Abstract
High loads of human papillomavirus (HPV) 16 and HPV 18/45 increase the risk of developing invasive cervical carcinoma, revealing higher risk in percentiles of highest viral loads for HPV 16 (odds ratio (OR) 58.7, 95% confidence interval (CI) 21.9–151.4) compared to HPV 18/45 (OR 3.3, 95% CI 1.5–7.2). Thus, HPV load is a type-dependent risk marker for invasive carcinoma.
Keywords: human papillomavirus, invasive cervical cancer, viral load
Human papillomavirus (HPV) is a necessary cause of cervical cancer, but the high prevalence of transient infections makes detection of the presence or absence of virus an inefficient means of identifying women at risk of developing cervical cancer (Ho et al, 1998; Jacobs et al, 2000). Viral load has been suggested as a marker of nontransient infection, and high HPV load in smears with normal cytology has been associated with the risk of developing dysplasia and carcinoma in situ (CIS) (Josefsson et al, 2000; van Duin et al, 2002; Dalstein et al, 2003; Moberg et al, 2004). However, it is not known whether high viral load is also predictive of invasive cervical cancer, as past studies have pooled the outcomes of invasive cancers together with earlier stages of the disease (Lorincz et al, 2002; Sherman et al, 2002, 2003; Sun et al, 2002; Dalstein et al, 2003; Gravitt et al, 2003). Integration of high-risk HPV into the cell genome has been suggested to play a pivotal role in malignant transformation (Boshart et al, 1984; Cullen et al, 1991; Daniel et al, 1997). As a consequence, the association between viral load and development of CIS may not automatically imply a strong association of high viral load and progression into invasive carcinoma. Here, we determine whether viral load in cervical smears is a risk marker of later developing invasive cervical cancer.
MATERIALS AND METHODS
Cases diagnosed with invasive squamous-cell carcinoma of the cervix and with registered smears taken at least 1 year prior to the diagnosis date were identified from the organised screening program (PAP-smear register) and the tumour register at the Clinic of Pathology, Uppsala Academic Hospital and Uppsala University, Sweden. Controls were selected from the same organised screening programme and have been described in detail elsewhere (Ylitalo et al, 2000). Birth years of cases and controls ranged from 1922 to 1968. Two strategies were used to select smears from both cases and controls. The screening history of individual women was examined to identify suitable smears for inclusion. First, we attempted to study an early phase of the infection. Therefore, we started from the date of diagnosis and stepped backwards in the screening history until reaching a smear fulfilling the criteria of (a) being negative itself, (b) being preceded by a normal smear or being preceded by an abnormal smear taken at least 3 years earlier, or being the first registered smear. The time span of 3 years was chosen to increase the likelihood of spontaneous regression of the preceding lesion (Schlecht et al, 2003), and consequently decreasing the risk of selecting false-negative smears. Secondly, up to three smears per woman were chosen from cases and controls at random to increase the chance of including an existing HPV infection. Smears collected less than 1 year prior to diagnosis were excluded. DNA was extracted from archival PAP smears using previously described methods (Josefsson et al, 1999), and amounts of human genomic DNA, HPV 16, HPV 31 and a combined load for HPV 18 and 45 were estimated using published methods (Moberg et al, 2003). Viral load is reported as HPV genomes per cell equivalent. An estimate of 6.6 pg per human cell was used to convert human DNA estimates into cell equivalents (CE). The strategy of a combined load of HPV 18 and 45 was employed to minimise the number of parallel quantitative PCR reactions. Odds ratios (ORs) were estimated by logistic regression (LOGISTIC procedure, SAS v6.12, SAS Institute Inc., Cary NC, USA).
RESULTS
A single, normal smear was obtained from 62 cases and 501 controls (Table 1). The smears were collected on average 9 years (range 1–28) prior to the diagnosis date and classified as negative for squamous cell abnormalities. A total of 10 cases had registered abnormal cytology during the screening interval before the collection of the normal smear included in this study. Four cases had diagnoses equivalent to ASCUS or LSIL, and six cases HSIL, as the worst registered lesion prior to the normal smear. However, in these four cases, the smear taken closest before the selected smear was classified as negative for lesions.
Table 1. Characteristics of the case-control material.
|
Single smear cohort
|
Random smear cohort
|
|||
|---|---|---|---|---|
| Cases | Controls | Cases | Controls | |
| n | 62 | 501 | 139 | 548 |
| Year of birth | ||||
| Mean | 1946.8 | 1949.0 | 1946.6 | 1949.0 |
| Range | 1922–1965 | 1922–1968 | 1922–1968 | 1922–1968 |
| Age at smear collection | ||||
| Mean | 34.7 | 30.9 | 34.6 | 29.8 |
| Range | 20–62 | 15–63 | 15–62 | 15–63 |
| Human DNA | ||||
| Meana | 159.8 | 219.2 | 175.7 | 206.2 |
| Range | 5.0–1354 | 5.4–6264 | 5.0–2465 | 5.0–15914 |
| HPV 16 | ||||
| No of positive women | 28 | 28 | 87 | 56 |
| No of positive smears | 28 | 28 | 120 | 66 |
| Mean viral loadb | 138.3 | 17.7 | 97.0 | 12.9 |
| Range | 0.05–2812 | 0.02–300.3 | 0.03–2812 | 0.01–300.3 |
| HPV 31 | ||||
| No of positive women | 5 | 19 | 9 | 34 |
| No of positive smears | 5 | 19 | 15 | 39 |
| Mean viral loadb | 17.7 | 37.4 | 29.8 | 50.5 |
| Range | 0.50–36.7 | 0.03–543.1 | 0.3–147.2 | 0.02–762.6 |
| HPV 18/45 | ||||
| No of positive women | 8 | 28 | 28 | 55 |
| No of positive smears | 8 | 28 | 39 | 64 |
| Mean viral loadb | 157.6 | 77.2 | 265.3 | 71.4 |
| Range | 0.11–1158 | 0.01–1402 | 0.02–5105 | 0.01–2044 |
Mean estimated number of human cells equivalents per reaction.
Mean number of HPV genomes per human cell equivalent.
In all, 45% of cases and 6% of controls test positive for HPV 16 (Table 1). In estimating the risk, crude ORs are presented since initial introduction of birth year and age at smear collection in the regression model did not alter the estimates appreciably. Odds ratio increases with HPV 16 viral load, reaching a maximum of OR=51 in the percentile with the highest viral load (Table 2). HPV 31 is found in 8% of cases and 4% of controls and HPV 18/45, detected together, are found in 13% of cases and 6% of controls. The ORs for HPV 31 and HPV 18/45 are significant for the percentiles with the highest viral load (Table 2).
Table 2. Odds ratios for invasive cervical carcinoma in relation to the mean normalised viral load of individual HPV types or group of closely related HPV types.
|
95% CI
|
|||||
|---|---|---|---|---|---|
| Limits | Cases/controls | OR | Lower limit | Upper limit | P-value |
| Single smear cohort | |||||
| HPV 16 | 34/473 | Ref.b | — | — | — |
| Negative | 4/10 | 5.565 | 1.658 | 18.673 | 0.0055 |
| 0<VLa⩽0.44 | 5/9 | 7.729 | 2.454 | 24.342 | 0.0005 |
| 0.44<VLa⩽2.47 | 8/6 | 18.549 | 6.087 | 56.524 | 0.0001 |
| 2.47<VLa⩽18.22 | 11/3 | 51.010 | 13.583 | 191.560 | 0.0001 |
| 18.22<VLa | |||||
| HPV 31 | 57/482 | Ref.b | — | — | — |
| Negative | 0/7 | 0 | 0 | 999 | 0.9866 |
| 0<VLa⩽0.50 | 2/7 | 2.416 | 0.490 | 11.910 | 0.2785 |
| 0.50<VLa⩽10.25 | 3/5 | 5.074 | 1.181 | 21.791 | 0.0290 |
| 10.25<VLa | |||||
| HPV 18/45 | 54/473 | Ref.b | — | — | — |
| Negative | 2/10 | 1.752 | 0.374 | 8.205 | 0.4767 |
| 0<VLa⩽0.11 | 2/9 | 1.947 | 0.410 | 9.243 | 0.4021 |
| 0.11<VLa⩽3.40 | 4/9 | 3.893 | 1.160 | 13.068 | 0.0278 |
| 3.40<VLa | |||||
| Random smear cohort | |||||
| HPV 16 | 52/492 | Ref.b | — | — | — |
| Negative | 13/23 | 5.348 | 2.557 | 11.183 | 0.0001 |
| 0<VLc⩽0.49 | 18/18 | 9.462 | 4.637 | 19.304 | 0.0001 |
| 0.49<VLc⩽3.09 | 25/10 | 23.654 | 10.767 | 51.967 | 0.0001 |
| 3.09<VLc⩽21.96 | 31/5 | 58.662 | 21.866 | 151.377 | 0.0001 |
| 21.96<VLc | |||||
| HPV 31 | 130/514 | Ref.b | — | — | — |
| Negative | 2/13 | 0.608 | 0.136 | 2.792 | 0.5163 |
| 0<VLc⩽1.29 | 3/10 | 1.186 | 0.322 | 4.372 | 0.7976 |
| 1.29<VLc⩽12.70 | 4/11 | 1.438 | 0.451 | 4.588 | 0.5397 |
| 12.70<VLc | |||||
| HPV 18/45 | 111/492 | Ref.b | — | — | — |
| Negative | 9/19 | 2.100 | 0.925 | 4.764 | 0.0760 |
| 0<VLc⩽0.65 | 7/21 | 1.477 | 0.613 | 3.561 | 0.3846 |
| 0.65<VLc⩽2.65 | 12/16 | 3.324 | 1.530 | 7.225 | 0.0024 |
| 2.65<VLc | |||||
Normalised viral load (number of HPV genomes per human cell equivalent).
Reference group.
Mean normalised viral load (number of HPV genomes per human cell equivalent) based on all HPV-positive smears per individual.
The random set includes 139 cases (261 smears) and 548 controls (1049 smears) (Table 1). In all, 168 (64%) of the included smears from cases were classified as negative for malignant cells. The corresponding number for controls is 819 (78%). Cytological information was missing in the archives for a total of five smears from cases and 188 smears from controls. In total, 63% of cases and 10% of controls test positive for HPV 16 (Table 2). The OR, calculated from the mean HPV 16 viral load per woman, increased with viral load and reached a maximum of OR=59 for the percentile with the highest viral load (Table 2). The ORs for HPV 31 are not significant, while the OR for HPV 18/45 is significant for the percentile with the highest viral load (Table 2).
DISCUSSION
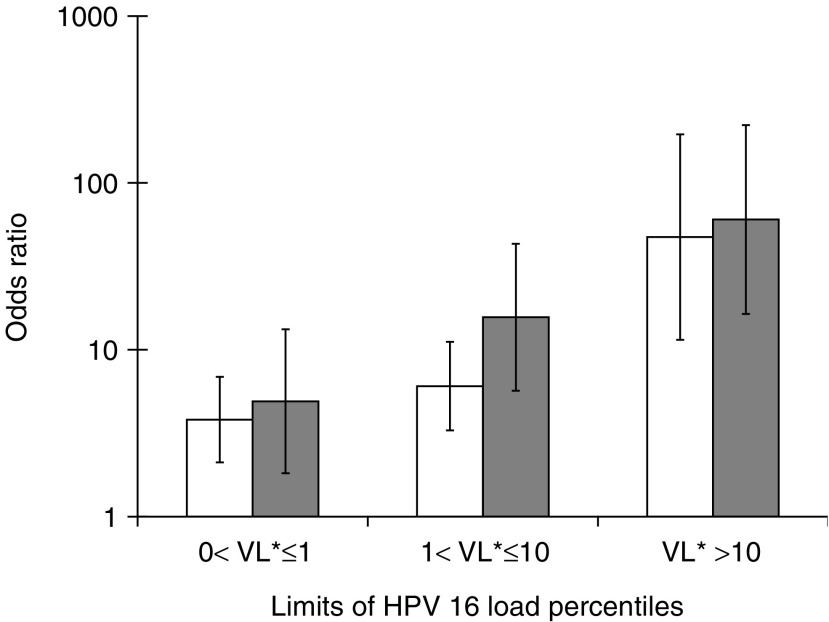
Our results indicate that increasing viral load of HPV 16 in cervical smears increases the risk of future invasive cervical cancer. For HPV 18/45, a significant OR was only seen for the highest viral-load percentile. The relationship between OR of invasive cancer and viral load of HPV 16 is similar to that previously described for CIS (Moberg et al, 2004) (Figure 1); consistent with this, CIS and invasive cervical cancer reflect the same basic aetiology. Thus, our results show that a high viral load in PAP smears is a risk factor not only for the development of CIS but also for invasive cancer. Our data support the hypothesis of Peitsaro et al (2002) that high viral loads are likely to increase the risk for events initiating dysplasia such as viral integration. Given that integration is a random event, the risk for such an event would increase with high viral loads, independent of whether a high viral load reflects widespread infection or high copy numbers per cell. HPV 16 displays a generally higher risk for invasive cervical cancer development over viral load categories than HPV 18 and HPV 31. Interestingly, HPV 16 appears to be able to induce malignant transformation without integration (Pirami et al, 1997; Badaracco et al, 2002; Hudelist et al, 2004). This indicates that additional factors to integration may be important for malignant transformation. Longitudinal studies including both the physical state and load of the virus are needed in order to determine their relative importance for development of cervical cancer. Judging from our data, HPV load provides information about the risk for subsequent development of invasive carcinomas, but the extent correlates strongly with the HPV type.
Figure 1.
Odds ratio with 95% CI of cervical CIS (empty bars) or invasive cervical carcinoma (filled bars) depending on HPV 16 load. VL* represents the number of HPV 16 genomes per human cell equivalent.
Acknowledgments
This study was supported by grants from the Swedish National Cancer Society (grant 3436-B97-04XAA) and by Quantovir AB, Sweden.
References
- Badaracco G, Venuti A, Sedati A, Marcante ML (2002) HPV16 and HPV18 in genital tumors: significantly different levels of viral integration and correlation to tumor invasiveness. J Med Virol 67: 574–582 [DOI] [PubMed] [Google Scholar]
- Boshart M, Gissmann L, Ikenberg H, Kleinheinz A, Scheurlen W, zur Hausen H (1984) A new type of papillomavirus DNA, its presence in genital cancer biopsies and in cell lines derived from cervical cancer. EMBO J 3: 1151–1157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullen AP, Reid R, Campion M, Lorincz AT (1991) Analysis of the physical state of different human papillomavirus DNAs in intraepithelial and invasive cervical neoplasm. J Virol 65: 606–612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalstein V, Riethmuller D, Pretet JL, Le Bail Carval K, Sautiere JL, Carbillet JP, Kantelip B, Schaal JP, Mougin C (2003) Persistence and load of high-risk HPV are predictors for development of high-grade cervical lesions: a longitudinal French cohort study. Int J Cancer 106: 396–403 [DOI] [PubMed] [Google Scholar]
- Daniel B, Rangarajan A, Mukherjee G, Vallikad E, Krishna S (1997) The link between integration and expression of human papillomavirus type 16 genomes and cellular changes in the evolution of cervical intraepithelial neoplastic lesions. J Gen Virol 78(Part 5): 1095–1101 [DOI] [PubMed] [Google Scholar]
- Gravitt PE, Burk RD, Lorincz A, Herrero R, Hildesheim A, Sherman ME, Bratti MC, Rodriguez AC, Helzlsouer KJ, Schiffman M (2003) A comparison between real-time polymerase chain reaction and hybrid capture 2 for human papillomavirus DNA quantitation. Cancer Epidemiol Biomarkers Prev 12: 477–484 [PubMed] [Google Scholar]
- Ho GY, Bierman R, Beardsley L, Chang CJ, Burk RD (1998) Natural history of cervicovaginal papillomavirus infection in young women. N Engl J Med 338: 423–428 [DOI] [PubMed] [Google Scholar]
- Hudelist G, Manavi M, Pischinger KI, Watkins-Riedel T, Singer CF, Kubista E, Czerwenka KF (2004) Physical state and expression of HPV DNA in benign and dysplastic cervical tissue: different levels of viral integration are correlated with lesion grade. Gynecol Oncol 92: 873–880 [DOI] [PubMed] [Google Scholar]
- Jacobs MV, Walboomers JM, Snijders PJ, Voorhorst FJ, Verheijen RH, Fransen-Daalmeijer N, Meijer CJ (2000) Distribution of 37 mucosotropic HPV types in women with cytologically normal cervical smears: the age-related patterns for high-risk and low-risk types. Int J Cancer 87: 221–227 [PubMed] [Google Scholar]
- Josefsson A, Livak K, Gyllensten U (1999) Detection and quantitation of human papillomavirus by using the fluorescent 5′ exonuclease assay. J Clin Microbiol 37: 490–496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josefsson AM, Magnusson PK, Ylitalo N, Sorensen P, Qwarforth-Tubbin P, Andersen PK, Melbye M, Adami HO, Gyllensten UB (2000) Viral load of human papilloma virus 16 as a determinant for development of cervical carcinoma in situ: a nested case–control study. Lancet 355: 2189–2193 [DOI] [PubMed] [Google Scholar]
- Lorincz AT, Castle PE, Sherman ME, Scott DR, Glass AG, Wacholder S, Rush BB, Gravitt PE, Schussler JE, Schiffman M (2002) Viral load of human papillomavirus and risk of CIN3 or cervical cancer. Lancet 360: 228–229 [DOI] [PubMed] [Google Scholar]
- Moberg M, Gustavsson I, Gyllensten U (2003) Real-time PCR-based system for simultaneous quantification of human papillomavirus types associated with high risk of cervical cancer. J Clin Microbiol 41: 3221–3228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moberg M, Gustavsson I, Gyllensten U (2004) Type-specific associations of human papillomavirus load with risk of developing cervical carcinoma in situ. Int J Cancer 112: 854–859 [DOI] [PubMed] [Google Scholar]
- Peitsaro P, Johansson B, Syrjanen S (2002) Integrated human papillomavirus type 16 is frequently found in cervical cancer precursors as demonstrated by a novel quantitative real-time PCR technique. J Clin Microbiol 40: 886–891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirami L, Giache V, Becciolini A (1997) Analysis of HPV16, 18, 31, and 35 DNA in pre-invasive and invasive lesions of the uterine cervix. J Clin Pathol 50: 600–604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlecht NF, Platt RW, Duarte-Franco E, Costa MC, Sobrinho JP, Prado JC, Ferenczy A, Rohan TE, Villa LL, Franco EL (2003) Human papillomavirus infection and time to progression and regression of cervical intraepithelial neoplasia. J Natl Cancer Inst 95: 1336–1343 [DOI] [PubMed] [Google Scholar]
- Sherman ME, Schiffman M, Cox JT (2002) Effects of age and human papilloma viral load on colposcopy triage: data from the randomized Atypical Squamous Cells of Undetermined Significance/Low-Grade Squamous Intraepithelial Lesion Triage Study (ALTS). J Natl Cancer Inst 94: 102–107 [DOI] [PubMed] [Google Scholar]
- Sherman ME, Wang SS, Wheeler CM, Rich L, Gravitt PE, Tarone R, Schiffman M (2003) Determinants of human papillomavirus load among women with histological cervical intraepithelial neoplasia 3: dominant impact of surrounding low-grade lesions. Cancer Epidemiol Biomarkers Prev 12: 1038–1044 [PubMed] [Google Scholar]
- Sun CA, Liu JF, Wu DM, Nieh S, Yu CP, Chu TY (2002) Viral load of high-risk human papillomavirus in cervical squamous intraepithelial lesions. Int J Gynaecol Obstet 76: 41–47 [DOI] [PubMed] [Google Scholar]
- van Duin M, Snijders PJ, Schrijnemakers HF, Voorhorst FJ, Rozendaal L, Nobbenhuis MA, van den Brule AJ, Verheijen RH, Helmerhorst TJ, Meijer CJ (2002) Human papillomavirus 16 load in normal and abnormal cervical scrapes: an indicator of CIN II/III and viral clearance. Int J Cancer 98: 590–595 [DOI] [PubMed] [Google Scholar]
- Ylitalo N, Josefsson A, Melbye M, Sorensen P, Frisch M, Andersen PK, Sparen P, Gustafsson M, Magnusson P, Ponten J, Gyllensten U, Adami HO (2000) A prospective study showing long-term infection with human papillomavirus 16 before the development of cervical carcinoma in situ. Cancer Res 60: 6027–6032 [PubMed] [Google Scholar]