Abstract
Loss of skeletal muscle in cancer cachexia has a negative effect on both morbidity and mortality. The role of nuclear factor-κB (NF-κB) in regulating muscle protein degradation and expression of the ubiquitin–proteasome proteolytic pathway in response to a tumour cachectic factor, proteolysis-inducing factor (PIF), has been studied by creating stable, transdominant-negative, muscle cell lines. Murine C2C12 myoblasts were transfected with plasmids with a CMV promoter that had mutations at the serine phosphorylation sites required for degradation of I-κBα, an NF-κB inhibitory protein, and allowed to differentiate into myotubes. Proteolysis-inducing factor induced degradation of I-κBα, nuclear accumulation of NF-κB and an increase in luciferase reporter gene activity in myotubes containing wild-type, but not mutant, I-κBα proteins. Proteolysis-inducing factor also induced total protein degradation and loss of the myofibrillar protein myosin in myotubes containing wild-type, but not mutant, plasmids at the same concentrations as those causing activation of NF-κB. Proteolysis-inducing factor also induced increased expression of the ubiquitin–proteasome pathway, as determined by ‘chymotrypsin-like’ enzyme activity, the predominant proteolytic activity of the β-subunits of the proteasome, protein expression of 20S α-subunits and the 19S subunits MSS1 and p42, as well as the ubiquitin conjugating enzyme, E214k, in cells containing wild-type, but not mutant, I-κBα. The ability of mutant I-κBα to inhibit PIF-induced protein degradation, as well as expression of the ubiquitin–proteasome pathway, confirms that both of these responses depend on initiation of transcription by NF-κB.
Keywords: protein degradation, proteolysis-inducing factor (PIF), NF-κB, proteasome proteolysis
Loss of skeletal muscle results in weakness, immobility and finally death of the cancer patient. Depletion of myofibrillar proteins in muscle results from a decreased protein synthesis (Lundholm et al, 1976) combined with an increased protein breakdown (Lundholm et al, 1982). The increase in protein breakdown is possibly the most important component of muscle cachexia, since anabolic stimuli, such as nutritional supplementation, fail to reverse the muscle wasting (Evans et al, 1985).
The major catabolic pathway involved in degradation of myofibrillar proteins in a range of catabolic conditions, including cancer cachexia, is the ubiquitin–proteasome proteolytic system (Attaix et al, 1998). In this process, protein substrates are conjugated with a polyubiquitin chain, which enables them to be recognised for degradation by the proteasome, a multi-subunit complex containing a range of proteolytic enzymes. The main mediators known to influence expression of polyubiquitin genes and proteasomal subunits are glucocorticoids (Wang et al, 1998), cytokines such as tumour necrosis factor-α (TNF-α) (Li et al, 1998) and proteolysis-inducing factor (PIF) (Lorite et al, 2001), a sulphated glycoprotein produced by cachexia-inducing murine and human tumours (Todorov et al, 1996), which specifically induces degradation of skeletal muscle (Lorite et al, 1998).
Several studies have investigated the role of the nuclear transcription factor, nuclear factor-κB (NF-κB), in induction of proteasome gene expression. The human proteasome C3 subunit promoter contains elements homologous to the consensus NF-κB-binding site (Du et al, 2000), suggesting that NF-κB may be involved in gene transcription. However, the mechanism by which this occurs appears to be diametrically opposite for glucocorticoids (Du et al, 2000) and TNF-α (Li and Reid, 2000). Thus, glucocorticoids stimulate proteasome expression (at least the C3 subunit) by antagonising the interaction of NF-κB with its response element in the proteasome promoter region. Glucocorticoids also induce gene transcription and protein synthesis of the NF-κB inhibitor, IκB, and inhibit the expression of cytokines (Almawi and Melemedjian, 2002). In contrast, induction of protein degradation by TNF-α, which is also mediated through the ubiquitin–proteasome proteolytic pathway (Li et al, 1998), appears to be mediated through proteasomal degradation of IκBα and translocation of NF-κB to the nucleus (Li and Reid, 2000). We have also recently shown that both PIF (Whitehouse and Tisdale, 2003) and 15(S)-hydroxyeicosatetraenoic acid (15(S)-HETE), an intracellular signal for the increased protein degradation induced by PIF (Whitehouse et al, 2003), cause degradation of IκBα and nuclear accumulation of NF-κB, associated with an increased proteasome expression. Moreover, attenuation of this process, either by the polyunsaturated fatty acid, eicosapentaenoic acid (EPA), or the NF-κB inhibitor peptide, SN50, also attenuated the PIF-induced increase in proteasome expression, suggesting that NF-κB may act as a transcription factor in the PIF-induced increase in proteasome expression.
To investigate the role of NF-κB in PIF-induced protein degradation and proteasome expression, murine myoblasts have been transfected with viral plasmid constructs that induce overexpression of mutant IκBα proteins, that are insensitive to degradation via the ubiquitin–proteasome pathway, and, which, selectively inhibit NF-κB activation (Li and Reid, 2000). The effect of PIF on protein degradation and expression of regulatory components of the ubiquitin–proteasome pathway has been compared in fully differentiated forms of these cells containing mutant I-κBα, with those transfected with the empty viral vector.
MATERIALS AND METHODS
Materials
L-[2,63H] phenylalanine (sp.act.2.00 TBq mmol−1) was purchased from Amersham International (Bucks, UK). Foetal calf serum (FCS), horse serum (HS), Dulbecco's modified Eagle's medium (DMEM), OPTI-MEM1 reduced medium and lipofectamine were purchased from Life Technologies (Paisley, Scotland). Mouse monoclonal antibodies to 20S proteasome α subunits, MSS1 and p42 were purchased from Affiniti Research Products, (Exeter, UK), while mouse monoclonal antibody to myosin heavy chain was from Novocastra (Newcastle, UK). Rabbit polyclonal antisera to murine I-κBα was from Calbiochem (Herts, UK), that to mouse actin was from Sigma Aldridge (Dorset, UK) and that to ubiquitin conjugating enzyme (E214k) was a gift from Dr Simon Wing, McGill University (Montreal, Canada). Peroxidase-conjugated rabbit anti-mouse antibody and peroxidase-conjugated goat anti-rabbit antibody were purchased from Dako Ltd (Cambridge, UK), Hybond A nitrocellulose membranes were from Amersham International (Bucks, UK). Electrophoretic-mobility shift (EMSA) gel shift assay kits were from Panomics (CA, USA). Escherichia coli DH5α cells were from Gibco BRL (Paisley, Scotland). Plasmid constructs were under the control of the cytomegalovirus (CMV) promoter and were gifts from Dr Yi-Ping Li (Baylor College of Medicine, Houston, TX, USA). These consisted of empty pCMV4 vector used for the control cell line I-κBαΔN (truncation of amino acids 1–36) and I-κBα S32/A36 (point mutations of Ser32 and Ser36 to alanine). Plasmid DNA was purified using WIZARD Magnesil™ purification kit (Promega, Southampton, UK) according to the manufacturer's protocol. Primers for PCR analysis were purchased from MWG Biotech (Ebersberg, Germany). Gene Juice for transfection studies was obtained from Gene Flow (Staffordshire, UK). The luciferase reporter assay kit was purchased from BD Biosciences Clontech, Oxford, UK. The kinetic-QCL endotoxin assay kit was from Bio Whittaker, MD, USA.
Purification of PIF
Proteolysis-including factor was purified from solid MAC16 tumours excised from mice with a weight loss between 20 and 25% as described previously (Todorov et al, 1996; Whitehouse and Tisdale, 2003). Tumours were homogenised in 10 mM Tris–HCl, pH 8.0, containing 0.5 mM phenylmethylsulphonyl fluoride, 0.5 mM EGTA and 1 mM dithiothreitol at a concentration of 5 ml g−1 tumour. The supernatant obtained after addition of ammonium sulphate (40% w v−1) was subjected to affinity chromatography using anti-PIF monoclonal antibody coupled to a solid matrix. The immunogenic fractions were concentrated and used for further studies. The purity of the PIF was confirmed by polyacrylamide gel electrophoresis and immunoblotting. This showed a band for PIF at Mr 24 000, sometimes accompanied by an albumin-bound band at Mr 69 000. No other bands were apparent. The endotoxin content of the preparation was below the level of detection.
Production of transformed colonies
Transformation of plasmid DNA into E. coli was achieved using DH5α cells. Plasmid DNA was serially diluted to 0.015 μg (μl)−1 to perform transformations and 5 μl of diluted DNA was added to 70 ml of competent DH5α cells in a chilled microcentrifuge tube and mixed before incubating on ice for 30 min. The cells were then heat shocked for 30 s at 37°C and immediately put back on ice for 2 min. LB medium (500 μl) was added and cells were further incubated at 37°C for 40 min. Aliquots (200 μl) of the transformed cells were spread on LB agar plates containing ampicillin and the plates were incubated overnight at 37°C. Controls for the transformation included a positive control of PUC19 and a negative control of DH5α alone. PCR analysis was employed to identify transformed colonies using primers directed against the I-κBα insert (forward: GCT GTG ATC ACC AAC CAG C; reverse: CTC TGG CAG CAT CTG AAG G) and for plasmid DNA for those containing pCMV4 (forward: GGT CTA TTC GGG AAC CAA G; reverse: CAC ATT CCA CAG AAG CTG C).
Myogenic cell culture and transfection
The C2C12 myoblast cell line was grown in DMEM supplemented with 10% FCS plus 1% penicillin and streptomycin under an atmosphere of 10% CO2 in air. Stable transfections were carried out on cells at 50–80% confluency using GeneJuice, according to the manufacturer's protocol, and selected by resistance to ampicillin (5 g l−1) as described previously (Smith et al, 2004). Transfected myoblasts were stimulated to differentiate by replacing the growth medium with DMEM supplemented with 2% HS, when the cells reached confluence. Differentiation was allowed to continue for 3–5 days until myotubes were clearly visible.
Luciferase reporter gene assay
The assay was performed using the method described by the supplier. In brief, C2C12 myoblasts containing each IκBα insert were seeded in 75 cm2 flasks without antibiotics and incubated until 80% confluent. Then, cells were washed twice with OPTI-MEM Reduced Medium, followed by the addition of 6 ml of OPTI-MEM Reduced Medium containing either 15 μg of NF-κB luciferase reporter plasmid or 15 μg of control luciferase plasmid and 45 μl of Lipofectamine reagent. After 24 h incubation, the cells were passaged into six-well plates at 1 × 105 cells per well, allowed to reach confluence, and differentiated into myotubes. Cells were treated with PIF for 1 h at varied concentrations between 0 and 16.8 nM and washed twice in PBS. 1 × cell lysis buffer was added to cells and cell extracts were immediately assayed for luciferase activity using a BioOrbit luminometer 1253 (Turku, Finland). This was the earliest time point at which an increase in luciferase activity was observed.
Measurement of protein degradation
This was determined as described previously (Whitehouse and Tisdale, 2003) by prelabelling cells for 24 h with L-[2,63H]phenylalanine (0.67 mCi mmole−1), followed by extensive washing in PBS and further incubation for 2 h in DMEM without phenol red, until no more radioactivity appeared in the supernatant. Protein degradation was determined by the release of [2,63H]phenylalanine into the medium after 24 h in the presence of various concentrations of PIF together with 2 mM cold phenylalanine to prevent reincorporation of radioactivity in the cells.
Measurement of proteasome activity
‘Chymotrypsin-like’ enzyme activity was determined fluorimetrically by the method of Orino et al (1991) by the release of aminomethyl coumarin (AMC) from the fluorogenic peptide succinyl-LLVY-AMC. This method has been described previously for C2C12 myotubes (Whitehouse and Tisdale, 2003). Activity was measured in the absence and presence of the specific proteasome inhibitor lactacystin (10 μM). Only lactacystin-suppressible activity was considered to be proteasome specific.
Western blot analysis
Myotubes were incubated with various concentrations of PIF as depicted in the figure legends, after which the medium was removed and the cells were washed with PBS and scraped from the plastic surface. They were then sonicated at 4°C in 500–2000 μl of 20 mM Tris–HCl, pH 7.5, 2 mM ATP, 5 mM MgCl2 and 1 mM dithiothreitol (DTT). Samples of cytosolic protein (5–30 μg), formed by centrifugation at 18 000 g for 5 min, were resolved on 12% sodium dodecylsulphate, polyacrylamide gels (SDS/PAGE) and transferred to 0.45 μm nitrocellulose membranes, which had been blocked with 5% Marvel in Tris-buffered saline, pH 7.5, at 4°C overnight. The primary antibodies were used at a dilution of 1 : 1000 except for actin (1 : 100), and the secondary antibodies were also used at a dilution of 1 : 1000. Incubation was for 1 h at room temperature and development was by enhanced chemiluminescence (ECL) (Amersham, UK). Blots were scanned by a densitometer to quantitate differences.
Electrophoresis mobility shift assay (EMSA)
DNA-binding proteins were extracted from myotubes according to the method of Andrews and Faller (1991), which utilises hypotonic lysis followed by high salt extraction of nuclei. The EMSA-binding assay was carried out using a Panomics EMSA ‘gel shift’ kit according to the manufacturer's instructions.
Statistical analysis
Differences in means between groups were determined by one-way ANOVA, followed by Tukey's post-test.
RESULTS
To determine whether NF-κB mediates PIF-induced protein degradation and upregulation of the ubiquitin–proteasome proteolytic pathway in muscle, C2C12 murine myoblasts were transfected with either of two dominant-negative mutants of I-κBα. In I-κBα ΔN, the phosphorylation sites required for degradation (Ser32 and Ser36) are absent (truncation of amino acids 1–36), while in I-κBα S32/A36 there are point mutations of Ser32 and Ser36 to alanine. This prevented ubiquitin conjugation and proteolysis of either protein (Brockman et al, 1993; Chen et al, 1995). The control cells were transfected with the empty pCMV4 vector. Transfected myoblasts were allowed to differentiate into myotubes for further studies.
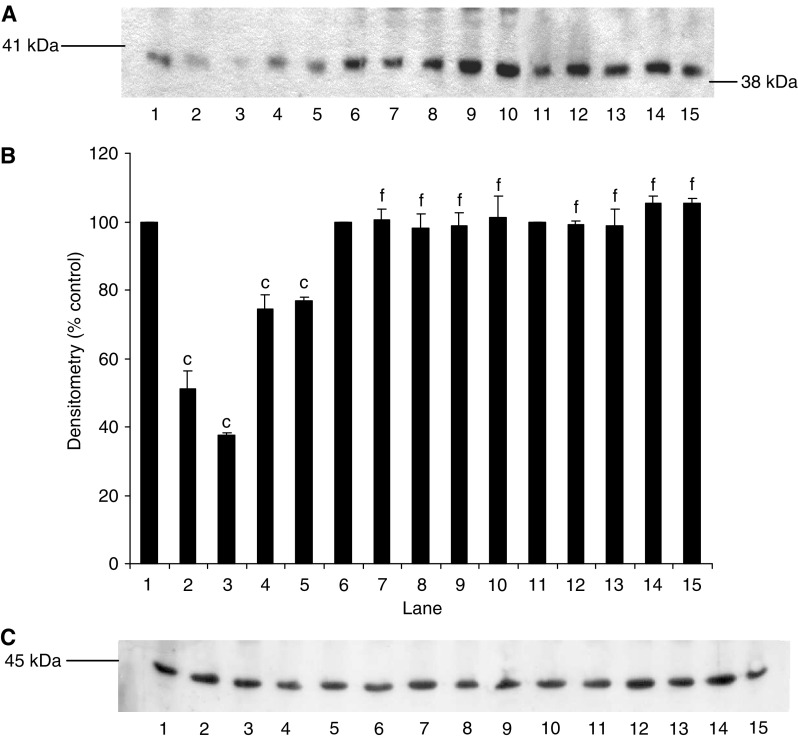
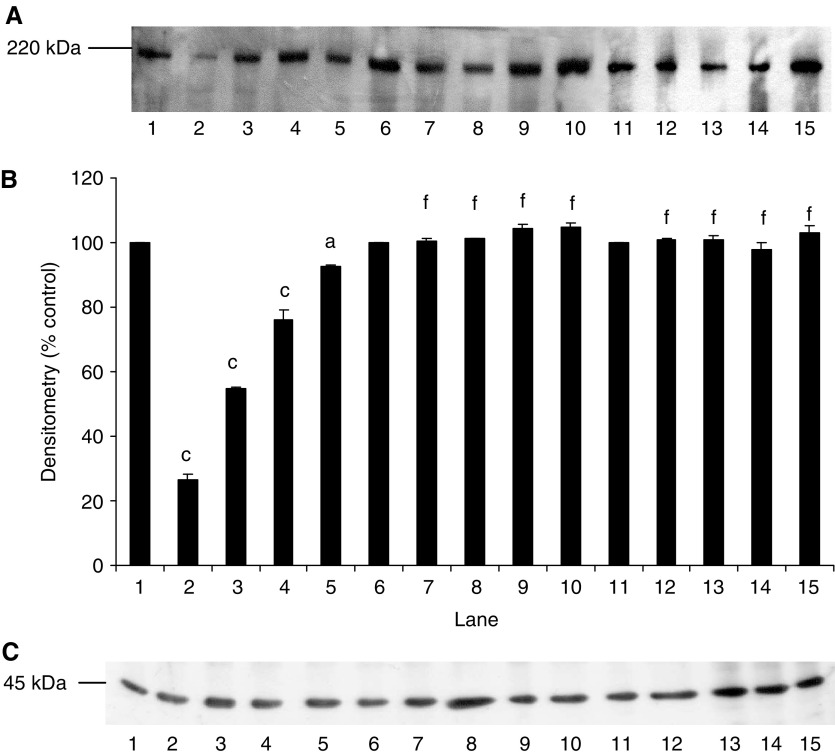
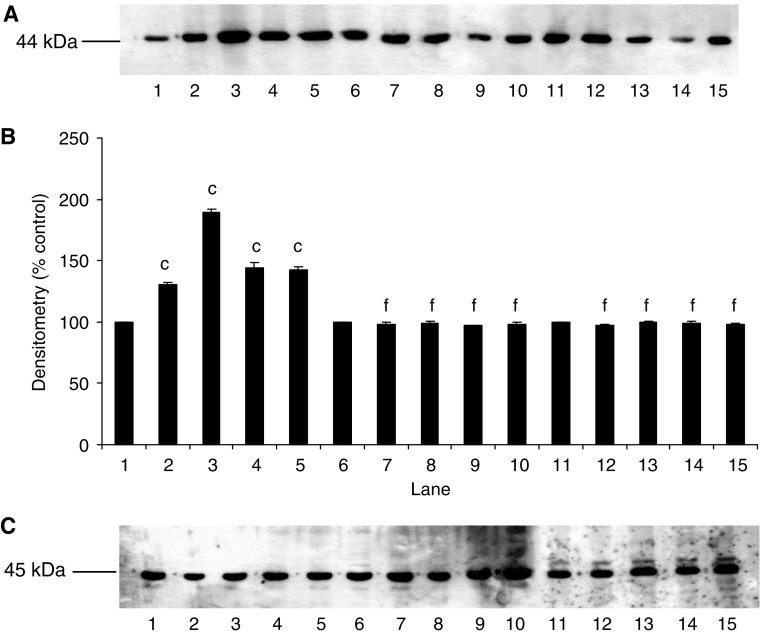
Proteolysis-inducing factor induced a decrease in I-κBα (Figure 1, lanes 2–5) and an increase in nuclear accumulation of NF-κB (Figure 2, lanes 2–4) in control myotubes transfected with the pCMV4 vector, which was the same as that previously observed in C2C12 myotubes (Whitehouse and Tisdale, 2003). In contrast, overexpression of I-κBα ΔN or I-κBα S32/A36 inhibited degradation of I-κBα in the presence of PIF (Figure 1, lanes 6–15), as well as nuclear translocation of NF-κB (Figure 2, lanes 6–10). Myoblasts transfected with these mutant plasmids have previously been shown not to respond to TNF-α with nuclear translocation of NF-κB (Li and Reid, 2000), in contrast with those containing the empty vector. Myotubes containing the truncated I-κBα (I-κBαΔN) showed a lower molecular weight for I-κBα (Figure 1, lanes 11–15) as expected.
Figure 1.
Effect of mutation on degradation of I-κBα in the presence of PIF. (A) Western blot analysis of I-κBα after 30 min incubation with 0 (lanes 1, 6 and 11), 2.1 (lanes 2, 7 and 12), 4.2 (lanes 3, 8 and 13), 10.5 (lanes 4, 9 and 14) and 16.8 (lanes 5, 10 and 15) nM PIF in wild-type cells transfected with pCMV4 (lanes 1–5), I-κBαS32/A36 (lanes 6–10) and I-κBαΔN (lanes 11–15). (B) Densitometric analysis of the blot shown in (A) as the mean±s.e.m. for three separate determinations. Differences from control are indicated as c, P<0.001, while differences from wild-type are shown as f, P<0.001. (C) Western blot of actin showing equal loading of samples in (A).
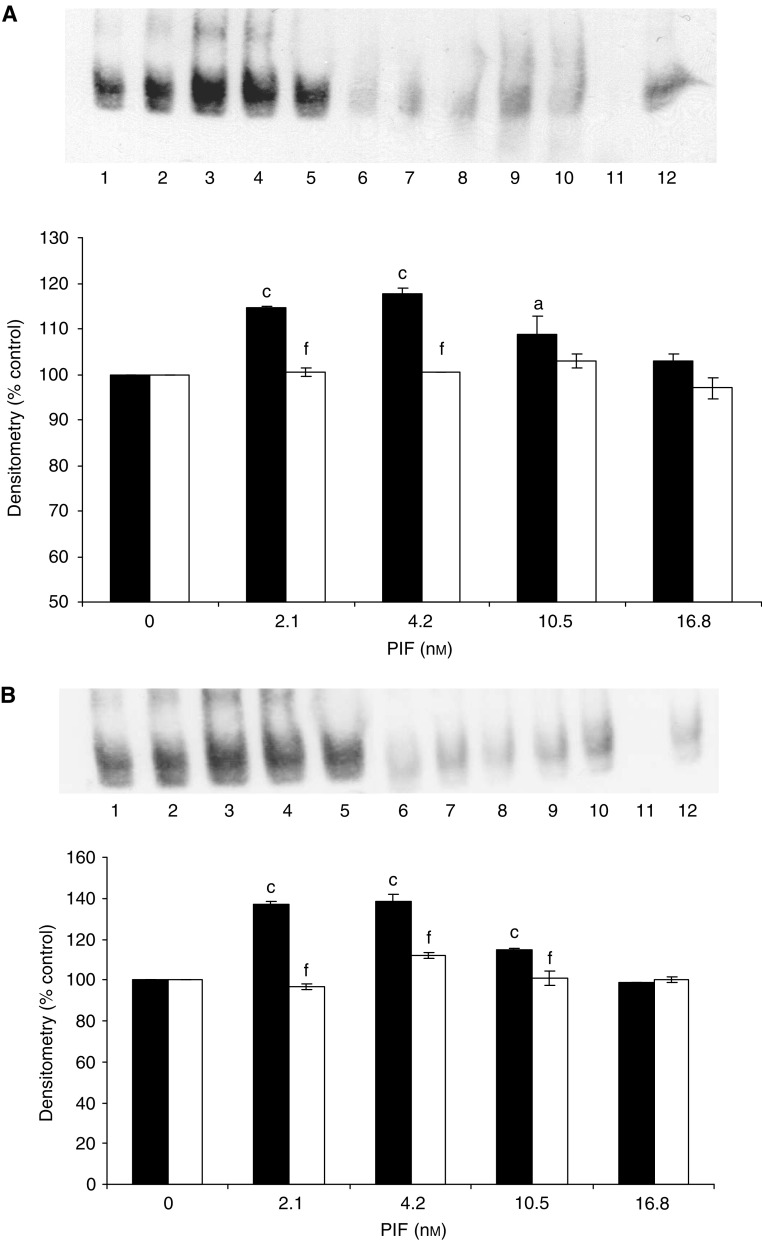
Figure 2.
Activation of NF-κB binding to DNA as demonstrated by EMSA. Only the band for bound NF-κB is shown. C2C12 myotubes were treated with 0 (lanes 1 and 6), 2.1 (lanes 2 and 7), 4.2 (lanes 3 and 8), 10.5 (lanes 4 and 9) and 16.8 (lanes 5 and 10) nM PIF. Binding of NF-κB to nuclear proteins was determined in cells transfected with pCMV4 (lanes 1–5) and I-κBα S32/A36 (lanes 6–10) (A) and in pCMV4 (lanes 1–5) and I-κBαΔN (lanes 6–10) (B). Lane 12 is a positive control for NF-κB (supplied by the manufacturer of the kit), while lane 11 contains the positive control for NF-κB together with a 100-fold excess of unlabelled NF-κB probe. The densitometric analysis of the blots is shown underneath the EMSA. Wild type is shown as solid boxes, while the mutants are shown as open boxes. Figures are means±s.e.m. for three separate determinations. Differences from control are indicated as a, P<0.05 and c, P<0.001, while differences from wild-type are shown as f, P<0.001.
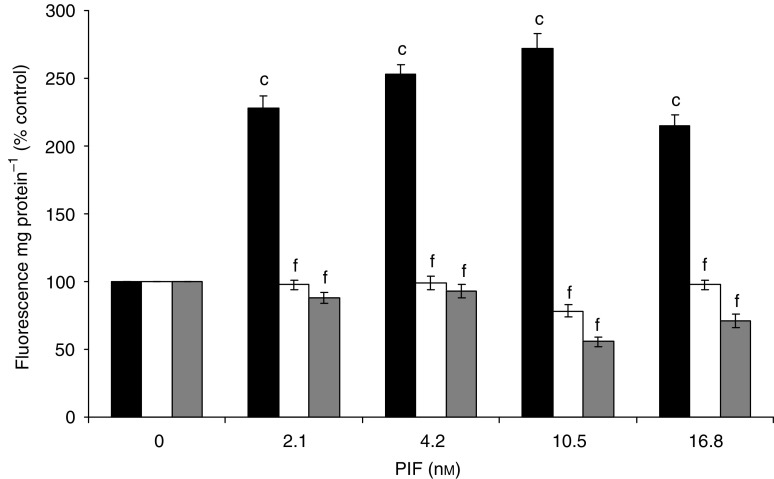
To determine whether the increased nuclear translocation of NF-κB was linked to an increased transcriptional activity, myoblasts containing the mutant plasmids or the empty vector were transfected with a plasmid vector with the NF-κB-binding site in the promoter region of the reporter luciferase gene and allowed to differentiate into myotubes. When treated with 2.1–10.5 nM PIF for 1 h, the luciferase activity was between 3.5- and 7.5-fold higher relative to untreated control in myotubes transfected with the empty pCMV4 vector, but was not increased in either of those containing the mutant plasmid (Figure 3). These results confirm that activation of NF-κB by PIF causes an increase in transcriptional activity.
Figure 3.
Effect of PIF on the transcriptional activity of NF-κB measured by the luciferase reporter gene assay in myotubes transfected with pCMV4 (▪), S32/A36 () and I-κBΔN (□) after 1 h incubation. Sample luciferase activity was normalised to control luciferase activity. The results shown are mean±s.e.m., where n=3. Differences from 0 nM PIF for pCMV4 is shown as a, P<0.05 or c, P<0.001, while differences from wild-type myotubes are indicated as e, P<0.01 or f, P<0.001.
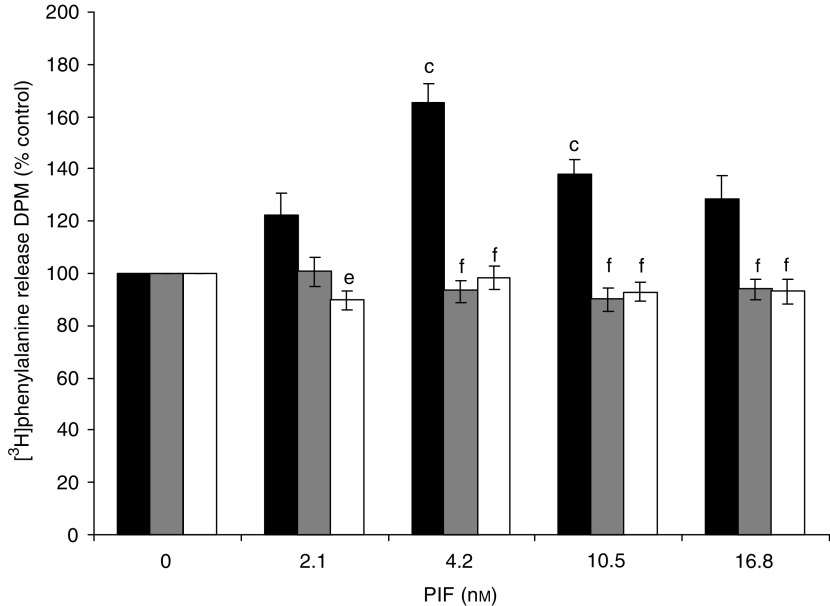
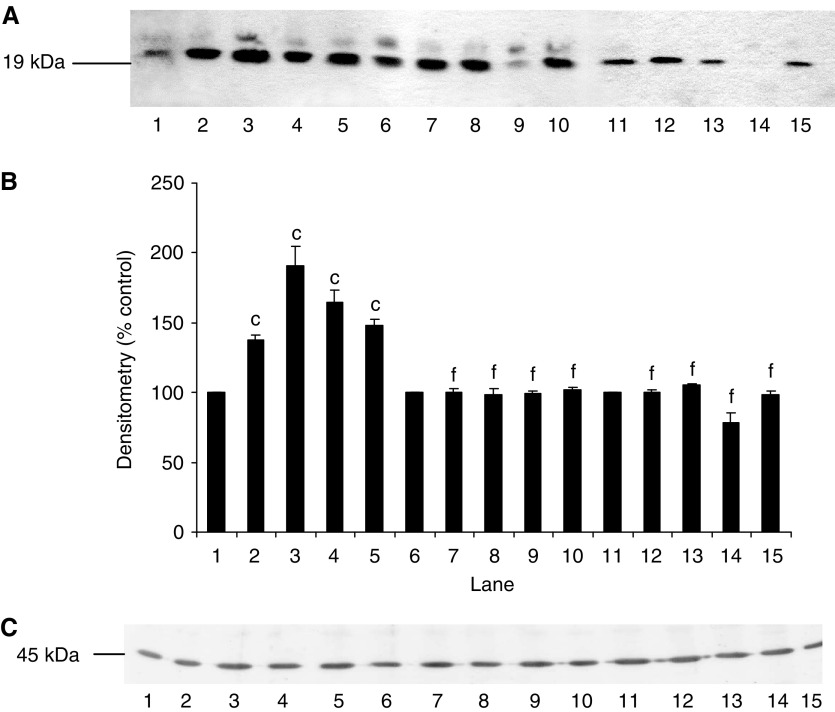
The effect of PIF on protein degradation in wild-type and mutant cell lines is shown in Figure 4. Proteolysis-inducing factor produced a significant increase in total protein loss, as measured by [3H] phenylalanine release, over the concentration range 2–16.8 nM, in wild-type, but not in mutant cells. The effect was seen over the same concentration range as that inducing I-κBα degradation (Figure 1, lanes 2–5) and nuclear accumulation of NF-κB (Figure 2, lanes 2–4) and the increase in luciferase reporter gene activity (Figure 3). The effect in wild-type cells was similar to that previously reported for nontransfected C2C12 myotubes (Gomes-Marcondes et al, 2003). Proteolysis-inducing factor also produced a decrease in the concentrations of the myofibrillar protein myosin in myotubes transfected with the wild-type pCMV4 vector, which was significant at all concentrations of PIF between 2 and 16.8 nM (Figure 5, lanes 1–5). A decrease in myosin was not seen in myotubes that overexpressed either I-κBα mutant (Figure 5, lanes 6–15). As previously reported (Acharyya et al, 2004) in cachectic mice bearing the colon 26 tumour and in myotubes treated with TNF-α and interferon-γ, PIF induced selective loss of myosin, while actin levels remained unchanged (Figure 5). This may be due to selective degradation of myosin by the ubiquitin–proteasome pathway. The ability of mutant I-κBα to inhibit PIF-induced protein loss indicates that this response depends on NF-κB signalling.
Figure 4.
Effect of PIF on total protein degradation in myotubes transfected with pCMV4 (▪), I-κBα S32/A36 () and I-κBαΔN (□) over a 24 h period. Results are shown as mean±s.e.m. of one experiment, where n=6, and the experiment was repeated twice on different days with similar results. Differences from 0 nM PIF for pCMV4 is shown as c, P<0.001, while differences between I-κBα S32/A36 and I-κBαΔN and pCMV4 are shown as e, P<0.01 and f, P<0.001.
Figure 5.
Effect of PIF on myosin content of myotubes containing wild-type and mutant I-κBα. (A) Wild-type myotubes transfected with pCMV4 (lanes 1–5), I-κBα S32/A36 (lanes 6–10) and I-κBαΔN (lanes 11–15) myotubes were incubated with 0 (lanes 1, 6 and 11), 2.1 (lanes 2, 7 and 12), 4.2 (lanes 3, 8 and 13), 10.5 (lanes 4, 9 and 14) or 16.8 (lanes 5, 10 and 15) nM PIF for 24 h and myosin content was determined by Western blotting. (B) Densitometric analysis of the blot shown in (A). Figures are mean±s.e.m. of three separate determinations. Differences from 0 nM PIF are shown as a, P<0.05 and c, P<0.001, while differences from wild-type myotubes are indicated as f, P<0.001. (C) Western blot of actin showing equal loading in (A).
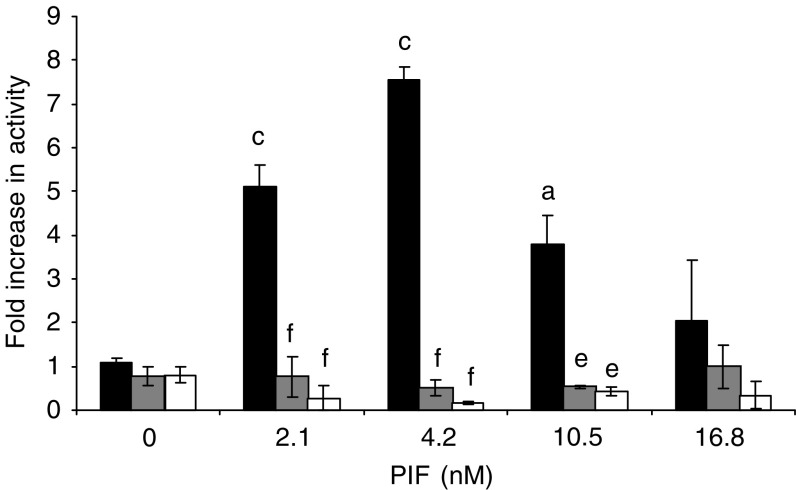
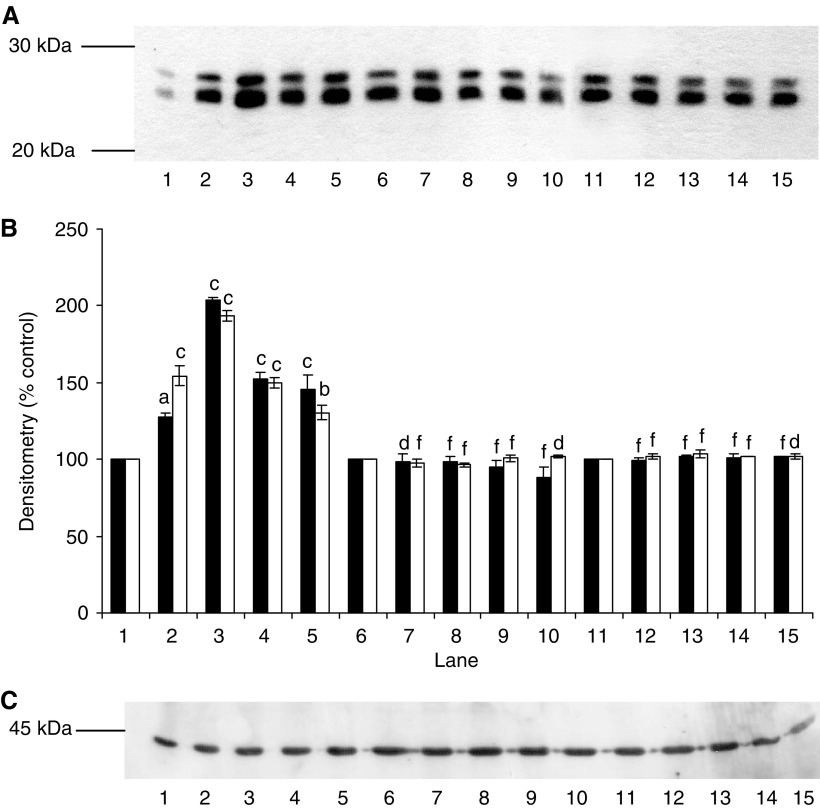
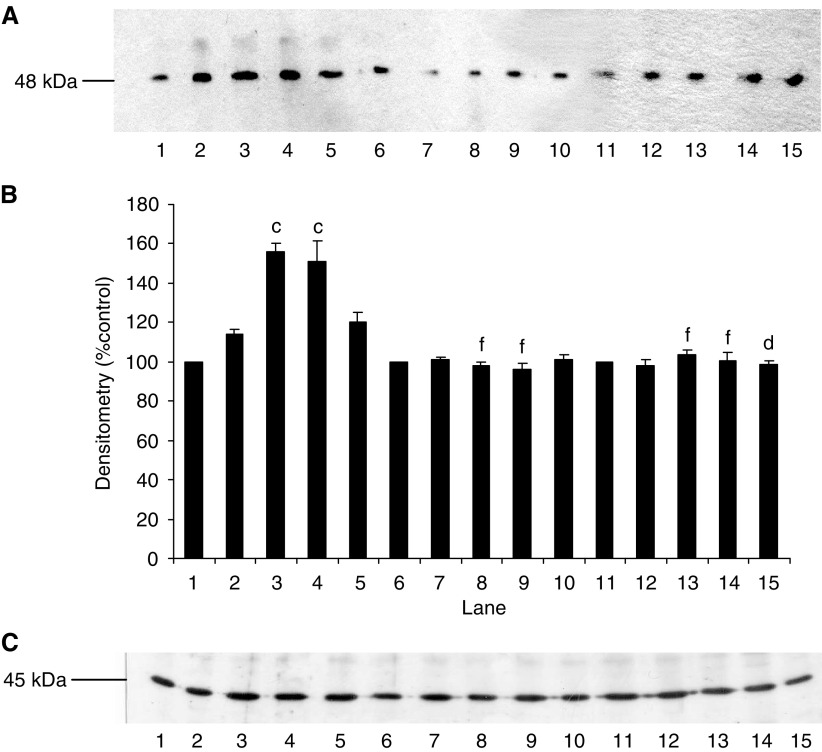
We have previously shown (Lorite et al, 2001; Gomes-Marcondes et al, 2003) that PIF-induced protein degradation was strongly correlated with an increase in expression of key regulatory components of the ubiquitin–proteasome proteolytic pathway. To determine the role of NF-κB in this process, functional proteasome activity was determined by measuring the ‘chymotrypsin-like’ enzyme activity, the major proteolytic activity of the β-subunits. Using the fluorogenic substrate succinyl LLVY-MCA, an increase in enzyme activity was seen at concentrations of PIF between 2 and 16.8 nM in myotubes transfected with the control vector pCMV4 (Figure 6), with a bell-shaped dose–response curve as previously reported (Gomes-Marcondes et al, 2003). In contrast, myotubes transfected with both mutant constructs showed no increase in ‘chymotrypsin-like’ enzyme activity in the presence of PIF (Figure 6). Expression of proteasome subunits was determined by Western blotting of cellular supernatants. The effect of PIF on expression of 20S α-subunits in wild-type and mutant I-κBα transfected myotubes is shown in Figure 7. Proteolysis-inducing factor induced a significant increase in 20S α-subunit expression at concentrations between 2 and 10.5 nM in wild-type (Figure 7, lanes 1–5), but not mutant cells (Figure 7, lanes 6–15). Proteolysis-inducing factor also increased expression of MSS1, an ATPase subunit of the 19S regulatory complex, in myotubes transfected with control vector, pCMV4 (Figure 8, lanes 1–5), but not in those transfected with either type of mutant I-κBα (Figure 8, lanes 6–15). MSS1, appearing as a single band at Mr∼50 000, was increased in pCMV4 myotubes in the same concentration range as that previously reported in untransfected myotubes (Gomes-Marcondes et al, 2003). Proteolysis-inducing factor also increased expression of p42, an ATPase subunit of the 19S regulator that promotes ATP-dependent association of the 20S proteasome with the 19S regulator to form the 26S proteasome (Tanahashi et al, 1999) in wild-type (Figure 9, lanes 1–5), but not in mutant myotubes (Figure 9, lanes 6–15). The concentrations of PIF producing an increase in p42 were the same as those producing an increase in 20S α-subunit expression (Figure 7). Proteolysis-inducing factor also produced an increase in expression of the Mr 14 000 ubiquitin-conjugating enzyme (E214k) in wild type (Figure 10, lanes 1–5), but not in myotubes expressing the mutant form of I-κBα (Figure 10, lanes 6–15). Previous studies (Gomes-Marcondes et al, 2003) have shown E214k expression to parallel that of proteasome subunits. The ability of mutant I-κBα to inhibit PIF-induced proteasome expression confirms that this response also depends on initiation of transcription by NF-κB.
Figure 6.
The effect of mutation of I-κBα on the chymotrypsin-like enzyme activity in murine myotubes after treatment with PIF. pCMV4 myotubes (▪); I-κBα S32/A36 (□) and I-κBαΔN() were treated with the indicated concentrations of PIF for 24 h and the ‘chymotrypsin-like’ enzyme activity was determined fluorimetrically as described in Materials and Methods. Differences from 0 nM PIF for pCMV4 are indicated as c, P<0.001, while differences from wild-type myotubes are indicated as f, P<0.001.
Figure 7.
(A) Effect of PIF on 20S proteasome α-subunit expression in myotubes transfected with pCMV4 (lanes 1–5), I-κBα S32/A36 (lanes 6–10) and I-κBαΔN (lanes 11–15) plasmids. Myotubes were incubated for 24 h with 0 (lanes 1, 6 and 11), 2.1 (lanes 2, 7 and 12), 4.2 (lanes 3, 8 and 13), 10.5 (lanes 4, 9 and 14) and 16.8 (lanes 5, 10 and 15) nM PIF and proteasome expression was determined by Western blotting of 5 μg of cytosolic protein. (B) Densitometric analysis of three replicate blots as shown in (A) ▪ band 1; □ band 2. Differences from 0 nM PIF are indicated as a, P<0.05, b, P<0.01 and c, P<0.001, while differences from wild-type myotubes are indicated as d, P<0.05 and f, P<0.001. (C) Western blot of actin from the blot shown in (A).
Figure 8.
(A) Effect of PIF on MSS1 expression in myotubes transfected with wild type (lanes 1–5), I-κBα S32/A36 (lanes 6–10) and I-κBαΔN (lanes 11–15) plasmids. Myotubes were incubated for 24 h with 0 (lanes 1, 6 and 11), 2.1 (lanes 2, 7 and 12), 4.2 (lanes 3, 8 and 13), 10.5 (lanes 4, 9 and 14) and 16.8 (lanes 5, 10 and 15) nM PIF and MSS1 expression was determined by Western blotting of 5 μg of cytosolic protein. (B) Densitometric analysis of three replicate blots shown in (A). Differences from 0 nM PIF are indicated as c, P<0.001, while differences from wild-type controls are shown as d, P<0.05 and f, P<0.001. (C) Western blot of actin from the blot shown in (A).
Figure 9.
(A) Effect of PIF on p42 expression in myotubes transfected with pCMV4 (lanes 1–5), I-κBα S32/A36 (lanes 6–10) and I-κBαΔN (lanes 11–15) plasmids. Myotubes were incubated for 24 h with 0 (lanes 1, 6 and 11), 2.1 (lanes 2, 7 and 12), 4.2 (lanes 3, 8 and 13), 10.5 (lanes 4, 9 and 14) and 16.8 (lanes 5, 10 and 15) nM PIF and p42 expression was determined by western blotting. (B) Densitometric analysis of three replicate blots shown in (A). Differences from 0 nM PIF are shown as c, P<0.001, while differences from wild-type controls are shown as f, P<0.001. (C) Western blot of actin from the blot shown in (A).
Figure 10.
Effect of PIF on E214k expression in myotubes transfected with pCMV4 (lanes 1–5), I-κBα S32/A36 (lanes 6–10) and I-κBαΔN (lanes 11–15) plasmids. Myotubes were incubated for 24 h with 0 (lanes 1, 6 and 11), 2.1 (lanes 2, 7 and 12), 4.2 (lanes 3, 8 and 13), 10.5 (lanes 4, 9 and 14) and 16.8 (lanes 5, 10 and 15) nM PIF and p42 expression was determined by Western blotting. (B) Densitometric analysis of three replicate blots shown in (A). Differences from 0 nM PIF are shown as c, P<0.001, while differences from wild-type controls are shown as f, P<0.001. (C) Western blot of actin from the blot shown in (A).
DISCUSSION
Nuclear factor-κB plays an important role in cellular function including immune and inflammatory responses, regulation of cell growth and apoptosis and tumour induction (Karin et al, 2002). In this study, we have utilised I-κBα mutated at Ser32 and Ser36 to investigate a role for NF-κB in protein degradation and induction of the ubiquitin–proteasome proteolytic pathway by PIF. Phosphorylation of I-κBα at Ser32 and Ser36 by the I-κB kinase complex (IKK) leads to ubiquitination of I-κBα at nearby lysine residues and degradation by the proteasome (Karin, 1999). An alternative pathway has recently been reported (Fan et al, 2003) whereby activation of NF-κB occurs through C-Src-mediated tyrosine phosphorylation of I-κBα at residue 42, which is capable of activating NF-κB in the absence of ubiquitin-dependent degradation of I-κBα. Such a pathway normally occurs in the redox activation of NF-κB. That such a pathway is not operative in C2C12 myotubes in the presence of PIF is shown by the lack of nuclear accumulation of NF-κB in cells transfected with the I-κBα mutant plasmids in the presence of PIF. This confirms that a lack of response in myotubes containing the mutant plasmids means that the effect is mediated through NF-κB.
The present observations indicate that NF-κB activation is involved in PIF-induced degradation of cellular proteins in skeletal muscle. In this respect, PIF appears to be similar to TNF-α (Li and Reid, 2000). There are few studies which have looked at NF-κB activation in skeletal muscle under conditions of muscle wasting. However, a recent study (Hunter et al, 2002) showed nuclear extracts from the soleus muscle of rats undergoing disuse atrophy to show increased binding of NF-κB oligonucleotides. This complex bound antibody to p50, c-Rel and Bcl-3, but not other NF-κB members, and there was no evidence for the canonical NF-κB pathway involving activation of p65 or I-κBα. This pathway, therefore, seems to be different from that induced by PIF.
Disuse atrophy resembles cancer cachexia in that protein degradation is mediated primarily by the ubiquitin–proteasome pathway (De Martino and Ordway, 1998). This suggests that activation of NF-κB may be a common mechanism for increased gene expression of key regulatory components of this pathway by agents such as PIF and TNF-α (Li and Reid, 2000). However, this mechanism is opposite to that induced by glucocorticoids, which have been shown (Du et al, 2000), at least in L6 muscle cells, to induce expression of the C3 proteasome subunit by antagonising interaction of NF-κB with the response element in the promoter region. Studies on protein degradation during sepsis, which is also mediated through the ubiquitin–proteasome pathway (Tiao et al, 1994), showed that NF-κB is increased at early time points (4 h), but decreased at later time points (16 h) (Penner et al, 2001). Which of these changes in NF-κB expression are responsible for the increased muscle protein degradation is not known, but the glucocorticoid receptor antagonist RU38486 increases NF-κB, suggesting that the decreased expression is due to glucocorticoids. How apparently opposite changes in NF-κB expression in the same cell type produce the same effect is not known. It is known that NF-κB can have different effects in different cell types. Thus, while in most cell types NF-κB seems to promote cell proliferation and protect against apoptosis, in skin, it appears to oppose proliferation (Dajee et al, 2003). It is possible that different NF-κB pathways are affected by glucocorticoids from that of PIF and TNF-α. Interestingly, insulin-like growth factor II (IGF-II)-dependent NF-κB activation has been linked to myoblast differentiation (Canicio et al, 2001). It is possible that proteolysis of intracellular regulators is required for fusion and that this requires induction of the ubiquitin–proteasome pathway.
This study shows that increased expression of both proteasome subunits and E214k in the presence of PIF is mediated through activation of NF-κB. A recent study (Li et al, 2003) shows that TNF-α stimulates expression of the ubiquitin carrier protein, UbcH2, a homologue of murine E220k in skeletal muscle, in a process which is regulated at the transcriptional level by NF-κB. UbcH2 appears to act in parallel with E214k targeting a distinct pool of protein for degradation.
Proteolysis-inducing factor has also been shown to activate NF-κB in primary hepatocytes and the human cancer cell line HepG2, resulting in the increased production of interleukin-6 and -8 (IL-6 and IL-8) and C-reactive protein and the decreased production of transferrin (Watchorn et al, 2001). There was also an increase in ICAM-1, another NF-κB-inducible gene. These results suggest that the primary effects of PIF on gene expression are mediated through NF-κB. The mechanism by which this occurs has not been completely evaluated, but recent evidence (Smith and Tisdale, 2003) suggests that PIF induces activation of both phospholipases A and C (PLA2 and PC-PLC). The former causes the release of arachidonic acid from membrane phospholipids, while diacylglycerol, derived from PC-PLC, has been suggested to provide a positive feedback signal to protein kinase C (PKC) (Smith and Tisdale, 2003). We have shown PKC to be involved in PIF-induced proteasome expression (Smith et al, 2004) and PKC may act as a signal for NF-κB activation through phosphorylation and activation of IKK (Fullman et al, 1992).
Induction of protein degradation and expression of the ubiquitin–proteasome pathway through activation of NF-κB would provide a mechanism to explain the effect of EPA in attenuating the process in cachectic mice (Whitehouse et al, 2001). Eicosapentaenoic acid is also effective clinically in preventing loss of lean body mass in patients with pancreatic carcinoma (Barber et al, 1999). We have shown EPA to prevent both degradation of I-κBα and nuclear accumulation of NF-κB in murine myotubes in the presence of PIF (Whitehouse and Tisdale, 2003), through attenuation of upstream signalling pathways. Omega-3 polyunsaturated fatty acids have also been shown to inhibit I-κB phosphorylation and NF-κB activation in murine macrophages, although the mechanism is not known (Novak et al, 2003). This manuscript provides experimental evidence to support the claim that PIF induces proteasome expression through activation of nuclear binding of NF-κB. Thus, agents capable of inhibiting activation of NF-κB should potentially be capable of inhibiting muscle protein degradation in cancer cachexia if PIF is involved. One such agent, resveratrol, may prove useful for the treatment of muscle wasting in cancer cachexia (Wyke et al, 2004).
Acknowledgments
This work has been supported by a grant from the Lustgarten Foundation for Pancreatic Cancer Research.
References
- Acharyya S, Ladner KJ, Nelson LL, Damrauer J, Reiser PJ, Swoap S, Guttridge DC (2004) Cancer cachexia is regulated by selective targeting of skeletal muscle gene products. J Clin Invest 114: 370–378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almawi WY, Melemedjian OK (2002) Negative regulation of nuclear factor-κB activation and function by glucocorticoids. J Mol Endocrinol 28: 69–78 [DOI] [PubMed] [Google Scholar]
- Andrews NC, Faller DV (1991) A rapid micropreparation technique for extraction of DNA-binding proteins from limiting numbers of mammalian cells. Nucleic Acids Res 19: 2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attaix D, Aurousseau E, Combaret L, Kee A, Larbaud D, Ralliere C, Souveine B, Taillander D, Tilignac T (1998) Ubiquitin–proteasome dependent proteolysis in skeletal muscle. Reprod Nutr Dev 38: 153–165 [DOI] [PubMed] [Google Scholar]
- Barber MD, Ross JA, Voss AC, Tisdale MJ, Fearon KCH (1999) The effect of an oral nutritional supplement enriched with fish oil on weight loss patients with pancreatic cancer. Br J Cancer 81: 80–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brockman JA, Scherer DC, McKinsey TA, Hall SM, Qi X, Lee WY, Ballard DW (1993) Coupling of a signal response domain in I-κBα to multiple pathways for NF-κB activation. Mol Cell Biol 15: 2809–2818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canicio J, Ruiz-Lozano P, Carrasco M, Palacin M, Chien K, Zorzano A, Kaliman P (2001) Nuclear factor κB-inducing kinase and IκB kinase-α signal skeletal muscle cell differentiation. J Biol Chem 276: 20228–20233 [DOI] [PubMed] [Google Scholar]
- Chen Z, Hagler J, Palombella VJ, Melandri F, Scherer D, Ballard D, Maniatis T (1995) Signal-induced site-specific phosphorylation targets I-κBα to the ubiquitin-proteasome pathway. Genes Dev 9: 1586–1597 [DOI] [PubMed] [Google Scholar]
- Dajee M, Lazarov M, Zhang JY, Cai T, Green CL, Russell AJ, Marinkovich MP, Tao S, Lin Q, Kubo Y, Khavari PA (2003) NF-κB blockage and oncogenic Ras trigger invasive human epidermal neoplasia. Nature 421: 639–643 [DOI] [PubMed] [Google Scholar]
- De Martino GN, Ordway GA (1998) Ubiquitin–proteasome pathway of intracellular protein degradation: implications for muscle atrophy during unloading. Exerc Sport Sci Rev 26: 219–252 [PubMed] [Google Scholar]
- Du J, Mitch WE, Wang X, Price SR (2000) Glucocorticoids induce proteasome C3 subunit expression in L6 muscle cells by opposing the suppression of its transcription by NF-κB. J Biol Chem 275: 19661–19666 [DOI] [PubMed] [Google Scholar]
- Evans WK, Makuch R, Clamon GH, Feld K, Weiner RS, Moran E, Blum R, Shepherd FA, Jeejeebhoy KW, De Wys WD (1985) Limited impact of total parenteral nutrition on nutritional status during treatment for small cell lung cancer. Cancer Res 45: 3347–3353 [PubMed] [Google Scholar]
- Fan C, Li Q, Ross D, Engelhardt JF (2003) Tyrosine phosphorylation of IκBα activates NFκB through a redox-regulated and c-Src-dependent mechanism following hypoxia/reoxygenation. J Biol Chem 278: 2072–2080 [DOI] [PubMed] [Google Scholar]
- Fullman M, Gulberg M, Helberg C, Anderson T (1992) Complement receptor-mediated phagocytosis is associated with the accumulation of phosphatidyl-choline derived diglyceride in human neutrophils. Involvement of phospholipase D and direct evidence for a positive feedback signal of protein kinase C. J Biol Chem 267: 2656–2663 [PubMed] [Google Scholar]
- Gomes-Marcondes MCC, Smith HJ, Cooper JC, Tisdale MJ (2003) Development of an in vitro model system to investigate the mechanism of muscle protein catabolism induced by proteolysis-inducing factor. Br J Cancer 86: 1628–1633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter RB, Stevenson EJ, Koncarevic A, Mitchell-Felton H, Essig DA, Kandarian SC (2002) Activation of an alternate NF-κB pathway in skeletal muscle during disuse atrophy. FASEB J 16: 529–538 [DOI] [PubMed] [Google Scholar]
- Karin M (1999) The beginning of the end: IkappaB kinase (IKK) and NF-kappaB activation. J Biol Chem 274: 27339–27342 [DOI] [PubMed] [Google Scholar]
- Karin M, Cao Y, Greten FR, Li Z-W (2002) NF-κB in cancer: from innocent bystander to major culprit. Nat Rev Cancer 2: 301–310 [DOI] [PubMed] [Google Scholar]
- Li Y-P, Lecker SH, Chen Y, Waddell ID, Goldberg AL, Reid MB (2003) TNF-α increases ubiquitin-conjugating enzyme activity in skeletal muscle by up-regulating UbcH2/E220k. FASEB J 17: 1048–1057 [DOI] [PubMed] [Google Scholar]
- Li Y-P, Reid MB (2000) NF-κB mediates the protein loss induced by TNF-α in differentiated skeletal muscle myotubes. Am J Physiol 279: R1165–R1170 [DOI] [PubMed] [Google Scholar]
- Li Y-P, Schwartz RJ, Waddell ID, Holloway BR, Reid MB (1998) Skeletal muscle myotubes undergo protein loss and reactive oxygen-mediated NF-κB activation in response to tumour necrosis factor α. FASEB J 12: 871–880 [DOI] [PubMed] [Google Scholar]
- Lorite MJ, Smith HJ, Arnold JA, Morris A, Thompson MG, Tisdale MJ (2001) Activation of ATP-ubiquitin-dependent proteolysis in skeletal muscle in vivo and murine myoblasts in vitro by a proteolysis-inducing factor (PIF). Br J Cancer 85: 297–302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorite MJ, Thompson MG, Drake JL, Carling G, Tisdale MJ (1998) Mechanism of muscle protein degradation induced by a cancer cachectic factor. Br J Cancer 78: 850–856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundholm K, Bennegard K, Eden E, Rennie MJ (1982) Efflux of 3-methylhistidine from the leg of cancer patients who experience weight loss. Cancer Res 42: 4809–4818 [PubMed] [Google Scholar]
- Lundholm K, Bylund AC, Holm J, Schersten T (1976) Skeletal muscle metabolism in patients with malignant tumour. Eur J Cancer 12: 465–473 [DOI] [PubMed] [Google Scholar]
- Novak TE, Babcock TA, Jho DH, Hilton WS, Espat NJ (2003) NF-κB inhibition by ω-3 fatty acids modulates LPS-stimulated microphage TNF-α transcription. Am J Physiol 284: L84–L89 [DOI] [PubMed] [Google Scholar]
- Orino E, Tanaka K, Tamura T, Sone S, Ogura T, Ichihara A (1991) ATP-dependent reversible association of proteasomes with multiple protein components to form 26S complexes that degrade ubiquitinated proteins in human HL-60 cells. FEBS Lett 284: 206–210 [DOI] [PubMed] [Google Scholar]
- Penner CG, Gang G, Wray C, Fischer JE, Hasselgren P-O (2001) The transcription factors NF-κB and AP-1 are differentially regulated in skeletal muscle during sepsis. Biochem Biophys Res Commun 281: 1331–1336 [DOI] [PubMed] [Google Scholar]
- Smith HJ, Tisdale MJ (2003) Signal transduction pathways involved in proteolysis-inducing factor (PIF) induced proteasome expression in murine myotubes. Br J Cancer 89: 1783–1788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith HJ, Wyke SM, Tisdale MJ (2004) Role of protein kinase C and NF-κB in proteolysis-inducing factor induced proteasome expression in C2C12 myotubes. Br J Cancer 90: 1850–1857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanahashi N, Kawahara H, Murakami Y, Tanaka K (1999) The proteasome-dependent proteolytic system. Mol Biol Rep 26: 3–9 [DOI] [PubMed] [Google Scholar]
- Tiao G, Fagan JM, Sammuels N, James JH, Hudson K, Lieberman M, Fischer JE, Hasselgren P-O (1994) Sepsis stimulates non-lysomal, energy-dependent proteolysis and increases ubiquitin mRNA levels in rat skeletal muscle. J Clin Invest 94: 2255–2264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todorov P, Cariuk P, McDevitt T, Coles B, Fearon K, Tisdale MJ (1996) Characterization of a cancer cachectic factor. Nature 379: 739–742 [DOI] [PubMed] [Google Scholar]
- Wang L, Lus G-J, Wang JJ, Hasselgren P-O (1998) Dexamethasone stimulates proteasome- and calcium-dependent proteolysis in cultured L6 myotubes. Shock 10: 298–306 [DOI] [PubMed] [Google Scholar]
- Watchorn TM, Waddell ID, Dowidar N, Ross JA (2001) Proteolysis-inducing factor regulates hepatic gene expression via the transcription factors NF-κB and STAT3. FASEB J 15: 562–564 [DOI] [PubMed] [Google Scholar]
- Whitehouse AS, Khal J, Tisdale MJ (2003) Induction of protein catabolism in myotubes by 15(S)-hydroxyeicosatetraenoic acid through increased expression of the ubiquitin–proteasome pathway. Br J Cancer 89: 737–745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitehouse AS, Smith HJ, Drake JL, Tisdale MJ (2001) Mechanism of attenuation of skeletal muscle protein catabolism in cancer cachexia by eicosapentaenoic acid. Cancer Res 61: 3604–3609 [PubMed] [Google Scholar]
- Whitehouse AS, Tisdale MJ (2003) Increased expression of the ubiquitin-proteasome pathway in murine myotubes by proteolysis-inducing factor (PIF) is associated with activation of the transcription factor NF-κB. Br J Cancer 89: 1116–1122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyke SM, Russell ST, Tisdale MJ (2004) Induction of proteasome expression in skeletal muscle is attenuated by inhibitors of NF-κB activation. Br J Cancer 91: 1742–1750 [DOI] [PMC free article] [PubMed] [Google Scholar]