mann–Whitney and Fisher's exact tests were utilised as appropriate. Comparisons between tumour samples and patient-matched normal mucosa were made using the Wilcoxon test for matched pairs. Survival curves were generated using the Kaplan–Meier method, with significance assessed by the log-rank test. A P-value less than 0.05 was considered significant. Values are expressed as mean [−95% confidence interval (CI), +95% CI].
RESULTS
Patient characteristics are depicted in Table 2. All patients underwent resection with curative intent. As previously mentioned, all patients had positive lymph node metastases (AJCC stage III). There were no significant differences between the mucinous and nonmucinous groups for age at diagnosis, gender, tumour size, histologic grade, or number of positive lymph nodes. No mucinous tumours occurred in the rectum, whereas 20% of the nonmucinous tumours were rectal. However, differences in tumour location did not reach statistical significance. A total of 13 mucinous tumours occurred proximal to the splenic flexure, compared to 14 nonmucinous tumours (P=0.58).
Table 2. Patient characteristics.
| Mucinous | Nonmucinous | P-value | |
|---|---|---|---|
| N | 21 | 30 | — |
| Age (years) | 68.9 | 69.2 | 0.98 |
| Male:female | 12:9 | 16:14 | 1.00 |
| Tumour diameter (cm) | 5.3 | 5.1 | 0.31 |
| Location | 0.16 | ||
| Right | 9 (43%) | 12 (40%) | — |
| Transverse | 4 (19%) | 3 (10%) | — |
| Left/sigmoid | 8 (38%) | 9 (30%) | — |
| Rectum | 0 (0%) | 6 (20%) | — |
| Median no. LNs evaluated | 19 | 16.5 | 0.52 |
| Median no. positive LNs | 2 | 2 | 0.78 |
| Tumour differentiation | 0.71 | ||
| Poor | 5 (24%) | 7 (23%) | — |
| Moderate | 13 (62%) | 16 (53%) | — |
| Well | 3 (14%) | 7 (23%) | — |
All patients are stage III.
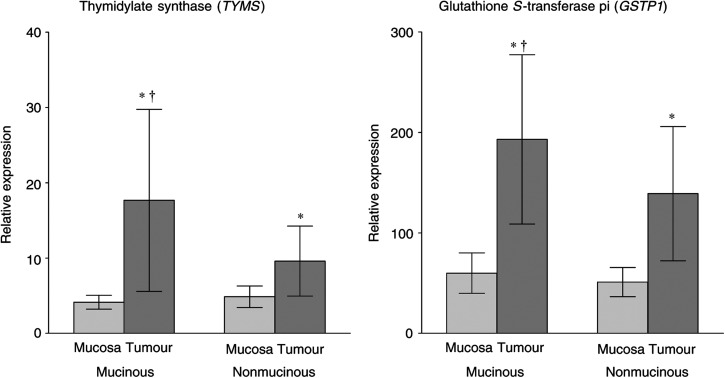
Relative gene expression of DPYD, ECGF1, ERCC1, ERCC2, ABCB1, ABCG2, CYP3A4, UGT1A1, CES2, and TOP1 did not differ significantly between mucinous and nonmucinous tumours, although the difference in ERCC2 expression approached statistical significance (P=0.083), with higher expression in the mucinous subgroup. However, as depicted in Figure 1, TYMS expression was significantly higher in mucinous tumours compared to nonmucinous tumours (17.7 arbitrary units [95% CI 5.8, 30.0] vs 9.6 [5.0, 14.3] respectively, P=0.013). Overexpression of TYMS was also observed in both subtypes of adenocarcinoma compared to normal mucosa. Relative to patient-matched mucosa, nonmucinous tumours demonstrated less overexpression (tumour mean 9.6 [5.0, 14.3] vs mucosal mean 4.9 [3.5, 6.3], P=0.01) than the mucinous variant (tumour mean 17.7 [5.8, 30.0] vs mucosal mean 4.1 [3.2, 5.1], P<0.001). There was no difference in nonmalignant mucosal expression of TYMS between patients with mucinous and nonmucinous cancers.
Figure 1.
Differential expression of TYMS and GSTP1 in mucinous and nonmucinous colorectal adenocarcinoma. *P<0.001 relative to patient-matched normal mucosa; †P<0.05 relative to nonmucinous adenocarcinoma. Error bars represent the 95% CI of the mean.
GSTP1 expression was also significantly greater in mucinous tumours (193.0 [108.8, 277.3]) than in nonmucinous tumours (139.0 [72.2, 205.8], P=0.029). Both histologic tumour subtypes had higher expression of GSTP1 than the matched normal mucosa. The degree of overexpression in mucinous tumours (tumour mean 193.0 [108.8, 277.3] vs mucosal mean 60.0 [39.9, 80.0], P<0.001) was greater than in nonmucinous tumours (tumour mean 139.0 [72.2, 205.8] vs mucosal mean 51.0 [36.4, 65.6], P<0.001). There was no difference in nonmalignant mucosal expression of GSTP1 in patients with mucinous and nonmucinous cancers.
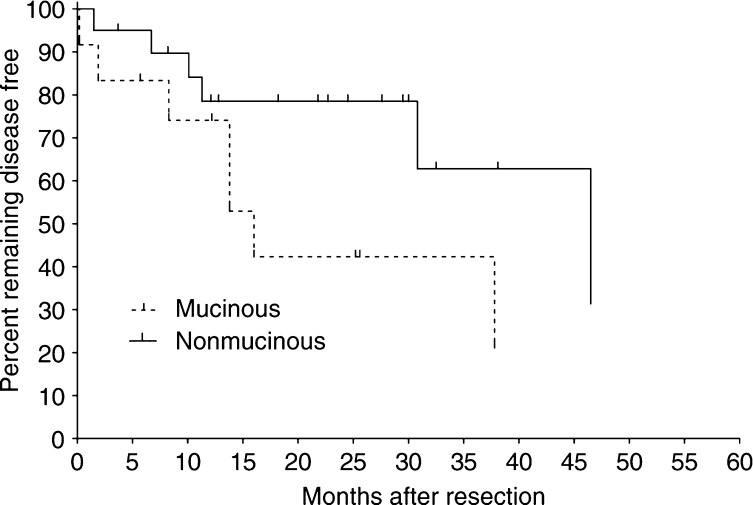
Clinical follow-up data were available for 44 of the 51 patients (86%); those without complete data were excluded from further analyses. Of the 44 patients, 17 experienced recurrence. Time to disease recurrence was evaluated for mucinous and nonmucinous tumours. Disease-free survival between the two groups was not significantly different, with median times to recurrence of 54.2 and 46.5 months (P=0.5) for patients with mucinous and nonmucinous adenocarcinoma, respectively. In all, 14 patients either did not complete a course of primary 5-FU-based chemotherapy (N=8) or were treated with other first-line treatment (N=6, irinotecan or neoadjuvant radiation only for rectal cancer). The median DFS for all 14 patients did not differ significantly from the entire cohort (52 months), nor was there a detectable difference between those with mucinous or nonmucinous tumours. However, subset analysis of the 30 patients who received 5-FU as primary adjuvant treatment revealed a shorter time to recurrence in patients with mucinous tumours (Figure 2). The median DFS was 13.8 months in the population of patients with mucinous adenocarcinoma (N=11) who received 5-FU chemotherapy. Conversely, patients with nonmucinous tumours (N=19) who underwent 5-FU treatment had a median DFS of 46.5 months; the trend of shorter time to recurrence in these patients approached statistical significance (P=0.053). Among patients treated with 5-FU, those with mucinous tumours had significantly higher TYMS expression (mean 20.8 vs 7.4 for patients with nonmucinous tumours who received 5-FU, P=0.014). Owing to limited patient numbers and clinical follow-up, subset analysis of patients receiving other chemotherapeutic agents (i.e. oxaliplatin or irinotecan) could not be performed. There were no differences in recurrence or survival when patients who were treated with adjuvant chemotherapy were stratified based on whether their tumour expressed greater than or less than the median level of either TYMS or GSTP1.
Figure 2.
Disease-free survival based on histologic subtype for all patients who received adjuvant 5-FU (N=30 total; P=0.053).
DISCUSSION
It is generally recognised that mucinous adenocarcinomas of the colon and rectum have a worse prognosis following resection than nonmucinous tumours (Symonds and Vickery, 1976; Umpleby et al, 1985; Green et al, 1993; Consorti et al, 2000; Nozoe et al, 2000). Compared to the more common nonmucinous variety, mucinous tumours metastasise to lymph nodes with increased frequency (Nozoe et al, 2000), are more prone to local recurrence (Umpleby et al, 1985; Connelly et al, 1991) and peritoneal carcinomatosis (Nozoe et al, 2000), are less likely to be resected with negative margins (Umpleby et al, 1985), and are typically diagnosed at an advanced stage (Younes et al, 1993). They also have altered p53 expression (Hanski et al, 1996) and increased microsatellite instability (Messerini et al, 1997). Although mucinous tumours occur more frequently in the proximal colon, left-sided and rectal mucinous tumours have an overall worse prognosis relative to location-matched nonmucinous tumours (Green et al, 1993). This finding may be at least partially explained by over-expression of both p53 (Lenz et al, 1996) and thymidylate synthase (Allegra et al, 2002) in left-sided tumours, although no previous study has examined expression in the mucinous subset alone. Based on these genetic differences and its more aggressive clinical behaviour, some authors have suggested that mucinous adenocarcinomas should be categorised and treated as a biological entity distinct from other colorectal adenocarcinomas (Consorti et al, 2000). The current study confirms the biological difference between mucinous and nonmucinous tumours. We found significantly higher expression of thymidylate synthase (TYMS) and GSTP1 in mucinous colorectal adenocarcinomas than in equivalent-stage nonmucinous tumours. Both mucinous and nonmucinous tumours also overexpressed these genes relative to normal colonic mucosa.
The association between high levels of TYMS in tumours and poor clinical response following 5-FU treatment of colorectal cancer is well documented (Johnston et al, 1995; Leichman et al, 1997; Salonga et al, 2000; Iacopetta et al, 2001). Elevated expression has also been correlated with time to recurrence in patients with stage II or III disease (Kornmann et al, 2002). The finding of overexpression of TYMS in mucinous tumours in our study offers a possible explanation as to why patients with these tumours tend to do worse. To our knowledge, response of mucinous tumours to 5-FU treatment has not been specifically investigated. In the recent literature regarding mucinous adenocarcinoma, the large majority of patients with locally advanced colorectal cancer would have received 5-FU. Perhaps decreased response to 5-FU due to overexpression of TYMS contributes to the relatively poorer prognosis for patients with mucinous adenocarcinoma. Among patients in our study who received 5-FU following initial surgical resection, there was a trend towards increased DFS only in the nonmucinous tumour group. Although the P-value did not reach the significance threshold of 0.05, this finding intuitively makes sense, given the fact that tumours in the nonmucinous group had significantly lower expression of TYMS. Owing to the small number of patients who either did not complete 5-FU-based chemotherapy or received other adjuvant treatment, no definitive conclusions can be drawn.
Oxaliplatin causes cell death by forming bulky, DNA helix-distorting adducts via crosslinking between guanine bases. GSTP1 belongs to the glutathione S-transferase superfamily and is a major mechanism of detoxification of platinum agents. It is highly overexpressed in colorectal cancer, with increased levels in drug-resistant tumours (Stoehlmacher et al, 2002). In the current study, GSTP1 expression was found to be similarly elevated in malignant tissue relative to normal colonic mucosa. Interestingly, expression was significantly greater in mucinous tumours. As our cohort of tumours was assembled in a retrospective manner from historically collected samples, none had been treated with oxaliplatin. However, the finding of elevated GSTP1 expression within mucin-producing tumours suggests that diminished clinical response may be expected from oxaliplatin-treated tumours. Further research examining this hypothesis is warranted.
Regarding the remaining ten markers, there were no significant differences in gene expression between mucinous and nonmucinous colorectal cancers. This negative finding does not imply they do not have a role in determining outcome following chemotherapy. Rather, they may not contribute to the differing clinical responses seen in the treatment of mucinous adenocarcinoma relative to the nonmucinous variant. In fact, it would be expected that altered expression of some genes contributes to the general drug-resistant phenotype seen in all colorectal adenocarcinomas, while expression of others distinguishes individual biologically distinct subtypes and determines their relative clinical responsiveness.
The intent of the current study was to examine the relative expression in colorectal cancer of certain genes believed to influence clinical response to adjuvant chemotherapy. It was not designed to characterise extensively the relationships between gene expression, response to chemotherapy, and patient outcome. The clinical follow-up data were limited, with a median follow-up of only 17.2 months. Since this is a retrospective study, chemotherapy selection and dosing were not standardised. Additionally, rectal cancers were somewhat under-represented, comprising 20% of the nonmucinous cohort vs approximately 30% of all colorectal adenocarcinomas. Nonetheless, several significant associations were found that may lead to further investigations. Longer patient follow-up and standardised treatment will be required to fully explore the role of TYMS and GSTP1 overexpression in mucinous adenocarcinoma. Protein analysis of these enzymes may validate our findings and provide further insight into their complex intracellular interactions. However, much of the data supporting the negative influence of overexpression of both TYMS and GSTP1 on patient outcome has been generated by examining either germline polymorphisms or transcriptional levels (Metzger et al, 1998a; Salonga et al, 2000; Stoehlmacher et al, 2002).
In addition to pharmacogenetic markers for outcome following specific drug treatment, there are a variety of patient characteristics (e.g. lymph node metastases, age), histologic findings (e.g. neurovascular invasion, differentiation), and genetic factors (e.g. p53 expression, microsatellite instability), which appear to influence patient prognosis in colorectal cancer. We chose not to perform an exhaustive examination of all such prognostic factors, but instead to examine the heterogeneity and differential expression of known pharmacogenetic markers in mucinous adenocarcinomas. Whether other markers of prognosis such as microsatellite instability can be utilised clinically to predict outcome in mucinous colorectal cancer remains to be determined.
In conclusion, mucinous tumours significantly overexpressed TYMS and GSTP1 relative to both normal mucosa and to nonmucinous adenocarcinomas. Both these genes are known markers for resistance to chemotherapy. Among those who received adjuvant 5-FU, DFS was shorter in patients with mucinous tumours. It is unclear whether the overexpression of TYMS contributed to these earlier recurrences. Clinical response may be improved for mucinous colorectal cancer if treated with combination chemotherapy or perhaps agents such as irinotecan, where no differences in drug pathway markers were identified. The mucin status of colorectal tumours should be carefully addressed in future trials of novel treatments.
Acknowledgments
This work supported in part by the NIH Pharmacogenetics Research Network (U01 GM63340; http://pharmacogenetics.wustl. edu) and the Siteman Cancer Center (P30CA091842).
References
- Allegra CJ, Parr AL, Wold LE, Mahoney MR, Sargent DJ, Johnston P, Klein P, Behan K, O'Connell MJ, Levitt R, Kugler JW, Tria Tirona M, Goldberg RM (2002) Investigation of the prognostic and predictive value of thymidylate synthase, p53, and Ki-67 in patients with locally advanced colon cancer. J Clin Oncol 20: 1735–1743 [DOI] [PubMed] [Google Scholar]
- Collie-Duguid ES, Johnston SJ, Boyce L, Smith N, Cowieson A, Cassidy J, Murray GI, McLeod HL (2001) Thymidine phosphorylase and dihydropyrimidine dehydrogenase protein expression in colorectal cancer. Int J Cancer 94: 297–301 [DOI] [PubMed] [Google Scholar]
- Connelly JH, Robey-Cafferty SS, Cleary KR (1991) Mucinous carcinomas of the colon and rectum. An analysis of 62 stage B and C lesions. Arch Pathol Lab Med 115: 1022–1025 [PubMed] [Google Scholar]
- Consorti F, Lorenzotti A, Midiri G, Di Paola M (2000) Prognostic significance of mucinous carcinoma of colon and rectum: a prospective case–control study. J Surg Oncol 73: 70–74 [DOI] [PubMed] [Google Scholar]
- Etienne MC, Cheradame S, Fischel JL, Formento P, Dassonville O, Renee N, Schneider M, Thyss A, Demard F, Milano G (1995) Response to fluorouracil therapy in cancer patients: the role of tumoral dihydropyrimidine dehydrogenase activity. J Clin Oncol 13: 1663–1670 [DOI] [PubMed] [Google Scholar]
- Gottesman MM, Fojo T, Bates SE (2002) Multidrug resistance in cancer: role of ATP-dependent transporters. Nat Rev Cancer 2: 48–58 [DOI] [PubMed] [Google Scholar]
- Green JB, Timmcke AE, Mitchell WT, Hicks TC, Gathright Jr JB, Ray JE (1993) Mucinous carcinoma – just another colon cancer? Dis Colon Rect 36: 49–54 [DOI] [PubMed] [Google Scholar]
- Halvorsen TB, Seim E (1988) Influence of mucinous components on survival in colorectal adenocarcinomas: a multivariate analysis. J Clin Pathol 41: 1068–1072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanski C, Tiecke F, Hummel M, Hanski M-L, Ogorek D, Rolfs A, Schmitt-Graff A, Stein H, Riecken E-O (1996) Low frequency of p53 gene mutation and protein expression in mucinous colorectal carcinomas. Cancer Lett 103: 163–170 [DOI] [PubMed] [Google Scholar]
- Iacopetta B, Grieu F, Joseph D, Elsaleh H (2001) A polymorphism in the enhancer region of the thymidylate synthase promoter influences the survival of colorectal cancer patients treated with 5-fluorouracil. Br J Cancer 85: 827–830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iyer L, Das S, Janisch L, Wen M, Ramirez J, Karrison T, Fleming GF, Vokes EE, Schilsky RL, Ratain MJ (2002) UGT1A1*28 polymorphism as a determinant of irinotecan disposition and toxicity. Pharmacogenom J 2: 43–47 [DOI] [PubMed] [Google Scholar]
- Iyer L, Hall D, Das S, Mortell MA, Ramirez J, Kim S, Di Rienzo A, Ratain MJ (1999) Phenotype–genotype correlation of in vitro SN-38 (active metabolite of irinotecan) and bilirubin glucuronidation in human liver tissue with UGT1A1 promoter polymorphism. Clin Pharmacol Ther 65: 576–582 [DOI] [PubMed] [Google Scholar]
- Johnston PG, Lenz H-J, Leichman CG, Danenberg KD, Allegra CJ, Danenberg PV, Leichman L (1995) Thymidylate synthase gene and protein expression correlate and are associated with response to 5-fluorouracil in human colorectal and gastric tumors. Cancer Res 55: 1407–1412 [PubMed] [Google Scholar]
- Kakar S, Aksoy S, Burgart L, Smyrk T (2004) Mucinous carcinoma of the colon: correlation of loss of mismatch repair enzymes with clinicopathologic features and survival. Mod Pathol 17: 696–700 [DOI] [PubMed] [Google Scholar]
- Kehrer DF, Mathijssen RH, Verweij J, de Bruijn P, Sparreboom A (2002) Modulation of irinotecan metabolism by ketoconazole. J Clin Oncol 20: 3122–3129 [DOI] [PubMed] [Google Scholar]
- Kornmann M, Link KH, Galuba I, Ott K, Schwabe W, Hausler P, Scholz P, Strater J, Polat S, Leibl B, Kettner E, Schlichting C, Baumann W, Schramm H, Hecker U, Ridwelski K, Vogt JH, Zerbian KU, Schutze F, Kreuser ED, Behnke D, Beger HG (2002) Association of time to recurrence with thymidylate synthase and dihydropyrimidine dehydrogenase mRNA expression in stage II and III colorectal cancer. J Gastrointest Surg 6: 331–337 [DOI] [PubMed] [Google Scholar]
- Leichman CG, Lenz HJ, Leichman L, Danenberg K, Baranda J, Groshen S, Boswell W, Metzger R, Tan M, Danenberg PV (1997) Quantitation of intratumoral thymidylate synthase expression predicts for disseminated colorectal cancer response and resistance to protracted–infusion fluorouracil and weekly leucovorin. J Clin Oncol 15: 3223–3229 [DOI] [PubMed] [Google Scholar]
- Lenz H-J, Leichman CG, Danenberg KD, Danenberg PV, Groshen S, Cohen H, Laine L, Crookes P, Silberman H, Baranda J, Garcia Y, Li J, Leichman L (1996) Thymidylate synthase mRNA level in adenocarcinoma of the stomach: a predictor for primary tumor response and overall survival. J Clin Oncol 14: 176–182 [DOI] [PubMed] [Google Scholar]
- McLeod HL, Murray GI (1999) Tumour markers of prognosis in colorectal cancer. Br J Cancer 79: 191–203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messerini L, Vitelli F, De Vitis LR, Mori S, Calzolari A, Palmirotta R, Calabro A, Papi L (1997) Microsatellite instability in sporadic mucinous colorectal carcinomas: relationship to clinico-pathological variables. J Pathol 182: 380–384 [DOI] [PubMed] [Google Scholar]
- Metzger R, Danenberg K, Leichman CG, Salonga D, Schwartz EL, Wadler S, Lenz HJ, Groshen S, Leichman L, Danenberg PV (1998a) High basal level gene expression of thymidine phosphorylase (platelet-derived endothelial cell growth factor) in colorectal tumors is associated with nonresponse to 5-fluorouracil. Clin Cancer Res 4: 2371–2376 [PubMed] [Google Scholar]
- Metzger R, Leichman CG, Danenberg KD, Danenberg PV, Lenz HJ, Hayashi K, Groshen S, Salonga D, Cohen H, Laine L, Crookes P, Silberman H, Baranda J, Konda B, Leichman L (1998b) ERCC1 mRNA levels complement thymidylate synthase mRNA levels in predicting response and survival for gastric cancer patients receiving combination cisplatin and fluorouracil chemotherapy. J Clin Oncol 16: 309–316 [DOI] [PubMed] [Google Scholar]
- Nita ME, Tominaga O, Nagawa H, Tsuruo T, Muto T (1998) Dihydropyrimidine dehydrogenase but not thymidylate synthase expression is associated with resistance to 5-fluorouracil in colorectal cancer. Hepato-Gastroenterol 45: 2117–2122 [PubMed] [Google Scholar]
- Nozoe T, Anai H, Nasu S, Sugimachi K (2000) Clinicopathological characteristics of mucinous carcinoma of the colon and rectum [comment]. J Surg Oncol 75: 103–107 [DOI] [PubMed] [Google Scholar]
- Park DJ, Stoehlmacher J, Zhang W, Tsao-Wei DD, Groshen S, Lenz HJ (2001) A xeroderma pigmentosum group D gene polymorphism predicts clinical outcome to platinum-based chemotherapy in patients with advanced colorectal cancer. Cancer Res 61: 8654–8658 [PubMed] [Google Scholar]
- Patel J, Mitra AK (2001) Strategies to overcome simultaneous P-glycoprotein mediated efflux and CYP3A4 mediated metabolism of drugs. Pharmacogenomics 2: 401–415 [DOI] [PubMed] [Google Scholar]
- Pfaffl MW (2001) A new mathematical model for relative quantification in real-time RT–PCR. Nucleic Acids Res 29: e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salonga D, Danenberg KD, Johnson M, Metzger R, Groshen S, Tsao-Wei DD, Lenz H-J, Leichman CG, Leichman L, Diasio RB, Danenberg PV (2000) Colorectal tumors responding to 5-fluorouracil have low gene expression levels of dihydropyrimidine dehydrogenase, thymidylate synthase, and thymidine phosphorylase. Clin Cancer Res 6: 1322–1327 [PubMed] [Google Scholar]
- Sasaki O, Atkins WS, Jass JR (1987) Mucinous carcinoma of the rectum. Histopathology 37: 1891–1900 [DOI] [PubMed] [Google Scholar]
- Stoehlmacher J, Park DJ, Zhang W, Groshen S, Tsao-Wei DD, Yu MC, Lenz H-J (2002) Association between glutathione S-transferase P1, T1, and M1 genetic polymorphism and survival of patients with metastatic colorectal cancer. J Natl Cancer Inst 94: 936–942 [DOI] [PubMed] [Google Scholar]
- Symonds DA, Vickery AL (1976) Mucinous carcinoma of the colon and rectum. Cancer 37: 1891–1900 [DOI] [PubMed] [Google Scholar]
- Umpleby HC, Ranson DL, Williamson RC (1985) Peculiarities of mucinous colorectal carcinoma. Br J Surg 72: 715–718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wierdl M, Wall A, Morton CL, Sampath J, Danks MK, Schuetz JD, Potter PM (2003) Carboxylesterase-mediated sensitization of human tumor cells to CPT-11 cannot override ABCG2-mediated drug resistance. Mol Pharmacol 64: 279–288 [DOI] [PubMed] [Google Scholar]
- Wu MH, Yan B, Humerickhouse R, Dolan ME (2002) Irinotecan activation by human carboxylesterases in colorectal adenocarcinoma cells. Clin Cancer Res 8: 2696–2700 [PubMed] [Google Scholar]
- Xu Y, Villalona-Calero MA (2002) Irinotecan: mechanisms of tumor resistance and novel strategies for modulating its activity. Ann Oncol 13: 1841–1851 [DOI] [PubMed] [Google Scholar]
- Younes M, Katikaneni PR, Lechago J (1993) The value of the preoperative mucosal biopsy in the diagnosis of colorectal mucinous adenocarcinoma. Cancer 72: 3588–3592 [DOI] [PubMed] [Google Scholar]
- Yu J, Marsh S, Ahluwalia R, McLeod HL (2003) Ferredoxin reductase: pharmacogenomic assessment in colorectal cancer. Cancer Res 63: 6170–6173 [PubMed] [Google Scholar]