Abstract
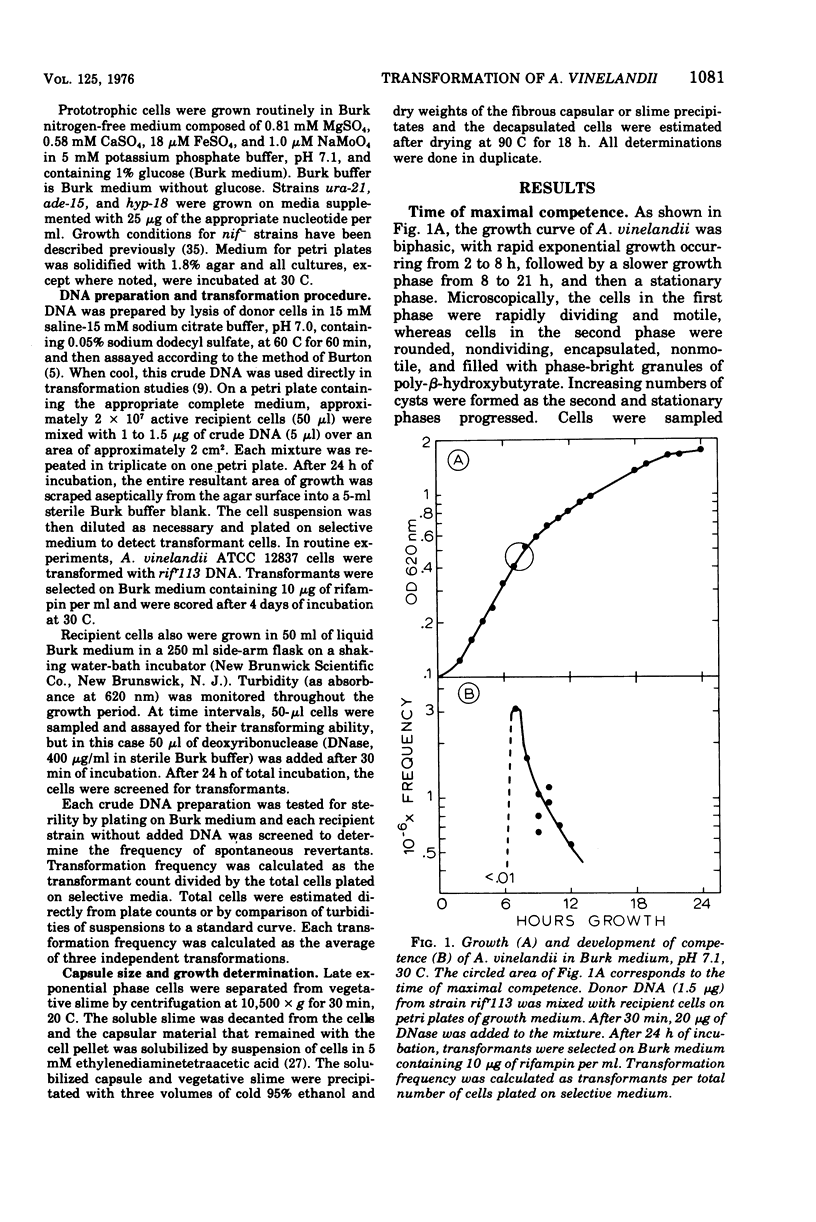
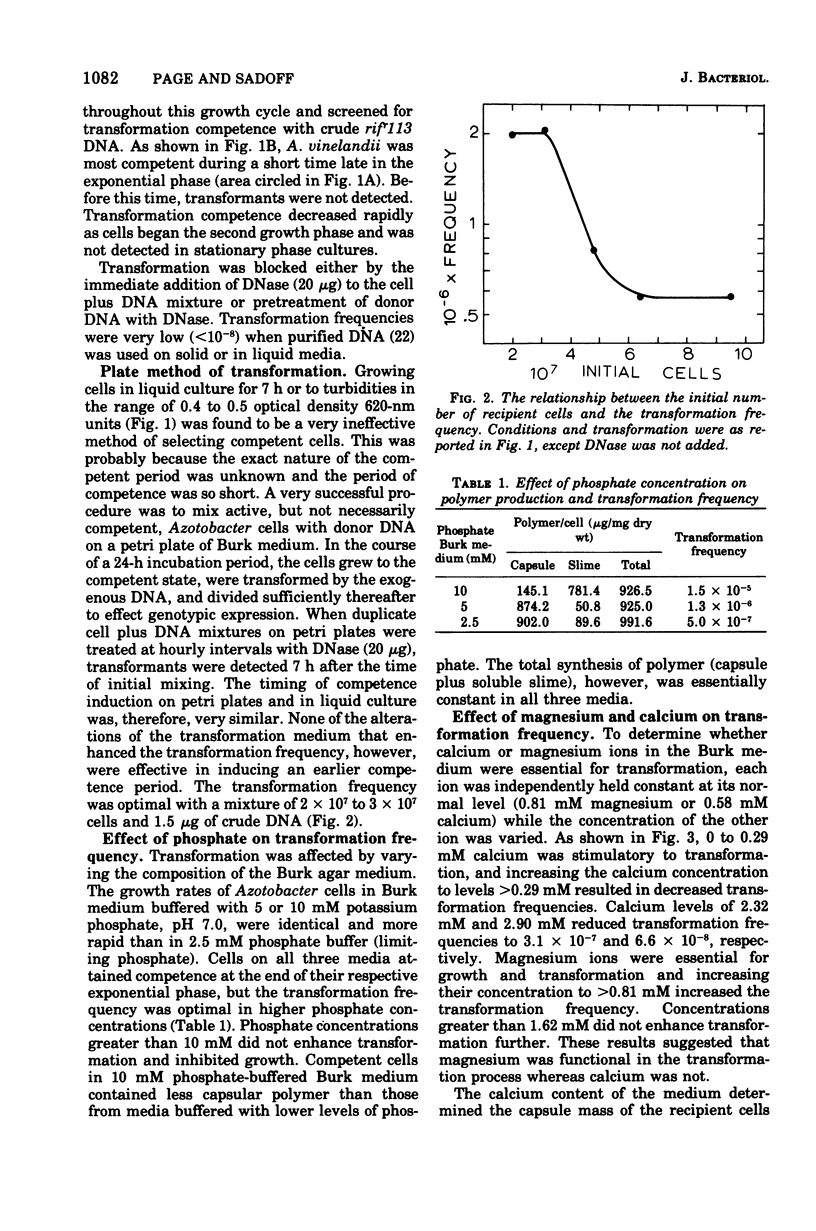
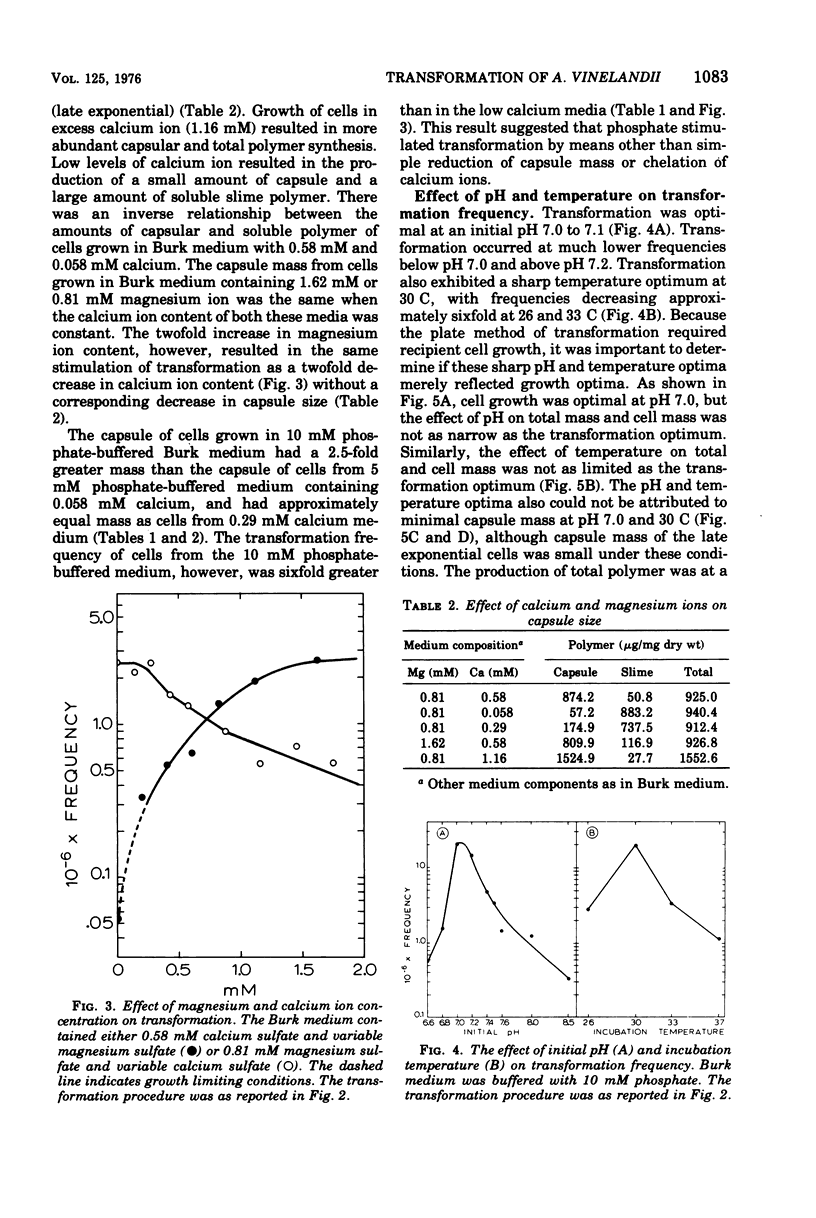
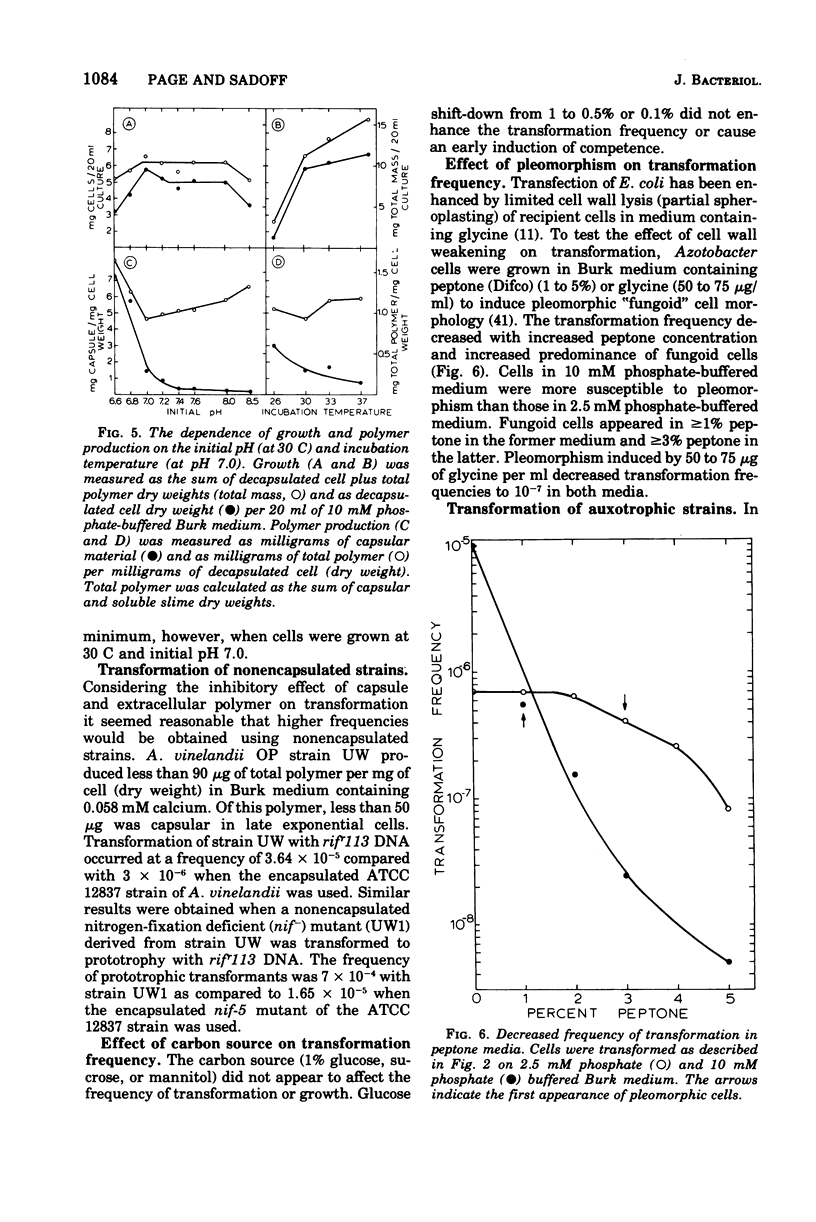
Cells of Azotobacter vinelandii (ATCC 12837) can be transformed by exogenous deoxyribonucleic acid towards the end of exponential growth. Transformation occurs at very low frequencies when the deoxyribonucleic acid is purified or when the transformation is carried out in liquid medium. Optimal transformation occurs on plates of Burk nitrogen-free glucose medium containing either high phosphate (10 mM) or low calcium (0 to 0.29 mM) content. Higher levels of calcium are inhibitory, whereas magnesium ions are essential for transformation and growth. Extracellular polymer and capsule are increasingly inhibitory to transformation and are most abundant when the calcium content of the medium is high. Transformation is optimal at pH 7.0 to 7.1 and at 30 C, conditions which also coincide with minimal extracellular polymer production. Nonencapsulated strains are excellent transformation recipients. Glycine-induced pleomorphism reduces the transformation frequency and the degree of inhibition is dependent on the phosphate concentration of the medium. Rifampin resistance and shifts from adenine, hypoxanthine, uracil, and nitrogenase auxotrophy to prototrophy can be achieved. Although single marker transfer is always greater than double marker transfer, the data suggest that rifampin resistance is linked to hypoxanthine, adenine and uracil protorophy at intervals of increasing distance. Rifampin resistance did not appear to be linked to nitrogenase.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aharonowitz Y., Ron E. Z. Transformation in Bacillus subtilis. A simple direct method. Mol Gen Genet. 1970;107(1):94–96. doi: 10.1007/BF00433227. [DOI] [PubMed] [Google Scholar]
- Anagnostopoulos C., Spizizen J. REQUIREMENTS FOR TRANSFORMATION IN BACILLUS SUBTILIS. J Bacteriol. 1961 May;81(5):741–746. doi: 10.1128/jb.81.5.741-746.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BURTON K. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J. 1956 Feb;62(2):315–323. doi: 10.1042/bj0620315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bettinger G. E., Young F. E. Transformation of Bacillus subtilis: transforming ability of deoxyribonucleic acid in lysates of L-forms or protoplasts. J Bacteriol. 1975 Jun;122(3):987–993. doi: 10.1128/jb.122.3.987-993.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fung P. H., Winter A. J. Effects of penicillin and glycine on cell wall glycopeptides of the two varieties of Vibrio fetus. J Bacteriol. 1968 Dec;96(6):1889–1894. doi: 10.1128/jb.96.6.1889-1894.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GWINN D. D., THORNE C. B. TRANSFORMATION OF BACILLUS LICHENIFORMIS. J Bacteriol. 1964 Mar;87:519–526. doi: 10.1128/jb.87.3.519-526.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haug A., Larsen B. Biosynthesis of alginate. II. Polymannuronic acid C-5-epimerase from Azotobacter vinelandii (Lipman). Carbohydr Res. 1971 Apr;17(2):297–308. doi: 10.1016/s0008-6215(00)82537-9. [DOI] [PubMed] [Google Scholar]
- ILIASHENKO B. N. SFEROPLASTY BAKTERI I KAK RETSIPIENTY INFEKTSIONNYKH DNK BAKTERIOFAGOV. Mikrobiologiia. 1964 Sep-Oct;33:812–818. [PubMed] [Google Scholar]
- Juni E., Janik A. Transformation of Acinetobacter calco-aceticus (Bacterium anitratum). J Bacteriol. 1969 Apr;98(1):281–288. doi: 10.1128/jb.98.1.281-288.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KELLY M. S., PRITCHARD R. H. UNSTABLE LINKAGE BETWEEN GENETIC MARKERS IN TRANSFORMATION. J Bacteriol. 1965 May;89:1314–1321. doi: 10.1128/jb.89.5.1314-1321.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly M. S. Physical and mapping properties of distant linkages between genetic markers in transformation of Bacillus subtilis. Mol Gen Genet. 1967;99(4):333–349. doi: 10.1007/BF00330909. [DOI] [PubMed] [Google Scholar]
- Kelly M. S. The causes of instability of linkage in transformation of Bacillus subtilis. Mol Gen Genet. 1967;99(4):350–361. doi: 10.1007/BF00330910. [DOI] [PubMed] [Google Scholar]
- LEONARD C. G., MATTHEIS M. J. DIFFERENT TRANSFORMING CHARACTERISTICS OF COLONIAL VARIANTS FROM AUXOTROPHIC MUTANTS OF BACILLUS LICHENIFORMIS. J Bacteriol. 1965 Aug;90:558–559. doi: 10.1128/jb.90.2.558-559.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacks S., Greenberg B., Neuberger M. Role of a deoxyribonuclease in the genetic transformation of Diplococcus pneumoniae. Proc Natl Acad Sci U S A. 1974 Jun;71(6):2305–2309. doi: 10.1073/pnas.71.6.2305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen B., Haug A. Biosynthesis of alginate. 1. Composition and structure of alginate produced by Azotobacter vinelandii (Lipman). Carbohydr Res. 1971 Apr;17(2):287–296. doi: 10.1016/s0008-6215(00)82536-7. [DOI] [PubMed] [Google Scholar]
- Lin L. P., Sadoff H. L. Encystment and polymer production by Azotobacter vinelandii in the presence of beta-hydroxybutyrate. J Bacteriol. 1968 Jun;95(6):2336–2343. doi: 10.1128/jb.95.6.2336-2343.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison D. A. Early intermediate state of transforming deoxyribonucleic acid during uptake by Bacillus subtilis. J Bacteriol. 1971 Oct;108(1):38–44. doi: 10.1128/jb.108.1.38-44.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NESTER E. W., LEDERBERG J. Linkage of genetic units of Bacillus subtilis in DNA transformation. Proc Natl Acad Sci U S A. 1961 Jan 15;47:52–55. doi: 10.1073/pnas.47.1.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page W. J., Sadoff H. L. Relationship between calcium and uroinic acids in the encystment of Azotobacter vinelandii. J Bacteriol. 1975 Apr;122(1):145–151. doi: 10.1128/jb.122.1.145-151.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RAVIN A. W. The significance of bacterial transformations to studies of the neoplastic process. Ann N Y Acad Sci. 1957 Oct 21;68(2):335–348. doi: 10.1111/j.1749-6632.1957.tb56089.x. [DOI] [PubMed] [Google Scholar]
- STROMINGER J. L., BIRGE C. H. NUCLEOTIDE ACCUMULATION INDUCED IN STAPHYLOCOCCUS AUREUS BY GLYCINE. J Bacteriol. 1965 Apr;89:1124–1127. doi: 10.1128/jb.89.4.1124-1127.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STUY J. H., STERN D. THE KINETICS OF DNA UPTAKE BY HAEMOPHILUS INFLUENZAE. J Gen Microbiol. 1964 Jun;35:391–400. doi: 10.1099/00221287-35-3-391. [DOI] [PubMed] [Google Scholar]
- Sadoff H. L., Berke E., Loperfido B. Physiological studies of encystment in Azotobacter vinelandii. J Bacteriol. 1971 Jan;105(1):185–189. doi: 10.1128/jb.105.1.185-189.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanderson K. E. Current linkage map of Salmonella typhimurium. Bacteriol Rev. 1970 Jun;34(2):176–193. doi: 10.1128/br.34.2.176-193.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sen M., Pal T. K., Sen S. P. Intergeneric transformation between Rhizobium and Azotobacter. Antonie Van Leeuwenhoek. 1969;35(4):533–540. doi: 10.1007/BF02219171. [DOI] [PubMed] [Google Scholar]
- Sen M., Sen S. P. Interspecific transformation in Azotobacter. J Gen Microbiol. 1965 Oct;41(1):1–6. doi: 10.1099/00221287-41-1-1. [DOI] [PubMed] [Google Scholar]
- Shah V. K., Davis I. C., Gordon J. K., Orme-Johnson W. H., Brill W. J. Nitrogenase. 3. Nitrogenaseless mutants of Azotobacter vinelandii: activities, cross-reactions and EPR spectra. Biochim Biophys Acta. 1973 Jan 18;292(1):246–255. doi: 10.1016/0005-2728(73)90269-7. [DOI] [PubMed] [Google Scholar]
- St John R. T., Johnston H. M., Seidman C., Garfinkel D., Gordon J. K., Shah V. K., Brill W. J. Biochemistry and genetics of Klebsiella pneumoniae mutant strains unable to fix N2. J Bacteriol. 1975 Mar;121(3):759–765. doi: 10.1128/jb.121.3.759-765.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanooka H. Transformation of germinated spores of Bacillus subtilis on agar plates. J Bacteriol. 1973 Apr;114(1):445–447. doi: 10.1128/jb.114.1.445-447.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tichy P., Landman O. E. Transformation in quasi spheroplasts of Bacillus subtilis. J Bacteriol. 1969 Jan;97(1):42–51. doi: 10.1128/jb.97.1.42-51.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vela G. R., Rosenthal R. S. Effect of peptone on Azotobacter morphology. J Bacteriol. 1972 Jul;111(1):260–266. doi: 10.1128/jb.111.1.260-266.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YOUNG F. E., SPIZIZEN J. BIOCHEMICAL ASPECTS OF COMPETENCE IN THE BACILLUS SUBTILIS TRANSFORMATION SYSTEM. II. AUTOLYTIC ENZYME ACTIVITY OF CELL WALLS. J Biol Chem. 1963 Sep;238:3126–3130. [PubMed] [Google Scholar]
- YOUNG F. E., SPIZIZEN J. INCORPORATION OF DEOXYRIBONUCLEIC ACID IN THE BACILLUS SUBTILIS TRANSFORMATION SYSTEM. J Bacteriol. 1963 Sep;86:392–400. doi: 10.1128/jb.86.3.392-400.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]



