Abstract
It is generally accepted that most colorectal carcinomas arise in pre-existing adenomas. Morphologically, colorectal adenomas can be divided into two groups, protruded type and flat type. The aim of this study was to clarify relevant alterations of gene expression associated with the early stage of colorectal carcinogenesis. Using cDNA array, we analysed the expression profiles of 550 cancer-related genes in 36 colorectal adenomas (18 flat-type and 18 protruded-type adenomas) and 14 early invasive carcinomas. Among the 550 genes, we chose 32 genes the average expression levels of which were at least three-fold up- or downregulated in tumour tissues compared with levels in matched normal tissues. A total of 13 and 19 genes were identified as up- and downregulated genes in tumour tissues, respectively. Among the upregulated genes, the average expression levels of E1AF, bone morphogenic protein (BMP)-4, insulin-like growth factor (IGF)-2, inducible nitric oxide synthase (iNOS), tissue inhibitors of metalloproteinase (TIMP)-1, Smad4, and nm23 in tumour tissues were over five times higher than those in matched normal tissues. Colorectal adenomas and early invasive carcinomas were divided into two major clusters by clustering analysis. Moreover, flat- and protruded-type adenomas were divided into two major clusters by clustering analysis. The expression profiles obtained by the cDNA array clearly indicate that colorectal adenomas and early invasive carcinomas have specific expression profiles. Likewise, the gene expression profiles of flat- and protruded-type adenomas are different. These results indicate that molecular classification of early colorectal tumours by a cDNA array is feasible.
Keywords: colorectal adenoma, early invasive colorectal carcinoma, cDNA array
Colorectal carcinoma is one of the most common human malignancies in the world. Although alternative pathways exist, it is generally accepted that most colorectal carcinomas arise in pre-existing adenomas (Jass et al, 2002). Despite a large number of studies, little is known about molecular alterations associated with the heterogeneity of colorectal carcinomas.
Morphologically, early colorectal tumors can be divided into two groups, protruded type and flat type. Recently, flat-type colorectal tumours have been reported not only in Japan (Sakashita et al, 2000) but also in Western countries (Olschwang et al, 1998). Previous studies showed that flat-type colorectal tumours tended to reach deeper layers earlier and to show higher rates of lymphatic invasion and lymph node metastasis than did protruded-type tumours (Kuramoto and Oohara, 1989; Mueller et al, 1998). Moreover, it has been thought that some flat-type cancers correspond to de novo cancers, which contain no observable adenomatous component and may develop through a distinct genetic pathway (Yashiro et al, 2001). Thus, it would be interesting to examine gene expression profiles of colorectal adenomas and early invasive colorectal carcinomas, because comparison of these two groups of tumours will provide information about genes that play an important role during progression from adenoma to carcinoma.
DNA array technology enables measure of the mRNA expression levels of thousands of genes in a single assay. Mainly advanced cancer has been analysed in gene expression profiling-based studies on colorectal cancer (Alon et al, 1999; Backert et al, 1999; Hegde et al, 2001; Kitahara et al, 2001; Notterman et al, 2001; Takemasa et al, 2001; Agrawal et al, 2002; Birkenkamp-Demtroder et al, 2002; Lin et al, 2002; Zou et al, 2002; Frederiksen et al, 2003; Muro et al, 2003; Tureci et al, 2003; Williams et al, 2003; Bertucci et al, 2004). Only a small number of adenoma and early invasive cancer tissues have been analysed in previous studies, and the issue of flat- and protruded-type adenoma tissues has not been directly addressed (Notterman et al, 2001; Agrawal et al, 2002; Lin et al, 2002; Williams et al, 2003).
In this study, we therefore applied cDNA array technology to analyse the gene expression profiles of 36 colorectal adenomas (18 flat-type and 18 protruded-type adenomas) and 14 early invasive carcinomas. This is the first study showing molecular classification of early colorectal tumours by a cDNA array analysis.
MATERIALS AND METHODS
Patients and tissue samples
A total of 50 paired specimens of colorectal tumour and nontumour tissues were obtained by polypectomy or surgical treatment. These tumour samples consisted of 36 colorectal adenomas and 14 early invasive carcinomas (pT1 in the TNM classification of the Union International Contre Cancer). Normal epithelial tissue samples and tumour tissue samples were carefully macrodissected by expert pathologists. In case of early invasive carcinomas, tumour tissue samples were taken from the macroscopically visible deepest invading part of the tumour after surgical resection. Each tissue specimen was divided into two pieces after resection. For total RNA extraction, one sample was immediately frozen in liquid nitrogen at the time of surgery and stored at −80°C until extraction. The other sample was processed for pathological examination using haematoxylin and eosin staining for the evaluation of the tumour cell content. Only specimens containing more than 80% tumour cells were used for analysis (Horiuchi et al, 2003). The histopathological features of the carcinoma specimens were classified according to the TNM classification system. Locations of the colorectal tumours were divided into proximal colon (caecum and ascending and transverse colon) and distal colon (descending and sigmoid colon and rectum). Macroscopic types were divided into protruded type (height of tumour ⩾3 mm) and flat type (height of tumour <3 mm). It was difficult to divide early invasive carcinoma into protruded type or flat type because colorectal tumours become thick when they have invaded the submucosal layer. Therefore, macroscopic type was classified in only colorectal adenomas. The clinicopathological characteristics of colorectal tumours are shown in Table 1. Informed consent was obtained from each subject, and the institutional review committee approved this study.
Table 1. Clinicopathological characteristics of patients with colorectal tumour.
|
Adenoma (n=36)
|
|||
|---|---|---|---|
| Characteristics | Early invasive carcinoma (n=14) | Protruded type (n=18) | Flat type (n=18) |
| Age (years, mean±s.d.) | 69.1±7.3 | 67.9±5.8 | 71.1±4.9 |
| Size (mm, mean±s.d.) | 25.7±10.0 | 10.5±5.4 | 14.2±12.7 |
| Gender | |||
| Male | 8 | 11 | 13 |
| Female | 6 | 7 | 5 |
| Location | |||
| Proximal | 9 | 5 | 10 |
| Distal | 5 | 13 | 8 |
cDNA array analysis
Total RNA was extracted from specimens using the acid guanidinum thiocyanate–phenol–chloroform extraction method and treated with DNase I. Biotin-labelled cDNA targets were made from 2.5 μg of total RNA using Gene Navigator cDNA amplification system (Toyobo, Osaka, Japan), including random 9-mer, biotin-16-dUTP, and ReverTraAceTM reverse transcriptase. Free biotin-16-dUTP in the reaction was removed by ethanol precipitation. Gene Navigator cDNA array filter (human cancer, Toyobo) consisted of 550 cancer-related genes and 11 housekeeping genes in duplicate. A complete list is available on the Internet (http://www.toyobo.co.jp). Hybridisation was performed overnight at 68°C in PerfectHybTM (Toyobo). Filters were washed three times in 2 × SSC/0.1% SDS at 68°C for 10 min each, followed by three washes in 0.1 × SSC/0.1% SDS at 68°C for 10 min each. Specific signals on the filters were detected by the chemiluminescence detection kit (Imaging highTM, Toyobo). CDP-Star was used as the chemiluminescent substrate. Quantitative assessment of the signals on the filters was performed by scanning on a Fluor-S MultiImager System (Bio-Rad, Richmond, CA, USA) followed by image analysis using ImaGene software (BioDiscovery, Los Angeles, CA, USA). The data were analysed with normalisation to glyceraldehyde-3-phosphate dehydrogenase (GAPDH) expression. The average of three experiments was calculated.
Statistical analysis
Expression of each target gene was assessed for associations with clinicopathological characteristics using Mann–Whitney U-test for average tumour expressions.
RESULTS
cDNA array analysis
To clarify relevant alterations of gene expression associated with early colorectal carcinogenesis, we analysed the gene expression profiles of tumour tissues by a cDNA array (Yamamoto et al, 2002). Gene Navigator cDNA array filter (human cancer, Toyobo) consisted of 550 cancer-related genes and 11 housekeeping genes in duplicate. Among the 550 genes the expression profiles of which were analysed, we chose 32 genes the average expression levels of which were at least three-fold up- or downregulated in 50 tumour tissues compared with levels in 50 matched normal tissues. Besides, since many colorectal tumours have been demonstrated to have a diversity of gene expression profiles, we examined the ratios of the selected 32 genes in all 50 tumour/normal pairs individually. The selected genes were classified as commonly changed if their ratio was three-fold up- or downregulated in more than one-third (15 of 50) of the patients. Among the selected 32 genes, all genes satisfied these criteria. A total of 13 genes (insulin-like growth factor (IGF)-2 bone morphogenic protein (BMP)-4, ECGF-1, E1AF, FAK, Rho GDIβ, nm23, tissue inhibitors of metalloproteinase (TIMP)-1, GSTP1, GST-II, Smad4, inducible nitric oxide synthase (iNOS), and c-jun) and 19 genes (Egr-2, PMS1, Eph, gp130, Rho 8, Ras-GAP, p120, GAK, Erk1, Lamin β3, Cdc42, αN-catenin, MMP-15, Galectin-1, HLA-DQ, MUC-2, MDR1, Mucin3, and p21) were identified as up- and downregulated genes in tumour tissues, respectively. These genes were associated with transcription (Egr-2 and E1AF), DNA repair or protection (PMS1, GSTP1, and GST-II), cell signalling (Eph, gp130, Rho 8, Ras-GAP, p120, GAK, Rho GDIβ, Erk1, FAK, and Lamin β3), cell cycle (Cdc42), growth factor (IGF-2, BMP-4, and ECGF-1), tumour suppressor (nm23 and Smad4), oncogene (c-jun), cell adhesion (αN-catenin), extracellular matrix-degrading enzymes (MMP-15, TIMP-1, and Galectin-1), human leucocyte antigen (HLA-DQ), glycoprotein (MUC-2, MDR1, and Mucin3), CDK inhibitor (p21), and angiogenesis (iNOS).
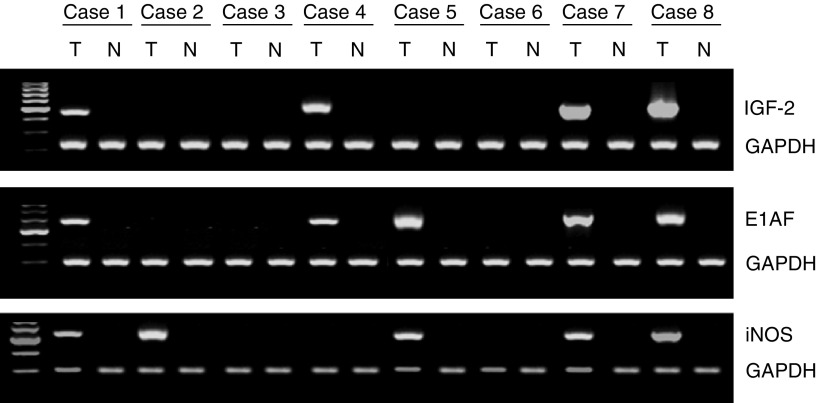
Among the 13 genes the average expression levels of which were at least three-fold upregulated in tumour tissues compared with levels in matched normal tissues, the average expression levels of E1AF, BMP-4, IGF-2, iNOS, TIMP-1, Smad4, and nm23 genes in tumour tissues were over five times higher than those in matched normal tissues (Table 2). Semiquantitative reverse transcriptase–polymerase chain reaction (RT–PCR) analysis of these differentially expressed genes gave results consistent with those by a cDNA array analysis (Figure 1).
Table 2. Average expression levels of E1AF, BMP4, IGF-2, iNOS, TIMP-1, Smad4, and nm23 genes in tumour tissues were over five times higher than those in the matched normal tissues.
| Gene name | Function | T average/N average |
|---|---|---|
| E1AF | Transcription | 8.11 |
| BMP4 | Growth factor | 7.64 |
| IGF-2 | Growth factor | 6.80 |
| iNOS | Angiogenesis | 6.55 |
| TIMP-1 | Inhibitor of MMPs | 6.39 |
| Smad4 | Tumour suppressor | 6.03 |
| nm23 | Tumour suppressor | 5.33 |
T average=average expression levels in tumour tissues; N average,=average expression levels in matched normal tissues. The average of three experiments is shown.
Figure 1.
Reverse transcriptase–polymerase chain reaction analysis of mRNA expression for IGF-2, E1AF, and iNOS in colorectal tumour tissues. T and N, matched samples from tumour and nontumour tissue, respectively. Cases 1–4 are colorectal adenomas and cases 5–8 are colorectal carcinomas (pT1).
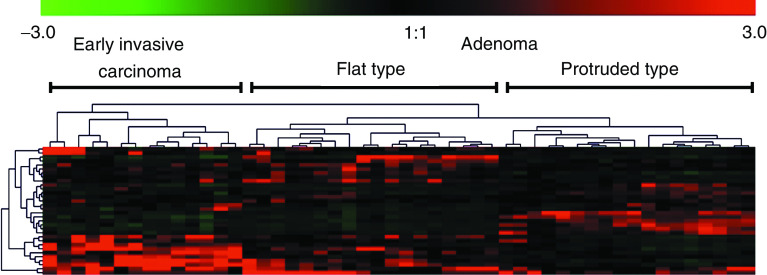
Colorectal adenomas (n=36) and early invasive carcinomas (n=14) were divided into two major clusters by clustering analysis (Figure 2). The average expression levels of 10 (IGF-2, E1AF, iNOS, Rho GDIβ, GSTP1, c-jun, ECGF1, nm23, Smad4, and TIMP-1) of the 32 genes were significantly higher in the early invasive carcinoma group than in the adenoma group. On the other hand, the average expression levels of 12 (Eph, gp130, GST-II, Rho 8, MUC-2, Ras-GAP, p120, MDR1, αN-catenin, Egr-2, PMS1, and GAK) of the 32 genes were significantly lower in the early invasive carcinoma group than in the adenoma group (Table 3).
Figure 2.
A two-dimensional hierarchical clustering of 32 genes across 50 colorectal tumours. The colour in each well represents relative expression of each gene (vertical axis) in each paired sample (horizontal axis); red, increased in tumour tissues; green, decreased in the tumour tissues. In the sample axis, early invasive carcinomas and adenomas were separated into two different trunks. In the gene axis, 32 genes were clustered in different branches according to their similarity; the shorter the branches, the greater the similarity. In adenomas, subclusters of flat type and protruded type were selected for further analysis (see Figure 3).
Table 3. Genes the expression levels of which differed significantly in the early invasive carcinoma group and the adenoma group (Mann–Whitney U-test).
| Gene name | Accession no. | Gene function | P-value | |
|---|---|---|---|---|
| Eph | M18391 | Cell signalling | 0.0342 | CA<AD |
| Gp130 | M57230 | Cell signalling | 0.0122 | CA<AD |
| GST-II | U77604 | DNA repair or protection | 0.0108 | CA<AD |
| Rho 8 | X95282 | Cell signalling | 0.0069 | CA<AD |
| MUC-2 | M74027 | Glycoprotein | 0.0046 | CA<AD |
| Ras-GAP | AF051311 | Cell signalling | 0.0035 | CA<AD |
| P120 | AF062324 | Cell signalling | 0.0029 | CA<AD |
| MDR1 | AF016535 | Glycoprotein | 0.0019 | CA<AD |
| αN-catenin | M94151 | Cell adhesion | 0.0014 | CA<AD |
| Egr-2 | X53700 | Transcription | 0.0011 | CA<AD |
| PMS1 | U13695 | DNA repair or protection | 0.0002 | CA<AD |
| GAK | D88435 | Cell signalling | 0.0001 | CA<AD |
| IGF-2 | M29645 | Growth factor | 0.0491 | CA>AD |
| E1AF | D12765 | Transcription | 0.0187 | CA>AD |
| iNOS | AB022318 | Angiogenesis | 0.0084 | CA>AD |
| Rho GDIβ | L20688 | Cell signalling | 0.0002 | CA>AD |
| GSTP1 | X06547 | DNA repair or protection | <0.0001 | CA>AD |
| c-jun | J04111 | Oncogene | <0.0001 | CA>AD |
| ECGF1 | M63193 | Growth factor | <0.0001 | CA>AD |
| nm23 | X17620 | Tumour suppressor | <0.0001 | CA>AD |
| Smad4 | U44378 | Tumour suppressor | <0.0001 | CA>AD |
| TIMP1 | X03124 | Extracellular matrix-Degrading enzymes | <0.0001 | CA>AD |
| BMP-4 | D30751 | Growth factor | 0.7494 | |
| Cdc42 | M57298 | Cell cycle | 0.65 | |
| Erk1 | X60188 | Cell signalling | 0.2101 | |
| FAK | L13616 | Cell signalling | 0.0878 | |
| Galectin-1 | J04456 | Extracellular matrix-degrading enzymes | 0.136 | |
| HLA-DQ | U77589 | Human leucocyte antigen | 0.8289 | |
| Laminin β-3 | D37766 | Cell signalling | 0.7134 | |
| MMP-15 | Z48482 | Extracellular matrix-degrading enzymes | 0.8289 | |
| Mucin 3 | AF143371 | Glycoprotein | 0.2897 | |
| P21 | U03106 | CDK inhibitor | 0.1734 | |
CA=early invasive carcinoma group; AD=adenoma group.
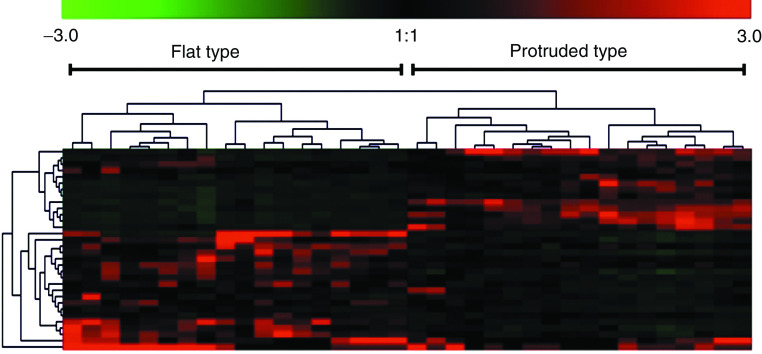
Flat-type (n=18) and protruded-type (n=18) adenomas were divided into two major clusters by clustering analysis (Figure 3). The average expression levels of 16 (PMS1, nm23, p21, FAK, Smad4, c-jun, ECGF-1, Erk1, GAK, GSTP1, IGF-2, Laminin β-3, MMP-15, Mucin3, Rho GDIβ, and TIMP-1) of the 32 genes were significantly higher in the flat-type group than in the protruded-type group, while the average expression levels of 11 (HLA-DQ, Cdc42, Egr-2, Eph, Galectin-1, gp130, GST-II, MDR1, p120, Ras-GAP, and Rho 8) of the 32 genes were significantly lower in the flat-type group than in the protruded-type group (Table 4).
Figure 3.
A two-dimensional hierarchical clustering of 32 genes across 36 colorectal adenomas. The colour in each well represents relative expression of each gene (vertical axis) in each paired sample (horizontal axis); red, increased in adenoma tissues; green, decreased in adenoma tissues. In the sample axis, flat- and protruded-type adenomas were separated into two different trunks.
Table 4. Genes the expression levels of which differed significantly in the flat-type adenoma group and the protruded-type adenoma group (Mann–Whitney U-test).
| Gene name | Accession no. | Gene function | P-value | |
|---|---|---|---|---|
| PMS1 | U13695 | DNA repair or protection | 0.0136 | P<F |
| nm23 | X17620 | Tumour suppressor | 0.0004 | P<F |
| p21 | U03106 | CDK inhibitor | 0.001 | P<F |
| FAK | L13616 | Cell signalling | 0.0017 | P<F |
| Smad4 | U44378 | Tumour suppressor | 0.0429 | P<F |
| c-jun | J04111 | Oncogene | <0.0001 | P<F |
| ECGF1 | M63193 | Growth factor | <0.0001 | P<F |
| Erk1 | X60188 | Cell signalling | <0.0001 | P<F |
| GAK | D88435 | Cell signalling | <0.0001 | P<F |
| GSTP1 | X06547 | DNA repair or protection | <0.0001 | P<F |
| IGF-2 | M29645 | Growth factor | <0.0001 | P<F |
| Laminin β-3 | D37766 | Cell signalling | <0.0001 | P<F |
| MMP-15 | Z48482 | Extracellular matrix-degrading enzymes | <0.0001 | P<F |
| Mucin 3 | AF143371 | Glycoprotein | <0.0001 | P<F |
| Rho GDIβ | L20688 | Cell signalling | <0.0001 | P<F |
| TIMP1 | X03124 | Extracellular matrix-degrading enzymes | <0.0001 | P<F |
| HLA-DQ | U77589 | Human leucocyte antigen | 0.0003 | P>F |
| Cdc42 | M57298 | Cell cycle | <0.0001 | P>F |
| Egr-2 | X53700 | Transcription | <0.0001 | P>F |
| Eph | M18391 | Cell signalling | <0.0001 | P>F |
| Galectin-1 | J04456 | Extracellular matrix-degrading enzymes | <0.0001 | P>F |
| Gp130 | M57230 | Cell signalling | <0.0001 | P>F |
| GST-II | U77604 | DNA repair or protection | <0.0001 | P>F |
| MDR1 | AF016535 | Glycoprotein | <0.0001 | P>F |
| p120 | AF062324 | Cell signalling | <0.0001 | P>F |
| Ras-GAP | AF051311 | Cell signalling | <0.0001 | P>F |
| Rho 8 | X95282 | Cell signalling | <0.0001 | P>F |
| E1AF | D12765 | Transcription | 0.1488 | |
| iNOS | AB022318 | Angiogenesis | 0.1639 | |
| BMP-4 | D30751 | Growth factor | 0.1966 | |
| MUC-2 | M74027 | Glycoprotein | 0.4107 | |
| αN-catenin | M94151 | Cell adhesion | 0.5478 | |
F=flat-type adenoma group; P=protruded-type adenoma group.
Among the 32 genes, the average expression levels of eight genes (Rho GDIβ, c-jun, iNOS, TIMP-1, GSTP1, ECGF1, nm23, and Smad4) and four genes (MUC-2, αN-catenin, PMS1, and GAK) were significantly lower and higher, respectively, in both the flat- and protruded-type adenoma groups than in the early invasive carcinoma group. The average expression levels of two genes (Cdc42 and Galectin-1) and four genes (p21, Erk1, Mucin 3, and Laminin β-3) were significantly lower and higher, respectively, in the flat-type adenoma group than those in the early invasive carcinoma group (Table 5). On the other hand, the average expression levels of seven genes (Erk1, Mucin 3, Laminin β-3, IGF-2, FAK, MMP-15, and E1AF) and nine genes (Cdc42, Eph, gp130, GST-II, Rho 8, Ras-GAP, p120, MDR1, and Egr-2) were significantly lower and higher, respectively, in the protruded-type adenoma group than in the early invasive carcinoma group (Table 5).
Table 5. Genes the expression levels of which differed significantly in the early invasive carcinoma group and the protruded-type (28 genes) or flat-type (18 genes) adenoma group (Mann-Whitney U-test).
| Gene name | Accession no. | P-value | P-value | ||
|---|---|---|---|---|---|
| BMP-4 | D30751 | 0.6128 | 0.2968 | ||
| Cdc42 | M57298 | 0.001 | CA<AD (P) | 0.0122 | CA>AD (F) |
| c-jun | J04111 | <0.0001 | CA>AD (P) | 0.0135 | CA>AD (F) |
| E1AF | D12765 | 0.0086 | CA>AD (P) | 0.13 | |
| ECGF1 | M63193 | <0.0001 | CA>AD (P) | 0.0002 | CA>AD (F) |
| Egr-2 | X53700 | <0.0001 | CA<AD (P) | 0.3423 | |
| Eph | M18391 | 0.0005 | CA<AD (P) | 0.7903 | |
| Erk1 | X60188 | <0.0001 | CA>AD (P) | 0.0276 | CA<AD (F) |
| FAK | L13616 | 0.0304 | CA>AD (P) | 0.4033 | |
| GAK | D88435 | 0.0227 | CA<AD (P) | <0.0001 | CA<AD (F) |
| Galectin-1 | J04456 | 0.704 | 0.0027 | CA>AD (F) | |
| Gp130 | M57230 | <0.0001 | CA<AD (P) | 0.9093 | |
| GST-II | U77604 | <0.0001 | CA<AD (P) | 0.8197 | |
| GSTP1 | X06547 | <0.0001 | CA>AD (P) | 0.0006 | CA>AD (F) |
| HLA-DQ | U77589 | 0.1489 | 0.0682 | ||
| IGF-2 | M29645 | 0.0034 | CA>AD (P) | 0.5937 | |
| iNOS | AB022318 | 0.0402 | CA>AD (P) | 0.0098 | CA>AD (F) |
| Laminin β-3 | D37766 | 0.0016 | CA>AD (P) | 0.0001 | CA<AD (F) |
| MDR1 | AF016535 | <0.0001 | CA<AD (P) | 0.4941 | |
| MMP-15 | Z48482 | 0.025 | CA>AD (P) | 0.0627 | |
| MUC-2 | M74027 | 0.0044 | CA<AD (P) | 0.0334 | CA<AD (F) |
| Mucin 3 | AF143371 | <0.0001 | CA>AD (P) | 0.0227 | CA<AD (F) |
| nm23 | X17620 | <0.0001 | CA>AD (P) | 0.0001 | CA>AD (F) |
| p120 | AF062324 | <0.0001 | CA<AD (P) | 0.4941 | |
| p21 | U03106 | 0.7324 | 0.0402 | CA<AD (F) | |
| PMS1 | U13695 | 0.0044 | CA<AD (P) | 0.0002 | CA<AD (F) |
| Ras-GAP | AF051311 | <0.0001 | CA<AD (P) | 0.7324 | |
| Rho 8 | X95282 | <0.0001 | CA<AD (P) | 0.8494 | |
| Rho GDIβ | L20688 | <0.0001 | CA>AD (P) | 0.0304 | CA>AD (F) |
| Smad4 | U44378 | <0.0001 | CA>AD (P) | <0.0001 | CA>AD (F) |
| TIMP1 | X03124 | <0.0001 | CA>AD (P) | 0.0024 | CA>AD (F) |
| αN-catenin | M94151 | 0.0014 | CA<AD (P) | 0.0151 | CA<AD (F) |
CA=early invasive carcinoma group; AD (P)=protruded-type adenoma group; AD (F)=flat-type adenoma group.
DISCUSSION
Using a cDNA array, we analysed the gene expression profiles of 36 colorectal adenoma and 14 early invasive carcinoma tissues to clarify characteristic changes associated with the early stage of colorectal carcinogenesis. The reason why we chose early invasive carcinoma is that it represents the early stage of colorectal carcinoma. Among the 550 genes the expression profiles of which were analysed, we chose 32 genes the average expression levels of which were at least three-fold up- or downregulated in 50 tumour tissues compared with levels in 50 matched normal tissues. Among the 13 upregulated genes, the expression levels of E1AF, BMP-4, IGF-2, iNOS, TIMP-1, Smad4, and nm23 genes were over five-times higher than those in matched normal tissues.
E1AF (human PEA3/ETV4) is an ets family transcriptional factor. We recently reported that E1AF plays a key role in the progression of colorectal carcinoma (Horiuchi et al, 2003). Thus, our results of cDNA array analysis extend roles of E1AF in the late stage to early stage of colorectal carcinogenesis. BMP-4 is a member of the TGF-β superfamily of growth factors. It has been reported that BMP-4 is overexpressed and secreted by human colon cancer cells with mutant APC genes (Kim et al, 2002). Our results suggest that BMP-4 overexpression plays an important role in the early stage of colorectal carcinogenesis. iNOS has been reported to play a crucial role in cancer development by promoting angiogenesis (Jenkins et al, 1995). Our results are consistent with those of previous studies showing an important role of iNOS in the early stage of colorectal carcinogenesis (Xu et al, 2003). Interestingly, nitric oxide (NO), generated by iNOS, reportedly augments the synergistic interaction between E1AF and its transcription coactivator CBP/p300, resulting in the facilitation of induction of tumour-related genes, such as COX-2 (Liu et al, 2004).
Several lines of evidence suggest that IGF-2 plays an important role in the progression of colorectal tumours (Lambert et al, 1990). Moreover, expression of IGF-2 protein has been reported to be associated with advanced tumour stage and poor survival (Kawamoto et al, 1998; Peters et al, 2003). It has also been suggested that IGF-2 plays a role in the development of liver metastasis from colorectal cancer (Kawamoto et al, 1999). Thus, our results extend roles of IGF-2 in the late stage to early stage of colorectal carcinogenesis.
Among the four known tissue inhibitors of metalloproteinases (TIMPs), TIMP-1 has functions apart from its protease inhibitory action. Several investigators have reported that TIMP-1 has growth-promoting properties and might also stimulate tumour growth by inhibiting apoptosis (Holten-Andersen et al, 2002). Overexpression of TIMP-1 has been reported in colorectal cancer tissues (Murashige et al, 1996). Holten-Andersen et al (2005) recently reported that TIMP-1 mRNA was detected in all of 24 cases of colorectal cancer tissues by in situ hybridisation, but it was detected in only two of seven adenoma tissues. In the current study, we also found that the average expression levels of TIMP-1 were significantly higher in the early invasive carcinoma group than in the adenoma group. Besides, levels of TIMP-1 in blood were significantly elevated in colorectal cancer patients compared to healthy donors, and high plasma TIMP-1 levels were associated with short survival of colorectal cancer patients (Holten-Andersen et al, 2005). Therefore, TIMP-1 appears to be a novel marker for detection of early colorectal cancer and for prognostic stratification of colorectal cancer patients.
Smad4 is an intracellular transmitter of TGF-β signals and its tumour suppressor function is presumed to reside in its capacity to mediate TGF-β-induced growth inhibition. However, there is accumulating evidence that this hypothesis may be too simple (Muller et al, 2002). Although functional inactivation of Smad4 in colorectal cancer frequently occurs at late stages when tumours acquire invasive and metastatic capabilities, the roles of TGF-β signals in carcinogenesis are complex and also comprise tumour-promoting functions in colorectal carcinogenesis (Muller et al, 2002; Reinacher-Schick et al, 2004). Nevertheless, the reason for the overexpression of Smad4 in early colorectal tumour tissues remains unknown. It may be induced to inhibit tumour growth by some compensatory mechanisms. Further analysis is needed to clarify this issue.
The nm23 gene was first identified as a gene the expression level of which was reduced in highly metastatic rodent tumours relative to poorly metastatic tumour cells (Steeg et al, 1988). The transfection of nm23 cDNA into various cancer cell lines resulted in suppression of the metastatic potential of motility, invasion, or colonisation (Suzuki et al, 2004). However, Bertucci et al (2004) recently reported over- and downexpression of nm23 in colorectal cancer tissues and in those with poor prognosis, respectively. The reason why nm23 gene was highly expressed in tumour tissues in the current study may be due to the fact that all tumour samples consisted of early colorectal tumours without metastasis. Further analysis is needed to clarify this issue.
We identified 22 genes the expression levels of which differed significantly in colorectal adenomas and early invasive carcinomas. Colorectal adenomas and early invasive carcinomas were divided into two major clusters by clustering analysis. This result is consistent with that of a recent study showing that nine colorectal adenomas were separated from 11 differentiated colorectal carcinomas by using oligonucleotide arrays (Lin et al, 2002). The expression profiles obtained by our cDNA array demonstrated that colorectal adenomas and early invasive carcinomas have specific expression profiles. Among the seven upregulated genes the expression levels of which were over five times higher than those in the matched normal tissues, the expression levels of IGF-2, E1AF, iNOS, nm23, Smad4, and TIMP-1 genes were significantly higher in the early invasive carcinoma group than in the adenoma group. These results suggest that these genes play an important role in the progression from adenoma to carcinoma.
On the other hand, GAK was the most downregulated gene in the early invasive carcinoma group relative to the adenoma group. GAK is a serine/threonine kinase that shows high homology outside its kinase domain with auxilin. Like auxilin, GAK has been shown to be a cofactor for uncoating clathrin vesicles in vitro. Zhang et al (2004) reported that downregulation of GAK by small hairpin RNA increased the levels of epidermal growth factor (EGF) receptor expression and tyrosine kinase activity, resulting in a large increase in the levels of activated extracellular signal-regulated kinase 5 and Akt. Moreover, downregulation of GAK has been reported to result in outgrowth of monkey kidney CV1P cells in soft agar, raising the possibility that loss of GAK function may promote tumorigenesis. Thus, our results suggest that downregulation of GAK plays an important role in the progression from colorectal adenoma to carcinoma.
The adenoma–carcinoma sequence (ACS) is widely accepted as a pathogenesis of colorectal carcinoma. A multistep genetic model for colorectal carcinogenesis based on the ACS has been proposed (Vogelstein et al, 1988). In the ACS sequence, mutations in the K-ras gene and various tumour suppressor genes, such as APC and p53, are known to accumulate during the progression from normal to malignant tissue. Although coexistence of all three mutations has been reported to be a rare occurrence (Smith et al, 2002), the majority of sporadic colorectal carcinomas are still thought to develop and progress through this pathway. It has been thought that de novo cancers develop from normal colonic mucosa directly. However, critical genetic abnormality is not known. Most protruded-type colorectal cancers have adenomatous elements in the periphery when found at an early stage, suggesting that these cancers have arisen from pre-existing adenomas. On the other hand, adenomatous components are not detectable microscopically in some flat-type cancers, suggesting that flat-type cancers correspond to de novo cancer (Yashiro et al, 2001). The reason why we could not detect any changes in the expression of APC, p53, and K-ras genes may be due to the fact that mutations of these genes do not necessarily result in alterations of mRNA expression levels.
In the current study, flat- and protruded-type adenomas were divided into two major clusters by clustering analysis. We identified 27 genes the expression levels of which differed significantly in flat- and protruded-type adenomas. The expression levels of 16 genes (PMS1, nm23, p21, FAK, Smad4, c-jun, ECGF-1, Erk1, GAK, GSTP1, IGF-2, Laminin β-3, MMP-15, Mucin3, Rho GDIβ, and TIMP-1) were significantly higher in the flat-type group than in the protruded-type group. On the other hand, the expression levels of 11 genes (HLA-DQ, Cdc42, Egr-2, Eph, Galectin-1, gp130, GST-II, MDR1, p120, Ras-GAP, and Rho 8) were significantly lower in the flat-type group than in the protruded-type group.
Among the 18 genes the expression levels of which were significantly different in the early invasive carcinoma group and the flat-type adenoma group, the expression levels of eight genes (p21, MUC-2, Erk1, Mucin 3, αN-catenin, PMS1, Lamin β3, and GAK) and 10 genes (Rho GDIβ, c-jun, Cdc42, iNOS, Galectin-1, TIMP-1, GSTP1, ECGF1, nm23, and Smad4) were significantly higher and lower, respectively, in the flat-type adenoma group than in the early invasive cancer group. On the other hand, among the 28 genes the expression levels of which were significantly different in the early invasive carcinoma group and the protruded-type adenoma group, the expression levels of 13 genes (GAK, MUC-2, PMS1, αN-catenin, Cdc42, Eph, gp130, GST-II, Rho 8, Ras-GAP, p120, MDR1, and Egr-2) and 15 genes (iNOS, FAK, MMP-15, E1AF, IGF-2, Laminin β3, Smad4, TIMP1, nm23, Rho GDIβ, GSTP1, c-jun, ECGF1, Erk1, and Mucin 3) were significantly higher and lower, respectively, in the protruded-type adenoma group than in the early invasive cancer group. These results suggest that flat- and protruded-type adenomas have specific expression profiles and that genes that play a crucial role in the progression from each type of adenoma to carcinoma are different.
In conclusion, the expression profiles obtained by the cDNA array clearly indicate that colorectal adenomas and early invasive carcinomas have specific expression profiles. Likewise, the gene expression profiles of flat- and protruded-type adenomas are different. These results indicate that molecular classification of early colorectal tumours by a cDNA array is feasible.
Acknowledgments
This work was supported by Grants-in-Aid from the Ministry of Education, Culture, Sports, Science and Technology of Japan (HY and KI) and from the Ministry of Health, Labor and Welfare of Japan (HY and KI).
References
- Agrawal D, Chen T, Irby R, Quackenbush J, Chambers AF, Szabo M, Cantor A, Coppola D, Yeatman TJ (2002) Osteopontin identified as lead marker of colon cancer progression, using pooled sample expression profiling. J Natl Cancer Inst 94: 513–521 [DOI] [PubMed] [Google Scholar]
- Alon U, Barkai N, Notterman DA, Gish K, Ybarra S, Mack D, Levine AJ (1999) Broad patterns of gene expression revealed by clustering analysis of tumor and normal colon tissues probed by oligonucleotide arrays. Proc Natl Acad Sci USA 96: 6745–6750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Backert S, Gelos M, Kobalz U, Hanski ML, Bohm C, Mann B, Lovin N, Gratchev A, Mansmann U, Moyer MP, Riecken EO, Hanski C (1999) Differential gene expression in colon carcinoma cells and tissues detected with a cDNA array. Int J Cancer 82: 868–874 [DOI] [PubMed] [Google Scholar]
- Bertucci F, Salas S, Eysteries S, Nasser V, Finetti P, Ginestier C, Charafe-Jauffret E, Loriod B, Bachelart L, Montfort J, Victorero G, Viret F, Ollendorff V, Fert V, Giovaninni M, Delpero JR, Nguyen C, Viens P, Monges G, Birnbaum D, Houlgatte R (2004) Gene expression profiling of colon cancer by DNA microarrays and correlation with histoclinical parameters. Oncogene 23: 1377–1391 [DOI] [PubMed] [Google Scholar]
- Birkenkamp-Demtroder K, Christensen LL, Olesen SH, Frederiksen CM, Laiho P, Aaltonen LA, Laurberg S, Sorensen FB, Hagemann R, ORntoft TF (2002) Gene expression in colorectal cancer. Cancer Res 62: 4352–4363 [PubMed] [Google Scholar]
- Frederiksen CM, Knudsen S, Laurberg S, Orntoft TF (2003) Classification of Dukes' B and C colorectal cancers using expression arrays. J Cancer Res Clin Oncol 129: 263–271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hegde P, Qi R, Gaspard R, Abernathy K, Dharap S, Earle-Hughes J, Gay C, Nwokekeh NU, Chen T, Saeed AI, Sharov V, Lee NH, Yeatman TJ, Quackenbush J (2001) Identification of tumor markers in models of human colorectal cancer using a 19,200-element complementary DNA microarray. Cancer Res 61: 7792–7797 [PubMed] [Google Scholar]
- Holten-Andersen MN, Christensen IJ, Nielsen HJ, Stephens RW, Jensen V, Nielsen OH, Sorensen S, Overgaard J, Lilja H, Harris A, Murphy G, Brunner N (2002) Total levels of tissue inhibitor of metalloproteinases 1 in plasma yield high diagnostic sensitivity and specificity in patients with colon cancer. Clin Cancer Res 8: 156–164 [PubMed] [Google Scholar]
- Holten-Andersen MN, Hansen U, Brunner N, Nielsen HJ, Illemann M, Nielsen BS (2005) Localization of tissue inhibitor of metalloproteinases 1 (TIMP-1) in human colorectal adenoma and adenocarcinoma. Int J Cancer 113: 198–206 [DOI] [PubMed] [Google Scholar]
- Horiuchi S, Yamamoto H, Min Y, Adachi Y, Itoh F, Imai K (2003) Association of ets-related transcriptional factor E1AF expression with tumour progression and overexpression of MMP-1 and matrilysin in human colorectal cancer. J Pathol 200: 568–576 [DOI] [PubMed] [Google Scholar]
- Jass JR, Whitehall VL, Young J, Leggett BA (2002) Emerging concepts in colorectal neoplasia. Gastroenterology 123: 862–876 [DOI] [PubMed] [Google Scholar]
- Jenkins DC, Charles IG, Thomsen LL, Moss DW, Holmes LS, Baylis SA, Rhodes P, Westmore K, Emson PC, Moncada S (1995) Roles of nitric oxide in tumor growth. Proc Natl Acad Sci USA 92: 4392–4396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamoto K, Onodera H, Kan S, Kondo S, Imamura M (1999) Possible paracrine mechanism of insulin-like growth factor-2 in the development of liver metastases from colorectal carcinoma. Cancer 85: 18–25 [DOI] [PubMed] [Google Scholar]
- Kawamoto K, Onodera H, Kondo S, Kan S, Ikeuchi D, Maetani S, Imamura M (1998) Expression of insulin-like growth factor-2 can predict the prognosis of human colorectal cancer patients: correlation with tumor progression, proliferative activity and survival. Oncology 55: 242–248 [DOI] [PubMed] [Google Scholar]
- Kim JS, Crooks H, Dracheva T, Nishanian TG, Singh B, Jen J, Waldman T (2002) Oncogenic beta-catenin is required for bone morphogenetic protein 4 expression in human cancer cells. Cancer Res 62: 2744–2748 [PubMed] [Google Scholar]
- Kitahara O, Furukawa Y, Tanaka T, Kihara C, Ono K, Yanagawa R, Nita ME, Takagi T, Nakamura Y, Tsunoda T (2001) Alterations of gene expression during colorectal carcinogenesis revealed by cDNA microarrays after laser-capture microdissection of tumor tissues and normal epithelia. Cancer Res 61: 3544–3549 [PubMed] [Google Scholar]
- Kuramoto S, Oohara T (1989) Flat early cancers of the large intestine. Cancer 64: 950–955 [DOI] [PubMed] [Google Scholar]
- Lambert S, Vivario J, Boniver J, Gol-Winkler R (1990) Abnormal expression and structural modification of the insulin-like growth-factor-II gene in human colorectal tumors. Int J Cancer 46: 405–410 [DOI] [PubMed] [Google Scholar]
- Lin YM, Furukawa Y, Tsunoda T, Yue CT, Yang KC, Nakamura Y (2002) Molecular diagnosis of colorectal tumors by expression profiles of 50 genes expressed differentially in adenomas and carcinomas. Oncogene 21: 4120–4128 [DOI] [PubMed] [Google Scholar]
- Liu Y, Borchert GL, Phang JM (2004) Polyoma enhancer activator 3, an ets transcription factor, mediates the induction of cyclooxygenase-2 by nitric oxide in colorectal cancer cells. J Biol Chem 279: 18694–18700 [DOI] [PubMed] [Google Scholar]
- Mueller JD, Haegle N, Keller G, Mueller E, Saretzky G, Bethke B, Stolte M, Hofler H (1998) Loss of heterozygosity and microsatellite instability in de novo versus ex-adenoma carcinomas of the colorectum. Am J Pathol 153: 1977–1984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller N, Reinacher-Schick A, Baldus S, van Hengel J, Berx G, Baar A, van Roy F, Schmiegel W, Schwarte-Waldhoff I (2002) Smad4 induces the tumor suppressor E-cadherin and P-cadherin in colon carcinoma cells. Oncogene 21: 6049–6058 [DOI] [PubMed] [Google Scholar]
- Murashige M, Miyahara M, Shiraishi N, Saito T, Kohno K, Kobayashi M (1996) Enhanced expression of tissue inhibitors of metalloproteinases in human colorectal tumors. Jpn J Clin Oncol 26: 303–309 [DOI] [PubMed] [Google Scholar]
- Muro S, Takemasa I, Oba S, Matoba R, Ueno N, Maruyama C, Yamashita R, Sekimoto M, Yamamoto H, Nakamori S, Monden M, Ishii S, Kato K (2003) Identification of expressed genes linked to malignancy of human colorectal carcinoma by parametric clustering of quantitative expression data. Genome Biol 4: R21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Notterman DA, Alon U, Sierk AJ, Levine AJ (2001) Transcriptional gene expression profiles of colorectal adenoma, adenocarcinoma, and normal tissue examined by oligonucleotide arrays. Cancer Res 61: 3124–3130 [PubMed] [Google Scholar]
- Olschwang S, Slezak P, Roze M, Jaramillo E, Nakano H, Koizumi K, Rubio CA, Laurent-Puig P, Thomas G (1998) Somatically acquired genetic alterations in flat colorectal neoplasias. Int J Cancer 77: 366–369 [DOI] [PubMed] [Google Scholar]
- Peters G, Gongoll S, Langner C, Mengel M, Piso P, Klempnauer J, Ruschoff J, Kreipe H, von Wasielewski R (2003) IGF-1R, IGF-1 and IGF-2 expression as potential prognostic and predictive markers in colorectal-cancer. Virchows Arch 443: 139–145 [DOI] [PubMed] [Google Scholar]
- Reinacher-Schick A, Baldus SE, Romdhana B, Landsberg S, Zapatka M, Monig SP, Holscher AH, Dienes HP, Schmiegel W, Schwarte-Waldhoff I (2004) Loss of Smad4 correlates with loss of the invasion suppressor E-cadherin in advanced colorectal carcinomas. J Pathol 202: 412–420 [DOI] [PubMed] [Google Scholar]
- Sakashita M, Aoyama N, Maekawa S, Kuroda K, Shirasaka D, Ichihara T, Kuroda Y, Minami R, Maeda S, Kasuga M (2000) Flat-elevated and depressed, subtypes of flat early colorectal cancers, should be distinguished by their pathological features. Int J Colorectal Dis 15: 275–281 [DOI] [PubMed] [Google Scholar]
- Smith G, Carey FA, Beattie J, Wilkie MJ, Lightfoot TJ, Coxhead J, Garner RC, Steele RJ, Wolf CR (2002) Mutations in APC, Kirsten-ras, and p53-alternative genetic pathways to colorectal cancer. Proc Natl Acad Sci USA 99: 9433–9438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steeg PS, Bevilacqua G, Kopper L, Thorgeirsson UP, Talmadge JE, Liotta LA, Sobel ME (1988) Evidence for a novel gene associated with low tumor metastatic potential. J Natl Cancer Inst 80: 200–204 [DOI] [PubMed] [Google Scholar]
- Suzuki E, Ota T, Tsukuda K, Okita A, Matsuoka K, Murakami M, Doihara H, Shimizu N (2004) nm23-H1 reduces in vitro cell migration and the liver metastatic potential of colon cancer cells by regulating myosin light chain phosphorylation. Int J Cancer 108: 207–211 [DOI] [PubMed] [Google Scholar]
- Takemasa I, Higuchi H, Yamamoto H, Sekimoto M, Tomita N, Nakamori S, Matoba R, Monden M, Matsubara K (2001) Construction of preferential cDNA microarray specialized for human colorectal carcinoma: molecular sketch of colorectal cancer. Biochem Biophys Res Commun 285: 1244–1249 [DOI] [PubMed] [Google Scholar]
- Tureci O, Ding J, Hilton H, Bian H, Ohkawa H, Braxenthaler M, Seitz G, Raddrizzani L, Friess H, Buchler M, Sahin U, Hammer J (2003) Computational dissection of tissue contamination for identification of colon cancer-specific expression profiles. FASEB J 17: 376–385 [DOI] [PubMed] [Google Scholar]
- Vogelstein B, Fearon ER, Hamilton SR, Kern SE, Preisinger AC, Leppert M, Nakamura Y, White R, Smits AM, Bos JL (1988) Genetic alterations during colorectal tumor development. N Engl J Med 319: 525–532 [DOI] [PubMed] [Google Scholar]
- Williams NS, Gaynor RB, Scoggin S, Verma U, Gokaslan T, Simmang C, Fleming J, Tavana D, Frenkel E, Becerra C (2003) Identification and validation of genes involved in the pathogenesis of colorectal cancer using cDNA microarrays and RNA interference. Clin Cancer Res 9: 931–946 [PubMed] [Google Scholar]
- Xu MH, Deng CS, Zhu YQ, Lin J (2003) Role of inducible nitric oxide synthase expression in aberrant crypt foci–adenoma–carcinoma sequence. World J Gastroenterol 9: 1246–1250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto H, Imsumran A, Fukushima H, Adachi Y, Min Y, Iku S, Horiuchi S, Yoshida M, Shimada K, Sasaki S, Itoh F, Endo T, Imai K (2002) Analysis of gene expression in human colorectal cancer tissues by cDNA array. J Gastroenterol 37(Suppl 14): 83–86 [DOI] [PubMed] [Google Scholar]
- Yashiro M, Carethers JM, Laghi L, Saito K, Slezak P, Jaramillo E, Rubio C, Koizumi K, Hirakawa K, Boland CR (2001) Genetic pathways in the evolution of morphologically distinct colorectal neoplasms. Cancer Res 61: 2676–2683 [PubMed] [Google Scholar]
- Zhang L, Gjoerup O, Roberts TM (2004) The serine/threonine kinase cyclin G-associated kinase regulates epidermal growth factor receptor signaling. Proc Natl Acad Sci USA 101: 10296–10301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou TT, Selaru FM, Xu Y, Shustova V, Yin J, Mori Y, Shibata D, Sato F, Wang S, Olaru A, Deacu E, Liu TC, Abraham JM, Meltzer SJ (2002) Application of cDNA microarrays to generate a molecular taxonomy capable of distinguishing between colon cancer and normal colon. Oncogene 21: 4855–4862 [DOI] [PubMed] [Google Scholar]