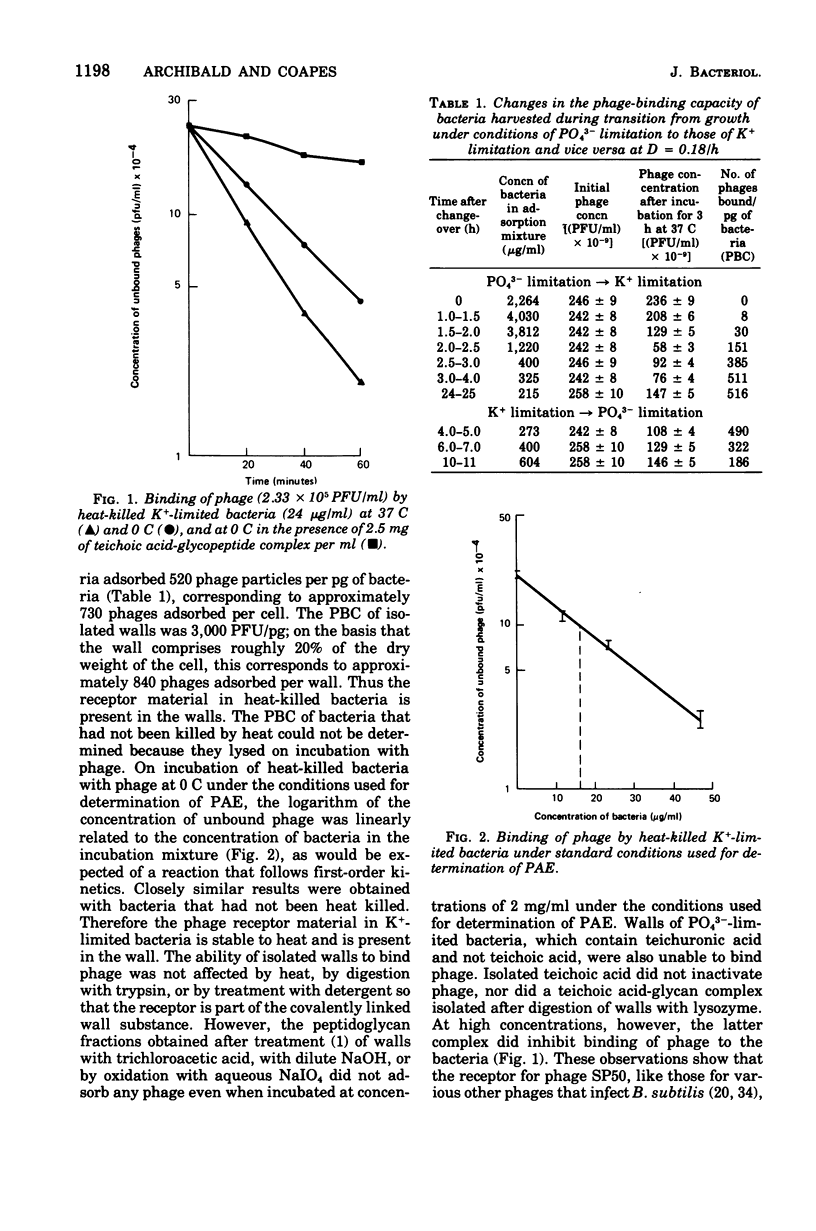
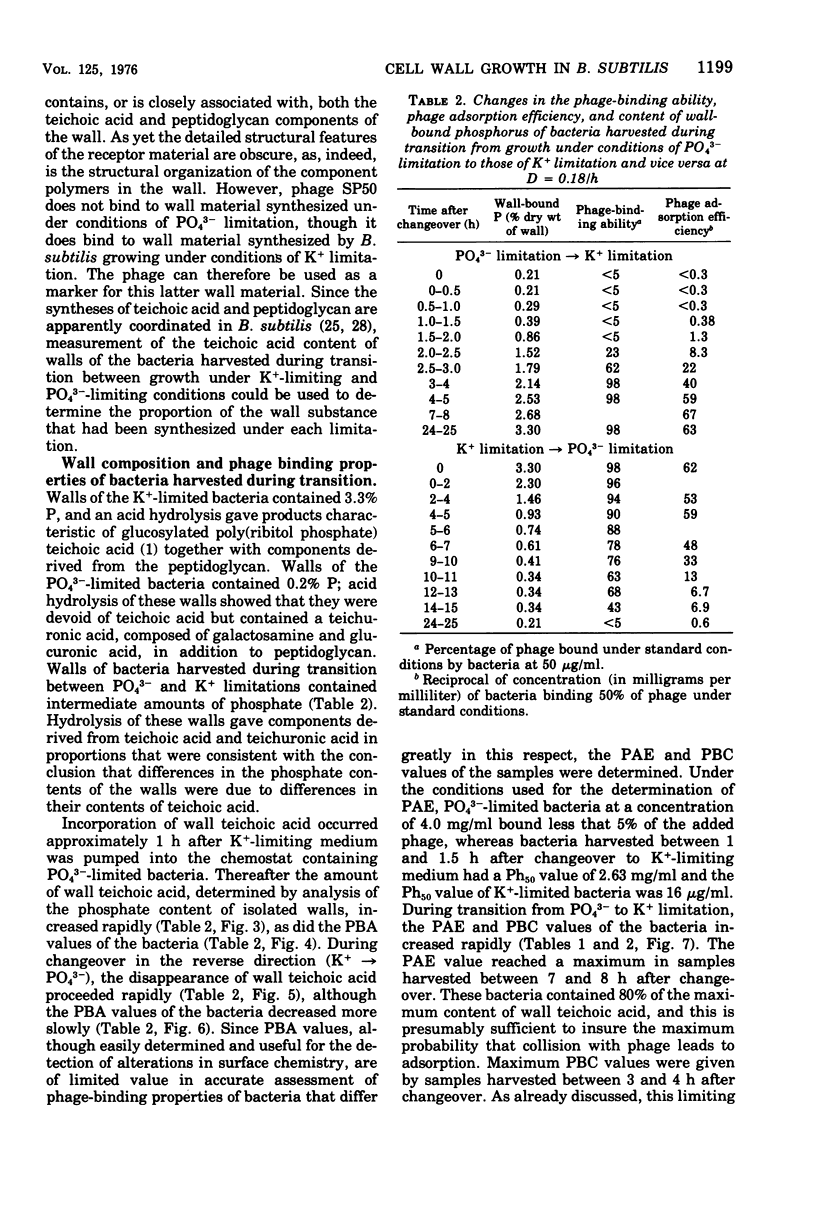
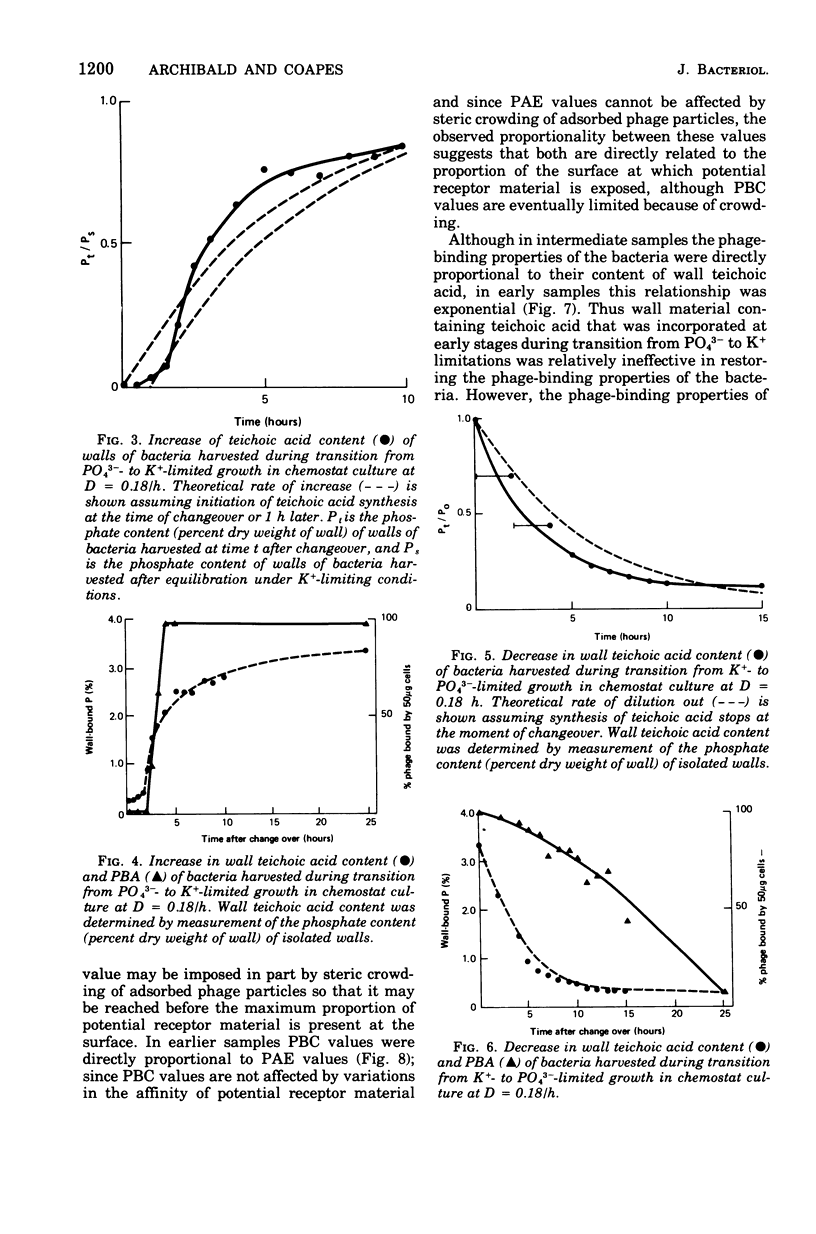
Abstract
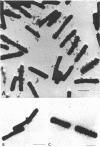
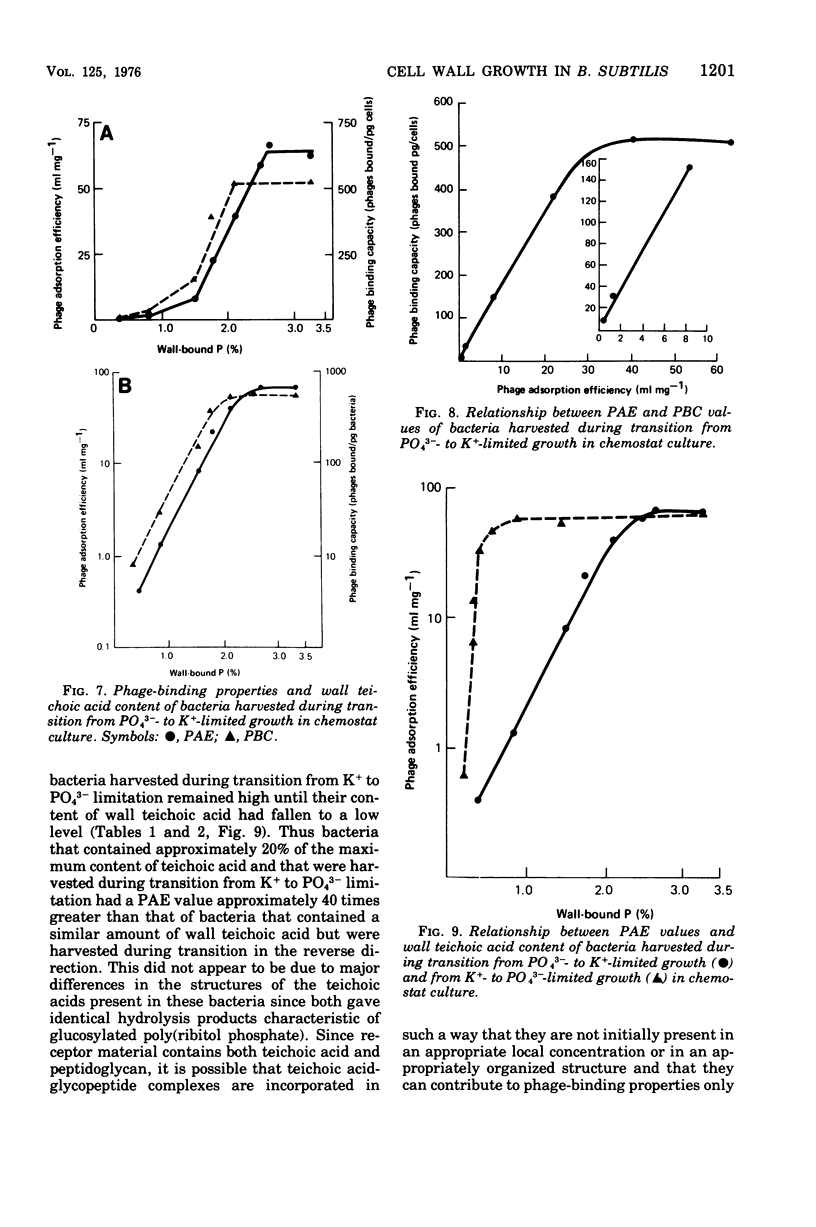
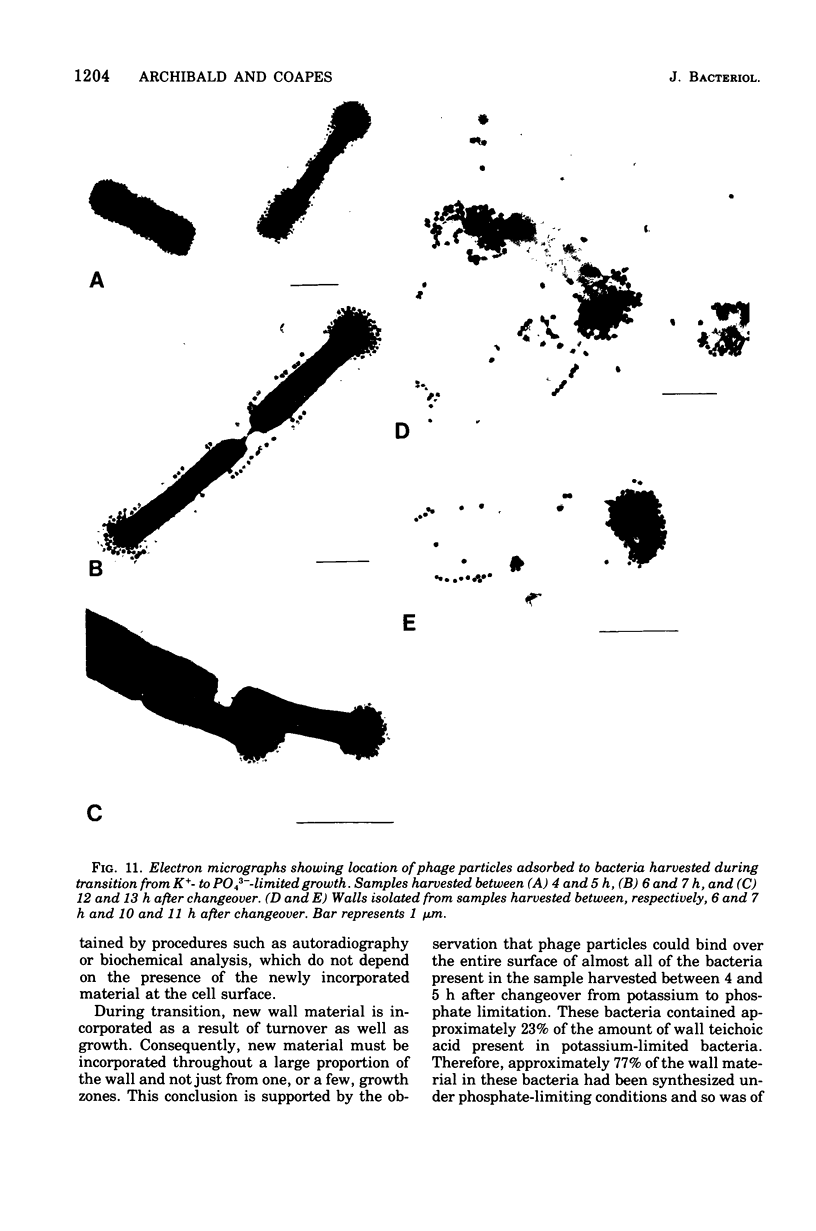
When grown under conditions of phosphate limitation, Bacillus subtilis W23 lacked wall teichoic acid and did not adsorb phage SP50. During transition from growth under conditions of phosphate limitation to those of potassium limitation, the bacteria developed an ability to adsorb phage which increased exponentially in relation to their content of wall teichoic acid. During transition in the reverse direction, the bacteria retained near-maximum phage-binding properties until their content of wall teichoic acid had fallen to a fairly low level. These observations suggest that newly incorporated wall material does not immediately appear at the cell surface in a structure to which phage can adsorb. Examination of the location of adsorbed phage particles showed that recently incorporated receptor material appeared at the cell surface first along the length of the cylindrical portion of the cell. The results are consistent with models of wall assembly in which newly synthesized wall material is intercalated at a large number of sites that are distributed along the length of the cell. This newly incorporated material may be located initially at a level underlying the surface of the cell and may become exposed at the surface only during subsequent growth. Incorporation of new material may also proceed rapidly into the developing septa, but new wall material is incorporated into existing polar caps more slowly, or perhaps not at all.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Archibald A. R., Coapes H. E. Blocking of bacteriophage receptor sites by Concanavalin A. J Gen Microbiol. 1972 Dec;73(3):581–585. doi: 10.1099/00221287-73-3-581. [DOI] [PubMed] [Google Scholar]
- Archibald A. R., Coapes H. E. The influence of growth conditions on the presence of bacteriophage-receptor sites in walls of Bacillus subtilis W23. Biochem J. 1971 Nov;125(2):667–669. doi: 10.1042/bj1250667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Archibald A. R., Stafford G. H. A polymer of N-acetylglucosamine 1-phosphate in the wall of Staphylococcus lactis 2102. Biochem J. 1972 Dec;130(3):681–690. doi: 10.1042/bj1300681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHUNG K. L., HAWIRKO R. Z., ISAAC P. K. CELL WALL REPLICATION. I. CELL WALL GROWTH OF BACILLUS CEREUS AND BACILLUS MEGATERIUM. Can J Microbiol. 1964 Feb;10:43–48. doi: 10.1139/m64-007. [DOI] [PubMed] [Google Scholar]
- Chaloupka J., Krecková P. Turnover of mucopeptide during the life cycle of Bacillus megaterium. Folia Microbiol (Praha) 1971;16(5):372–382. doi: 10.1007/BF02875757. [DOI] [PubMed] [Google Scholar]
- Chung K. L. Autoradiographic studies of bacterial cell wall replication. I. Cell wall growth of Bacillus cereus in the presence of chloramphenicol. Can J Microbiol. 1967 Apr;13(4):341–350. doi: 10.1139/m67-046. [DOI] [PubMed] [Google Scholar]
- Cole R. M. Symposium on the fine structure and replication of bacteria and their parts. 3. Bacterial cell-wall replication followed by immunofluorescence. Bacteriol Rev. 1965 Sep;29(3):326–344. doi: 10.1128/br.29.3.326-344.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellwood D. C., Tempest D. W. Control of teichoic acid and teichuronic acid biosyntheses in chemostat cultures of Bacillus subtilis var. niger. Biochem J. 1969 Jan;111(1):1–5. doi: 10.1042/bj1110001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FOELDES J., TRAUTNER T. A. INFECTIOUS DNA FROM A NEWLY ISOLATED B. SUBTILIS PHAGE. Z Vererbungsl. 1964 Apr 10;95:57–65. doi: 10.1007/BF00898184. [DOI] [PubMed] [Google Scholar]
- Fan D. P., Beckman B. E. Structural difference between walls from hemispherical caps and partial septa of Bacillus subtilis. J Bacteriol. 1973 May;114(2):790–797. doi: 10.1128/jb.114.2.790-797.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan D. P., Beckman M. M., Cunningham W. P. Ultrastructural studies on a mutant of Bacillus subtilis whose growth is inhibited due to insufficient autolysin production. J Bacteriol. 1972 Mar;109(3):1247–1257. doi: 10.1128/jb.109.3.1247-1257.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan D. P., Pelvit M. C., Cunningham W. P. Structural difference between walls from ends and sides of the rod-shaped bacterium Bacillus subtilis. J Bacteriol. 1972 Mar;109(3):1266–1272. doi: 10.1128/jb.109.3.1266-1272.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsberg C. W., Wyrick P. B., Ward J. B., Rogers H. J. Effect of phosphate limitation on the morphology and wall composition of Bacillus licheniformis and its phosphoglucomutase-deficient mutants. J Bacteriol. 1973 Feb;113(2):969–984. doi: 10.1128/jb.113.2.969-984.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frehel C., Beaufils A. M., Ryter A. Etude au microscope électronique de la croissance de la paroi chez B. subtilis et B. megaterium. Ann Inst Pasteur (Paris) 1971 Aug;121(2):139–148. [PubMed] [Google Scholar]
- GAREN A., PUCK T. T. The first two steps of the invasion of host cells by bacterial viruses. II. J Exp Med. 1951 Sep;94(3):177–189. doi: 10.1084/jem.94.3.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glaser L., Ionesco H., Schaeffer P. Teichoic acids as components of a specific phage receptor in Bacillus subtilis. Biochim Biophys Acta. 1966 Aug 24;124(2):415–417. doi: 10.1016/0304-4165(66)90211-x. [DOI] [PubMed] [Google Scholar]
- Higgins M. L., Shockman G. D. Procaryotic cell division with respect to wall and membranes. CRC Crit Rev Microbiol. 1971 May;1(1):29–72. doi: 10.3109/10408417109104477. [DOI] [PubMed] [Google Scholar]
- Highton P. J., Hobbs D. G. Penicillin and cell wall synthesis: a study of Bacillus cereus by electron microscopy. J Bacteriol. 1972 Mar;109(3):1181–1190. doi: 10.1128/jb.109.3.1181-1190.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Highton P. J., Hobbs D. G. Penicillin and cell wall synthesis: a study of Bacillus licheniformis by electron microscopy. J Bacteriol. 1971 May;106(2):646–658. doi: 10.1128/jb.106.2.646-658.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes R. C., Stokes E. Cell wall growth in Bacillus licheniformis followed by immunofluorescence with mucopeptide-specific antiserum. J Bacteriol. 1971 May;106(2):694–696. doi: 10.1128/jb.106.2.694-696.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes R. C., Tanner P. J., Stokes E. Cell-wall thickening in Bacillus subtilis. Comparison of thickened and normal walls. Biochem J. 1970 Nov;120(1):159–170. doi: 10.1042/bj1200159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauck J., Chan L., Glaser L. Turnover of the cell wall of Gram-positive bacteria. J Biol Chem. 1971 Mar 25;246(6):1820–1827. [PubMed] [Google Scholar]
- Mauck J., Chan L., Glaser L., Williamson J. Mode of cell wall growth of Bacillus megaterium. J Bacteriol. 1972 Jan;109(1):373–378. doi: 10.1128/jb.109.1.373-378.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauck J., Glaser L. On the mode of in vivo assembly of the cell wall of Bacillus subtilis. J Biol Chem. 1972 Feb 25;247(4):1180–1187. [PubMed] [Google Scholar]
- Rogers H. J. Bacterial growth and the cell envelope. Bacteriol Rev. 1970 Jun;34(2):194–214. doi: 10.1128/br.34.2.194-214.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schleifer K. H., Kandler O. Peptidoglycan types of bacterial cell walls and their taxonomic implications. Bacteriol Rev. 1972 Dec;36(4):407–477. doi: 10.1128/br.36.4.407-477.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tempest D. W., Dicks J. W., Ellwood D. C. Influence of growth condition on the concentration of potassium in Bacillus subtilis var. niger and its possible relationship to cellular ribonucleic acid, teichoic acid and teichuronic acid. Biochem J. 1968 Jan;106(1):237–243. doi: 10.1042/bj1060237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young F. E. Requirement of glucosylated teichoic acid for adsorption of phage in Bacillus subtilis 168. Proc Natl Acad Sci U S A. 1967 Dec;58(6):2377–2384. doi: 10.1073/pnas.58.6.2377. [DOI] [PMC free article] [PubMed] [Google Scholar]