Abstract
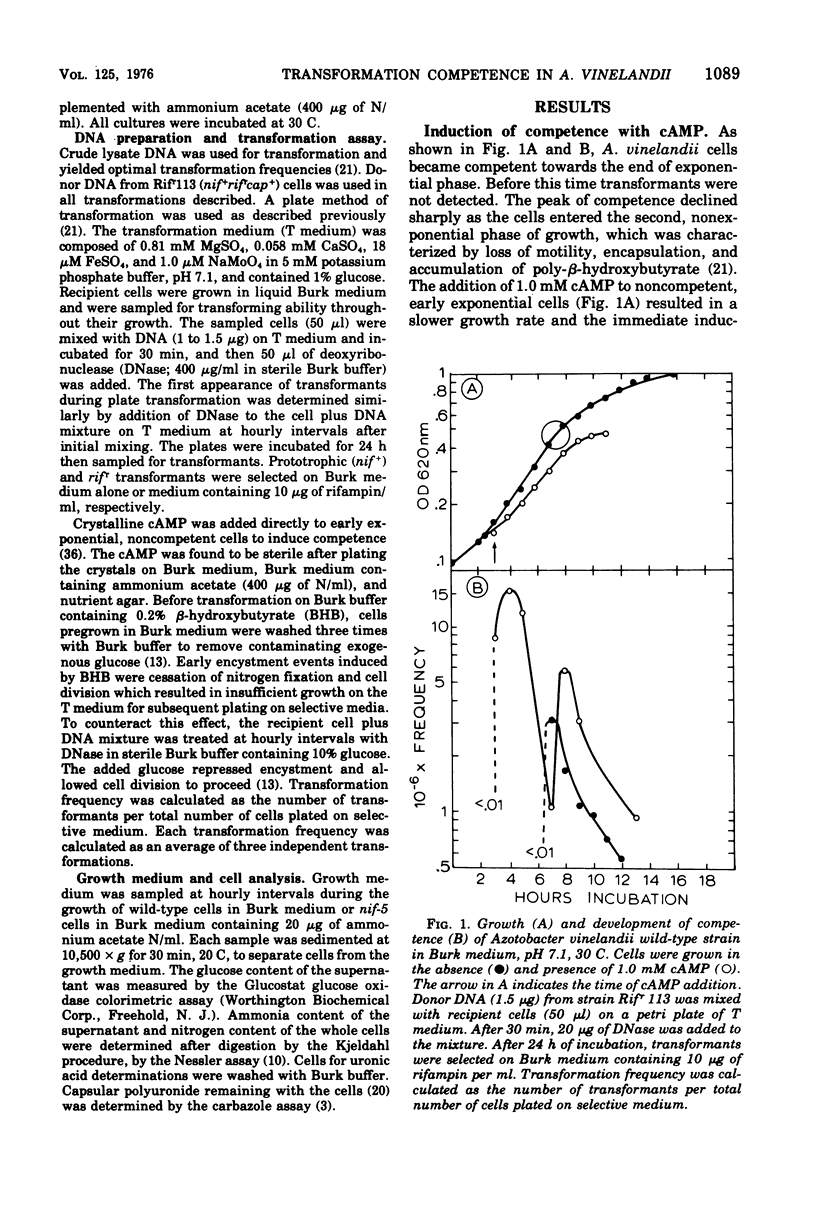
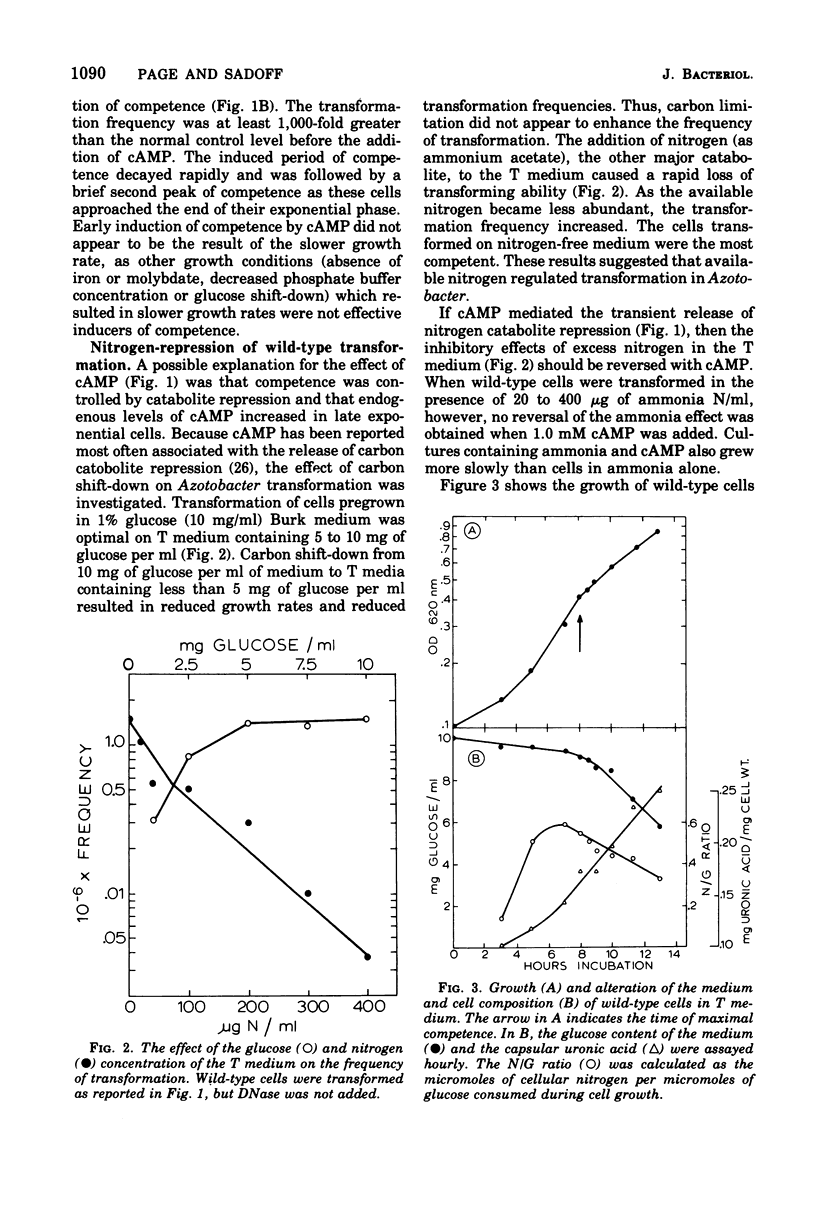
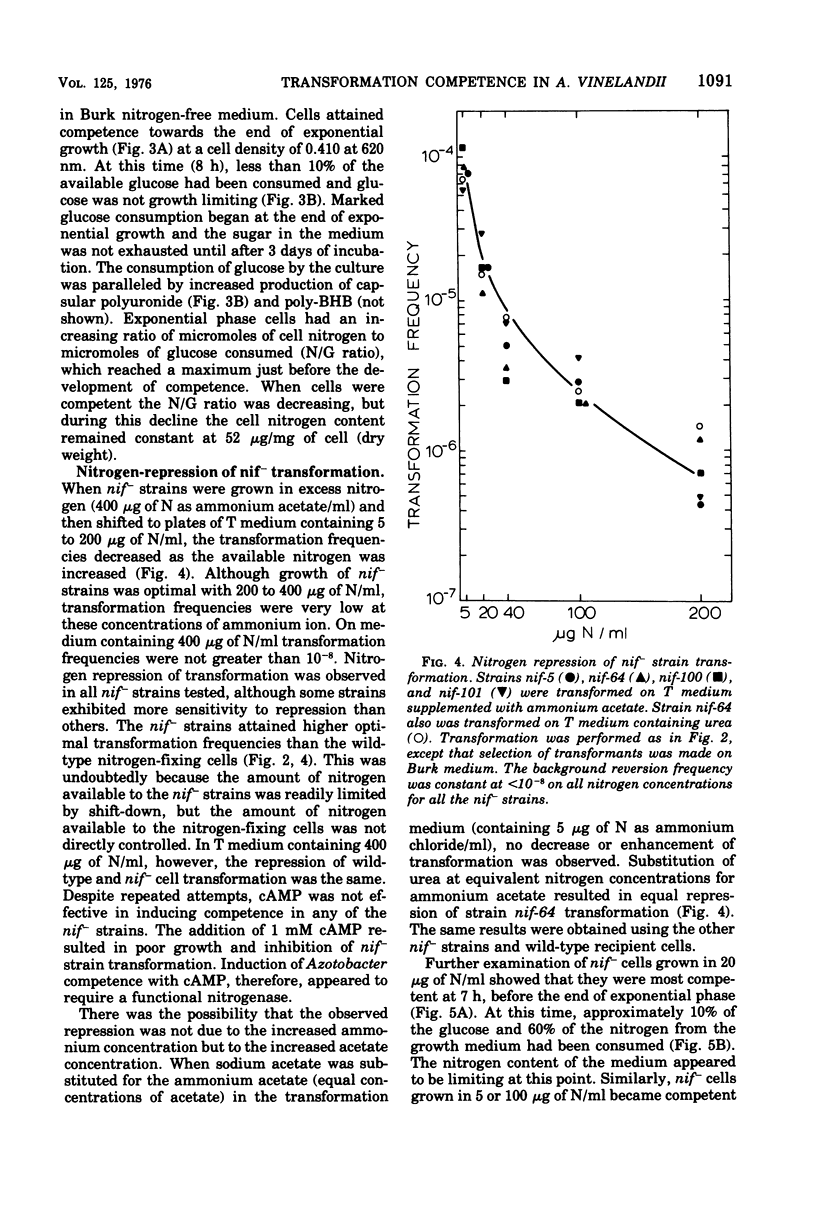
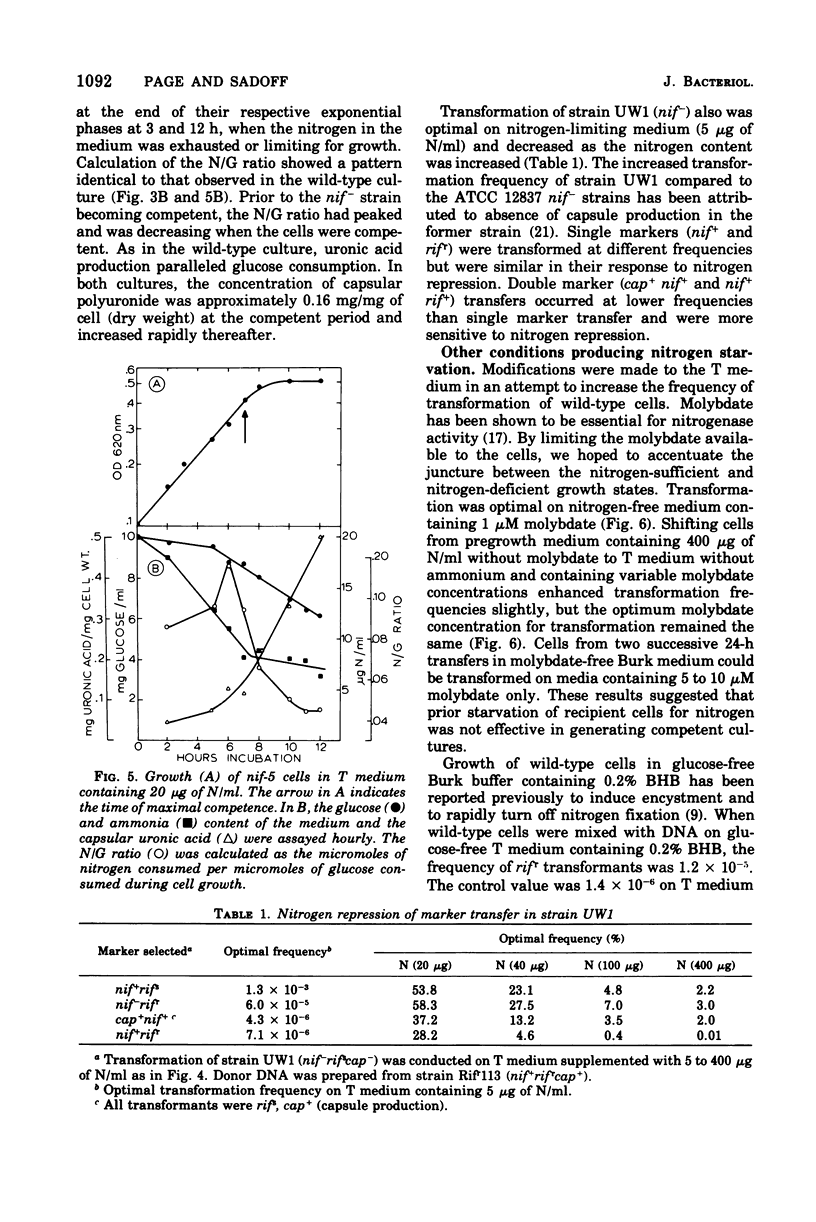
Azotobacter vinelandii (ATCC 12837) became competent to be transformed by exogenous deoxyribonucleic acid towards the end of exponential growth. Competence in wild-type and nitrogenase auxotrophic (nif-) strains was repressed by the addition of ammonium salts or urea to the transformation medium. Transformation of wild-type cells and nif- strains was optimal on nitrogen-free or nitrogen-limiting medium, respectively. Transformation of wild-type cells also was enhanced when the transformation medium had low molydbate content. During the development of competence, nitrogen was growth limiting, whereas carbon (glucose) was in excess. Carbon source shift-down was not effective in inducing competence. Shifting glucose-grown wild-type cells to medium containing 0.2% beta-hydroxybutyrate initiated encystment and also induced competence. The addition of glucose to this medium blocked encystment and early competence induction and reduced the transformation frequency to the basal level. Cyclic adenosine 3',5'-monophosphate induced competence in wild-type nitrogen-fixing cells and increased the transformation frequency 1,000-fold over the basal level. Exogenous cyclic adenosine 3',5'-monophosphate however, did not reverse nitrogen repression of competence in ammonia-grown wild-type or nif- strains.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anagnostopoulos C., Spizizen J. REQUIREMENTS FOR TRANSFORMATION IN BACILLUS SUBTILIS. J Bacteriol. 1961 May;81(5):741–746. doi: 10.1128/jb.81.5.741-746.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BITTER T., MUIR H. M. A modified uronic acid carbazole reaction. Anal Biochem. 1962 Oct;4:330–334. doi: 10.1016/0003-2697(62)90095-7. [DOI] [PubMed] [Google Scholar]
- ESPOSITO R. G., WILSON P. W. Trace metal requirements of Azotobacter. Proc Soc Exp Biol Med. 1956 Dec;93(3):564–567. doi: 10.3181/00379727-93-22820. [DOI] [PubMed] [Google Scholar]
- Eisenstadt E., Lange R., Willecke K. Competent Bacillus subtilis cultures synthesize a denatured DNA binding activity. Proc Natl Acad Sci U S A. 1975 Jan;72(1):323–327. doi: 10.1073/pnas.72.1.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GWINN D. D., THORNE C. B. TRANSFORMATION OF BACILLUS LICHENIFORMIS. J Bacteriol. 1964 Mar;87:519–526. doi: 10.1128/jb.87.3.519-526.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadkari D., Stolp H. Influence of nitrogen source on growth and nitrogenase activity in Azotobacter vinelandii. Arch Mikrobiol. 1974 Mar 4;96(2):135–144. doi: 10.1007/BF00590170. [DOI] [PubMed] [Google Scholar]
- Gordon J. K., Brill W. J. Derepression of nitrogenase synthesis in the presence of excess NH4+. Biochem Biophys Res Commun. 1974 Aug 5;59(3):967–971. doi: 10.1016/s0006-291x(74)80074-4. [DOI] [PubMed] [Google Scholar]
- Hitchins V. M., Sadoff H. L. Sequential metabolic events during encystment of Azobacter vinelandii. J Bacteriol. 1973 Mar;113(3):1273–1279. doi: 10.1128/jb.113.3.1273-1279.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KEELER R. F., VARNER J. E. Tungstate as an antagonist of molybdate in Azotobacter vinelandii. Arch Biochem Biophys. 1957 Aug;70(2):585–590. doi: 10.1016/0003-9861(57)90146-7. [DOI] [PubMed] [Google Scholar]
- Lacks S., Greenberg B., Neuberger M. Role of a deoxyribonuclease in the genetic transformation of Diplococcus pneumoniae. Proc Natl Acad Sci U S A. 1974 Jun;71(6):2305–2309. doi: 10.1073/pnas.71.6.2305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin L. P., Sadoff H. L. Encystment and polymer production by Azotobacter vinelandii in the presence of beta-hydroxybutyrate. J Bacteriol. 1968 Jun;95(6):2336–2343. doi: 10.1128/jb.95.6.2336-2343.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magasanik B., Prival M. J., Brenchley J. E., Tyler B. M., DeLeo A. B., Streicher S. L., Bender R. A., Paris C. G. Glutamine synthetase as a regulator of enzyme synthesis. Curr Top Cell Regul. 1974;8(0):119–138. doi: 10.1016/b978-0-12-152808-9.50010-9. [DOI] [PubMed] [Google Scholar]
- Marcus L., Kaneshiro T. Lipid composition of Azotobactervinelandii in which the internal membrane network is induced or repressed. Biochim Biophys Acta. 1972 Nov 2;288(2):296–303. doi: 10.1016/0005-2736(72)90250-7. [DOI] [PubMed] [Google Scholar]
- Nagatani H. H., Brill W. J. Nitrogenase V. The effect of Mo, W and V on the synthesis of nitrogenase components in Azotobacter vinelandii. Biochim Biophys Acta. 1974 Aug 7;362(1):160–166. doi: 10.1016/0304-4165(74)90037-3. [DOI] [PubMed] [Google Scholar]
- Oppenheim J., Marcus L. Correlation of ultrastructure in Azotobacter vinelandii with nitrogen source for growth. J Bacteriol. 1970 Jan;101(1):286–291. doi: 10.1128/jb.101.1.286-291.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orkwiszewski K. G., Kaney A. R. Genetic transformation of the blue-green bacterium, Anacystis nidulans. Arch Mikrobiol. 1974 Jun 7;98(1):31–37. doi: 10.1007/BF00425265. [DOI] [PubMed] [Google Scholar]
- PAKULA R., WALCZAK W. On the nature of competence of transformable streptococci. J Gen Microbiol. 1963 Apr;31:125–133. doi: 10.1099/00221287-31-1-125. [DOI] [PubMed] [Google Scholar]
- Page W. J., Sadoff H. L. Physiological factors affecting transformation of Azotobacter vinelandii. J Bacteriol. 1976 Mar;125(3):1080–1087. doi: 10.1128/jb.125.3.1080-1087.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page W. J., Sadoff H. L. Relationship between calcium and uroinic acids in the encystment of Azotobacter vinelandii. J Bacteriol. 1975 Apr;122(1):145–151. doi: 10.1128/jb.122.1.145-151.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prival M. J., Brenchley J. E., Magasanik B. Glutamine synthetase and the regulation of histidase formation in Klebsiella aerogenes. J Biol Chem. 1973 Jun 25;248(12):4334–4344. [PubMed] [Google Scholar]
- Prival M. J., Magasanik B. Resistance to catabolite repression of histidase and proline oxidase during nitrogen-limited growth of Klebsiella aerogenes. J Biol Chem. 1971 Oct 25;246(20):6288–6296. [PubMed] [Google Scholar]
- Prusiner S., Miller R. E., Valentine R. C. Adenosine 3':5'-cyclic monophosphate control of the enzymes of glutamine metabolism in Escherichia coli. Proc Natl Acad Sci U S A. 1972 Oct;69(10):2922–2926. doi: 10.1073/pnas.69.10.2922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rickenberg H. V. Cyclic AMP in prokaryotes. Annu Rev Microbiol. 1974;28(0):353–369. doi: 10.1146/annurev.mi.28.100174.002033. [DOI] [PubMed] [Google Scholar]
- STUY J. H. Transformability of Haemophilus influenzae. J Gen Microbiol. 1962 Nov;29:537–549. doi: 10.1099/00221287-29-3-537. [DOI] [PubMed] [Google Scholar]
- Sadoff H. L., Berke E., Loperfido B. Physiological studies of encystment in Azotobacter vinelandii. J Bacteriol. 1971 Jan;105(1):185–189. doi: 10.1128/jb.105.1.185-189.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah V. K., Davis I. C., Gordon J. K., Orme-Johnson W. H., Brill W. J. Nitrogenase. 3. Nitrogenaseless mutants of Azotobacter vinelandii: activities, cross-reactions and EPR spectra. Biochim Biophys Acta. 1973 Jan 18;292(1):246–255. doi: 10.1016/0005-2728(73)90269-7. [DOI] [PubMed] [Google Scholar]
- Shah V. K., Davis L. C., Brill W. J. Nitrogenase. I. Repression and derepression of the iron-molybdenum and iron proteins of nitrogenase in Azotobacter vinelandii. Biochim Biophys Acta. 1972 Feb 28;256(2):498–511. doi: 10.1016/0005-2728(72)90078-3. [DOI] [PubMed] [Google Scholar]
- Streicher S. L., Gurney E. G., Valentine R. C. The nitrogen fixation genes. Nature. 1972 Oct 27;239(5374):495–499. doi: 10.1038/239495a0. [DOI] [PubMed] [Google Scholar]
- Streicher S. L., Shanmugam K. T., Ausubel F., Morandi C., Goldberg R. B. Regulation of nitrogen fixation in Klebsiella pneumoniae: evidence for a role of glutamine synthetase as a regulator of nitrogenase synthesis. J Bacteriol. 1974 Nov;120(2):815–821. doi: 10.1128/jb.120.2.815-821.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TOMASZ A., HOTCHKISS R. D. REGULATION OF THE TRANSFORMABILITY OF PHEUMOCOCCAL CULTURES BY MACROMOLECULAR CELL PRODUCTS. Proc Natl Acad Sci U S A. 1964 Mar;51:480–487. doi: 10.1073/pnas.51.3.480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyler B., Deleo A. B., Magasanik B. Activation of transcription of hut DNA by glutamine synthetase. Proc Natl Acad Sci U S A. 1974 Jan;71(1):225–229. doi: 10.1073/pnas.71.1.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise E. M., Jr, Alexander S. P., Powers M. Adenosine 3':5'-cyclic monophosphate as a regulator of bacterial transformation. Proc Natl Acad Sci U S A. 1973 Feb;70(2):471–474. doi: 10.1073/pnas.70.2.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YOUNG F. E., SPIZIZEN J. Physiological and genetic factors affecting transformation of Bacillus subtilis. J Bacteriol. 1961 May;81:823–829. doi: 10.1128/jb.81.5.823-829.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]



