Abstract
Patients with metastatic nasopharyngeal carcinoma have variable survival outcomes. We previously designed a scoring system to better prognosticate these patients. Here, we report results on validation of this new prognostic index score in a separate cohort of patients. Clinical features and laboratory parameters were examined in 172 patients with univariate and multivariate analyses and a numerical score was derived for each independent prognostic variable. Significant independent prognostic variables and their scores assigned included poor performance status (score 5), haemoglobin <12 g dl−1 (score 4) and disease-free interval (DFI) (DFI⩽6 months (score 10) or metastases at initial diagnosis (score 1)). Maximum score was 19 and patients stratified into three prognostic groups: good, 0–3; intermediate, 4–8; poor, ⩾9. When applied to a separate cohort of 120 patients, 59 patients were good, 43 intermediate and 18 poor prognosis, with median survivals of 19.6 (95% CI 16.1, 23.1), 14.3 (95% CI 12.3, 16.2) and 7.9 (95% CI 6.6, 9.2) months, respectively. (logrank test: P=0.003). We have validated a new prognostic score with factors readily available in the clinics. This simple score will prove useful as a method to prognosticate and stratify patients as well as to promote consistent reporting among clinical trials.
Keywords: nasopharyngeal carcinoma, prognostic factors, metastatic survival
Nasopharyngeal carcinoma (NPC) occurs sporadically in the West but constitute a major health problem in several parts of Asia, including Southern China, Hong Kong and Singapore (Parkin, 2001). In Singapore, the incidence rate in Chinese is among the highest in Asia and the disease ranks as the fifth most common cancer among Chinese males (Chia et al, 2000). The histological pattern of NPC among the Chinese population comprise mainly the World Health Organization (WHO) types II (nonkeratinising) and III (undifferentiated) (Chan et al, 1998). Both histological types are often considered together as they share similar epidemiological, clinical and serologic characteristics, as distinct from the epidermoid carcinoma of the head and neck region seen in the western population (Shanmugaratnam and Sobin, 1993; Altun et al, 1995).
There is a high incidence of distant metastases with nonkeratinising or undifferentiated NPC, as compared to other epidermoid carcinoma arising in the head and neck region (Reddy et al, 1995). This is especially true for patients who present with locally advanced disease (Ahmad and Stefani, 1986). A prospective study showed a high rate of subclinical distant metastasis, with a distinctive feature of bone marrow invasion (Micheau et al, 1987). In addition, the incidence of distant failure after radiotherapy treatment for locally advanced disease can be as high as 57% in N3 disease (Lee et al, 1992). In view of the high rate of systemic relapse, chemotherapy has been incorporated into the primary treatment of locally advanced disease in order to improve the outcome. Concurrent chemoradiotherapy was shown to improve overall and progression-free survival for locally advanced NPC in the Intergroup study (Al-Sarraf et al, 1998) and this was replicated in studies carried out in the endemic areas of Singapore (Wee et al, 2004), Hong Kong (Lee et al, 2004) and Taiwan (Lin et al, 2003). However, a significant proportion of patients would still relapse systemically despite combined modality therapy and many of these patients would ultimately succumb to disseminated disease.
Patients with disseminated disease do not behave in a uniform manner. It is hence not surprising to see significantly variable results between studies of similar therapeutic manoeuvres in patients with metastatic NPC. We have shown in our previous study that by using several clinical and laboratory parameters, we were able to define three prognostically distinct groups of patients with disseminated NPC (Ong et al, 2003). We proposed that a prognostic index scoring system using these parameters could be used for a more accurate prognostic evaluation of a patient. However more importantly, it can also be used for a more accurate stratification of patients in prospective clinical studies and hopefully help to standardise reporting results of any therapeutic interventions.
We now report a follow-on study that reanalysed our previous findings to define a new prognostic index score and to validate this new score in a separate cohort of patients with disseminated NPC.
PATIENTS AND METHODS
Patients
All patients were treated at the Department of Medical Oncology, National Cancer Centre between January 1994 and January 2003. There were two different cohorts of patients: the first (Cohort 1) was the group on which the new prognostic index score was derived and included 220 patients treated between January 1994 and December 1999, while the second cohort (Cohort 2) was the group on which the new score was validated. Cohort 2 included 99 patients treated between January 2002 and January 2003 and 21 patients (not included in our previous analysis for Cohort 1) from a previous Institutional Review Board-approved phase II clinical trial conducted in 1996 (Au et al, 1998).
All patients had a histological confirmation of NPC and had computerised tomography (CT) scan of the posterior nasal space, chest X-ray and/or CT scan of the thorax, ultrasound or CT scan of the abdomen and bone radionuclide scan to identify the extent of systemic disease. Patients were classified into the International Union Against Cancer/American Joint Committee on Cancer (UICC/AJCC) stages using the clinical and radiological data.
Pretreatment patient and disease characteristics, disease-free interval (DFI) (time from the onset of primary radiotherapy or chemoradiotherapy to the time of distant relapse), type of chemotherapy given, best response to chemotherapy, type of salvage chemotherapy given and date of death were recorded for all patients.
Survival data
The primary end point of interest was metastatic survival. Metastatic survival was defined as survival subsequent to the development of distant relapse, that is, from the first diagnosis of distant metastases to the time of death. Locoregional recurrence was not considered a distant relapse. The survival status of all patients was verified with Singapore's national death registry for Cohorts 1 and 2 as on 30 June 2000 and 31 December 2003, respectively. The cohorts excluded patients who were nonresidents of Singapore and as notification is mandatory in the event of death, the mortality data obtained from the death registry was complete and exhaustive.
Statistical analysis
The analysis to derive a new prognostic score was performed on Cohort 1 and focused only on 172 patients who had received chemotherapy. The original prognostic index score was based on all patients including patients who were not treated with chemotherapy (Ong et al, 2003). As chemotherapy has an impact on survival outcome in patients with disseminated disease, there was a need to rederive a new prognostic index score using only patients from Cohort 1 who received palliative chemotherapy as all the patients from Cohort 2 were treated with chemotherapy. Univariate and multivariable analyses were performed using the Cox proportion hazards model. The multivariable analyses were undertaken with both forward and backward stepwise procedures for identifying the independent prognostic variables. Factors that were considered in the derivation of the new prognostic index score included age, gender, performance status according to Eastern Co-operative Oncology Group (ECOG) criteria, specific metastatic sites, number of metastatic sites, metastasis at presentation, stage at first diagnosis, DFI, leucocyte count, haemoglobin (Hb) level, and albumin level, on the basis of our previous analysis. In order to construct the new prognostic index score, factors were entered as categorical values as far as possible to keep the computations simple, although categorisation inevitably will result in some loss of information. P⩽0.05 was used as the cutoff value of statistical significance for variable selection in the multivariable modelling. The regression coefficient of each independent prognostic variable (the β in the Cox regression equation hazard ratio (HR)=eβ) is then modified into an integer numerical value to construct the new prognostic index score. The patients were stratified, based on the new prognostic index score, into three different risk groups with significantly different median metastatic survivals.
The validation of the new prognostic index score was subsequently performed on Cohort 2 patients. Overall and median metastatic survival estimates and curves were obtained using the Kaplan–Meier method and logrank test was used to compare among the three prognostic groups stratified by the new score.
RESULTS
Patient and disease characteristics (Table 1)
Table 1. Patient and disease characteristics.
|
Cohort 1
|
Cohort 2
|
||||
|---|---|---|---|---|---|
| Characteristics | No. of patients | % | No. of patients | % | P-value |
| No. of patients | 172 | 120 | |||
| Survival status | See logrank test | ||||
| Dead | 130 | 75.6 | 91 | 75.8 | |
| Alive | 42 | 24.4 | 29 | 24.2 | |
| Age, in years | <0.001 | ||||
| Median | 47 | 48 | |||
| Interquartile range | (40,54) | (42,54) | |||
| Gender | 0.32 | ||||
| Male | 144 | 83.7 | 95 | 79.2 | |
| Female | 28 | 16.3 | 25 | 20.8 | |
| ECOG status a | 0.07 | ||||
| 0 | 18 | 10.5 | 19 | 15.8 | |
| 1 | 133 | 77.3 | 96 | 80 | |
| 2 | 14 | 8.1 | 2 | 1.7 | |
| 3 | 6 | 3.5 | 3 | 2.5 | |
| 4 | 1 | 0.6 | 0 | 0 | |
| Laboratory parameters | |||||
| Albumin (g l−1) | 0.38 | ||||
| <40 | 128 | 74.4 | 90 | 75 | |
| ⩾40 | 32 | 18.6 | 29 | 24.1 | |
| Hemoglobin (g l−1) | 0.003 | ||||
| <12 | 106 | 61.6 | 54 | 45 | |
| ⩾12 | 63 | 36.6 | 66 | 55 | |
| UICC/AJCC stage at first diagnosisb | 0.002 | ||||
| I | 5 | 2.9 | 2 | 1.7 | |
| IIA | 1 | 0.6 | 4 | 3.3 | |
| IIB | 31 | 18 | 4 | 3.3 | |
| III | 39 | 22.7 | 39 | 32.5 | |
| IVA | 20 | 11.6 | 16 | 13.3 | |
| IVB | 27 | 15.7 | 16 | 13.3 | |
| IVC | 33 | 19.2 | 27 | 22.5 | |
| Disease-free interval | 0.16 | ||||
| Mets at diagnosis | 35 | 20.3 | 27 | 22.5 | |
| ⩽6 months | 11 | 6.4 | 15 | 12.5 | |
| >6 month | 125 | 72.7 | 78 | 65 | |
| Sites of metastasis | |||||
| Bone | 118 | 68.6 | 79 | 65.8 | 0.26 |
| Liver | 74 | 43 | 63 | 52.5 | 0.12 |
| Lung | 65 | 37.8 | 51 | 42.5 | 0.41 |
| Distant nodes | 63 | 36.6 | 39 | 32.5 | 0.43 |
| No. of metastatic sites | 0.3 | ||||
| Single | 69 | 40.1 | 41 | 34.2 | |
| Multiple | 103 | 59.9 | 79 | 65.8 | |
| Prior chemotherapy before mets | 0.58 | ||||
| Yes | 14 | 8.1 | 12 | 10 | |
| No | 158 | 91.9 | 108 | 90 | |
| Salvage chemotherapy | 0.02 | ||||
| Yes | 91 | 52.9 | 68 | 61.8 | |
| No | 81 | 47.1 | 42 | 38.2 | |
ECOG status refers to performance status as defined by the Eastern Cooperative Oncology Group.
UICC/AJCC refers to International Union against Cancer/American Joint Committee on Cancer.
Table 1 shows the characteristics of patients in both cohorts who had received at least one line of palliative chemotherapy at diagnosis of distant metastases. All patients had nonkeratinising or undifferentiated NPC. There was a male predominance in both cohorts. Most characteristics were similar between both cohorts. The median age at diagnosis of metastases was 47 years for Cohort 1 and 48 years for Cohort 2. In all, 35 (20.3%) patients in Cohort 1 and 27 (22.5%) patients in Cohort 2 had metastases at diagnosis. The majority had distant relapse after treatment for locally advanced disease previously. Bone was the most common site of metastasis, followed by liver, lung and distal nodes. Most of the patients had multiple sites involved at diagnosis of metastases.
The proportion of patients with good ECOG status of 0 and 1 was higher in Cohort 2 (95.8%) compared to Cohort 1 (87.8%) (P=0.07). Several chemotherapy regimens were used in the first-line setting and these included Cisplatin and Fluorouracil, Paclitaxel and Gemcitabine either alone or in combination with Carboplatin.
Survival distribution
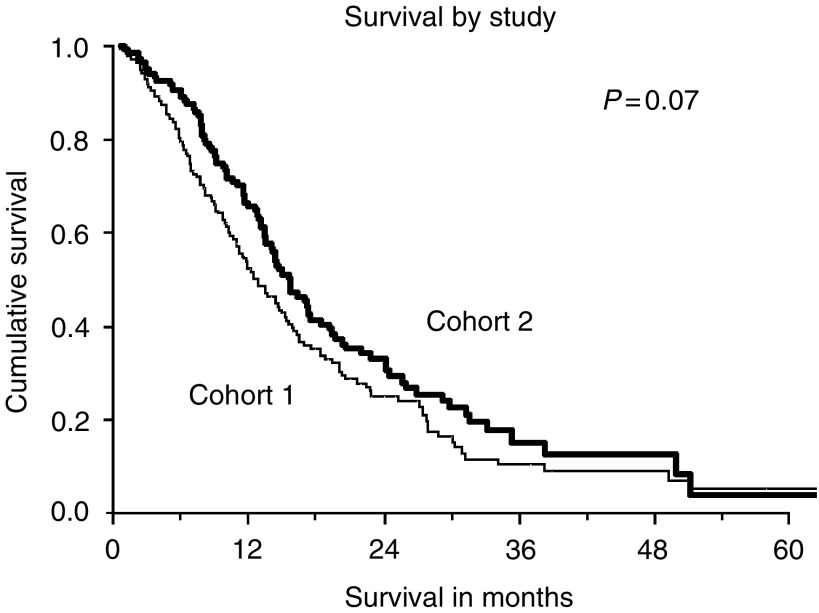
Patients from Cohort 2 appeared to have better survival, although this was not statistically significant (logrank test P-value=0.07). See Figure 1. The median metastatic survival for patients in Cohort 1 was 12.9 months (95% CI 10.5,15.3) and that for patients in Cohort 2 was 15.6 months (95% CI 13.2, 18.0). The 1-, 2- and 3-year survival proportions were 52, 25 and 10% for Cohort 1 and 66, 33 and 15% for Cohort 2, respectively.
Figure 1.
Survival curves for Cohorts 1 and 2. There is no statistically significant difference in metastatic survival between the two cohorts.
Univariate and multivariate analysis
The univariate analysis was performed on the 172 patients who were treated with chemotherapy in Cohort 1. Factors considered in the univariate analyses were based on our previous study and included age, gender, performance status, laboratory parameters such as Hb, leucocyte count and albumin level, stage at first diagnosis, sites and number of metastases and DFI. The factors associated with an adverse prognosis were anaemia (Hb<12g dl−1), ECOG ⩾2, DFI⩽6 months, presence of multiple metastases and liver metastases. See Table 2.
Table 2. Univariate analysis of patients treated with chemotherapy in Cohort 1 (N=172).
| Factor | No. of patients | No. alive | Hazard ratio (95% CI) | P-value |
|---|---|---|---|---|
| Gender | ||||
| Female | 28 | 6 | Baseline | |
| Male | 144 | 36 | 0.66 (0.41–1.04) | 0.075 |
| Age, in years | ||||
| ⩽54 | 78 | 23 | Baseline | |
| 46–65 | 84 | 16 | 1.31 (0.92–1.88) | 0.14 |
| >65 | 10 | 3 | 1.79 (0.81–3.96) | 0.15 |
| Albumin, g l −1 | ||||
| ⩾04 | 32 | 10 | Baseline | |
| <40 | 128 | 31 | 1.43 (0.89–1.88) | 0.13 |
| Haemoglobin, g dl −1 | ||||
| ⩾12 | 63 | 28 | Baseline | |
| <12 | 106 | 14 | 2.61 (1.76–3.87) | <0.001 |
| ECOG status a | ||||
| 0–1 | 151 | 41 | Baseline | |
| 2 | 14 | 1 | 2.24 (1.26–4.00) | 0.006 |
| 3–4 | 7 | 0 | 4.19 (1.93–9.18) | <0.001 |
| Leucocyte count, × 109 l−1 | ||||
| <4 | 26 | 4 | Baseline | |
| 4–11 | 112 | 35 | 0.77 (0.48–1.24) | 0.29 |
| >11 | 31 | 3 | 1.46 (0.83–2.56) | 0.19 |
| UICC/AJCC stage at first diagnosisb | ||||
| I–II | 37 | 8 | Baseline | |
| III–IVB | 86 | 23 | 1.21 (0.78–1.89) | 0.38 |
| IVC | 33 | 9 | 1.41 (0.81–2.45) | 0.22 |
| Bone metastasis | ||||
| No | 42 | 10 | Baseline | |
| Yes | 118 | 27 | 1.24 (0.82–1.85) | 0.3 |
| Liver metastasis | ||||
| No | 97 | 26 | Baseline | |
| Yes | 74 | 16 | 1.60 (1.13–2.28) | 0.008 |
| Lung metastasis | ||||
| No | 106 | 27 | Baseline | |
| Yes | 65 | 15 | 1.19 (0.83–1.70) | 0.35 |
| Distal node metastasis | ||||
| No | 107 | 19 | Baseline | |
| Yes | 63 | 22 | 0.81 (0.56–1.18) | 0.27 |
| Number of metastatic sites | ||||
| Single | 69 | 17 | Baseline | |
| Multiple | 103 | 25 | 1.55 (1.08–2.21) | 0.017 |
| Disease-free interval | ||||
| >6 months | 125 | 32 | Baseline | |
| ⩽6 months | 11 | 1 | 4.03 (2.02–8.02) | <0.001 |
| Metastases at diagnosis | 35 | 9 | 1.25 (0.81–1.94) | 0.31 |
ECOG status refers to performance status as defined by the Eastern Cooperative Oncology Group.
UICC/AJCC refers to International Union against Cancer/American Joint Committee on Cancer.
The variables included in the multivariate analysis were similar to that used in the univariate analysis. Using a significance level of 0.05, the significant independent variables were Hb level (Hb<12g dl−1 with HR: 2.1), performance status (ECOG⩾2 with HR: 2.6), DFI (metastasis at diagnosis and short DFI with HR: 1.2 and 7.7 respectively). See Table 3. These three variables predicted negatively for metastatic survival.
Table 3. Significant independent variables from multivariate analysis for patients treated with chemotherapy in Cohort 1 (N=172).
| Factor | Hazard ratio (95% CI) | P-value |
|---|---|---|
| Haemoglobin level (<12 g dl−1) | 2.067 (1.34–3.17) | 0.001 |
| Performance status (ECOG ⩾2a) | 2.585 (1.45–4.59) | 0.001 |
| Disease-free interval | ||
| Metastasis at diagnosis | 1.21 (0.75–1.95) | 0.031 |
| ⩽6 months | 7.656 (2.23–26.25) |
ECOG refers to performance status as defined by the Eastern Cooperative Oncology Group.
New prognostic index score and risk groups
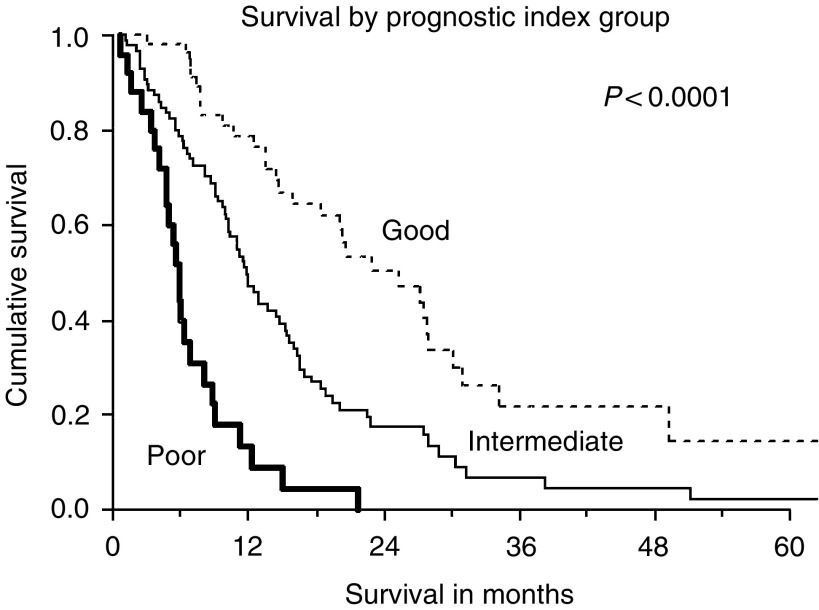
A numerical score was derived from the regression coefficient of each of the three independent prognostic variables derived above, namely, anaemia (Hb<12 g dl−1), ECOG⩾2 and DFI. A score of 0 was assigned if the factor was absent or 1, 4, 5 or 10 according to the factor present. See Table 4. The new prognostic index score for each individual patient was calculated by adding up the scores of each independent factor. The maximum score obtainable was 19, instead of 20 as metastasis at diagnosis and short DFI were mutually exclusive. In view of the gaps between the scores, the possible scores were 0–1, 4–6, 9–11, 14–16 and 19. The patients were stratified into three prognostic groups based on the new prognostic index score: 57 patients in the low-risk group (score: 0–3), 86 patients in intermediate-risk group (score: 4–8) and 25 patients in high-risk group (score: ⩾9). The median metastatic survivals for the three different risk groups were 25.3 (95% CI 17.7–33.9), 11.7 (95% CI 9.9–13.6) and 5.8 (95% CI 5.0–6.5) months, respectively. (logrank test, P<0.0001). See Figure 2.
Table 4. New prognostic index score and risk groups.
| Factor | Score | β (hazard ratio=e β )a |
|---|---|---|
| Haemoglobin level (<12 g dl−1) | 4 | 0.73 |
| Performance status (ECOG ⩾2b) | 5 | 0.95 |
| Disease-free interval | ||
| Metastasis at diagnosis | 1 | 0.19 |
| ⩽6 months | 10 | 2.04 |
| Maximum score | 19 |
Low risk: score 0–3; intermediate risk: score 4–8; high risk: score ⩾9.
β=Regression Coefficient.
ECOG refers to performance status as defined by the Eastern Cooperative Oncology Group.
Figure 2.
Survival by new prognostic index grouping derived from patients in Cohort 1. There is a clear demarcation of survival differences between the three risk groups.
Validation of new prognostic index score
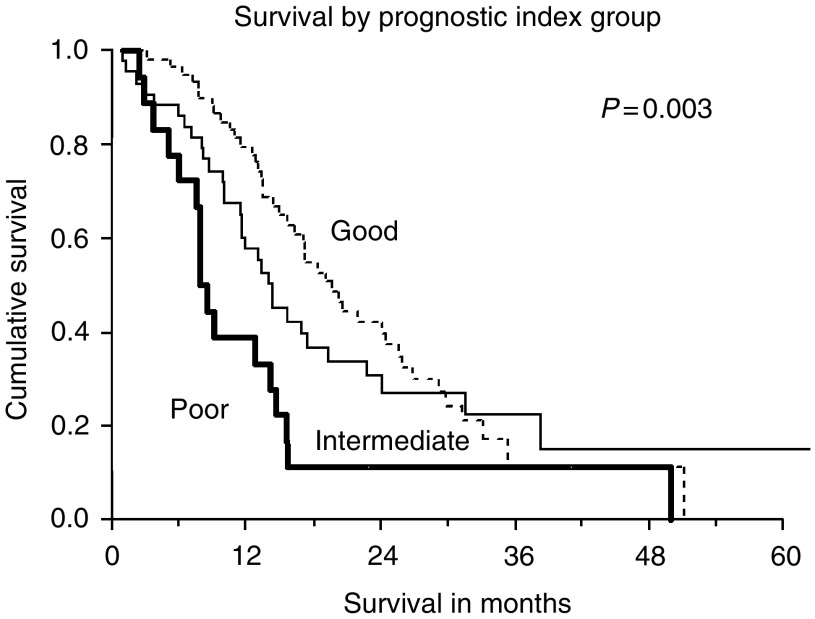
The new prognostic index score was applied to the patients in Cohort 2. The proportion of patients in the low-risk, intermediate-risk and high-risk groups were 49, 36 and 15%, respectively. The median metastatic survivals of these three different groups were as follows: 19.6 (95% CI 16.1–23.1), 14.3 (95% CI 12.3–16.2) and 7.9 (95% CI 6.6–9.2) months respectively (logrank, P=0.003). See Figure 3.
Figure 3.
Survival by new prognostic index grouping applied to patients in Cohort 2. There is a statistically significant difference between the three risk groups.
DISCUSSION
We previously presented our results on derivation of a prognostic index score derived from all patients in Cohort 1 that included those not treated with chemotherapy (Ong et al, 2003). However, as Cohort 2 was used for validation of the score and all the patients in Cohort 2 were treated, we elected to reanalyse Cohort 1 to restrict the derivation of a new prognostic index score to only patients given chemotherapy. The reason for the reanalysis is that chemotherapy has an impact on survival outcome in patients with disseminated NPC, although there are no randomised comparison studies between chemotherapy and best supportive care (Fandi et al, 1994; Hong et al, 1999). This is also supported by the fact that incorporation of chemotherapy in locally advanced disease has been shown to improve progression-free and overall survival (Al-Sarraf et al, 1998; Lin et al, 2003; Wee et al, 2004). Thus, with reanalysis, we have eliminated the bias with the use of chemotherapy and make the two cohorts more comparable.
We found that DFI, Hb level (<12g dl−1) and poor performance status (ECOG⩾2) were significant negative prognostic factors in patients treated with chemotherapy and these three independent variables were used in the derivation of the new prognostic index score. Metastasis at diagnosis and short DFI were regarded as two separate categories within the same DFI variable and thus were mutually exclusive. The natural history of a disease is dependent on the interaction of both patient and disease factors. Anaemia can be related to the disease process itself, host-related factors or treatment given and has been shown to be associated with significant reduction in survival in various cancers, other than NPC (Caro et al, 2001; Bokemeyer et al, 2002). Anaemia may reflect not only a biologically more aggressive tumour but may be a mediating factor to resistance to treatment and this has been demonstrated in retrospective studies on cervical cancer treated with chemoradiation (Obermair et al, 2001). The negative prognostic factor of a poor performance status has been shown in many other tumour types as well (Paesmans et al, 1995; Polee et al, 2003; Motzer et al, 2004). Patients with poor performance status do not tolerate treatment well and it may also be a reflection of the more advanced state of the cancer.
Patients who present with metastasis at diagnosis can be a result of delay in seeking medical attention or more likely, a reflection of the aggressive nature of the disease such that it is widespread by the time the patient becomes symptomatic. Having a short DFI is also reflective of an aggressive disease and has been convincingly shown to portend a poorer outcome in other tumours such as ovarian cancer (Markman et al, 1991). Our analysis showed that a short DFI after initial radiotherapy or chemoradiotherapy for the primary tumour had a worse prognostic score compared to metastasis at diagnosis and this is likely due to emerging chemoresistant clones within the tumour of those patients who relapse shortly after treatment.
A study by Teo et al (1996) showed that short DFI, the presence of liver metastasis and age at diagnosis were prognostic for metastatic survival in metastatic NPC. Short DFI is included as a factor in our new prognostic index score but the other two variables were found to be not statistically significant on multivariate analysis. This may be a result of our population of patients being limited to only those treated with chemotherapy and the treatment likely negated the prognostic significance of age and site of metastasis. Statistical issues, such as variations in modelling procedure in small to moderate size data sets between the different studies, could be an alternative explanation.
The metastatic survival of patients with disseminated NPC treated with chemotherapy is highly variable and our findings provide further supportive evidence for that. In Cohort 1, at the time of analysis in June 2000, the metastatic survival varied from 1 to 72 months. Similarly, for Cohort 2, the metastatic survival duration varied from 1 month to 103 months: 10 patients died within 3 months of diagnosis of metastatic disease despite chemotherapy, while 13 patients are still alive after 2 years. In fact, one patient survived 8 years 7 months while another three lived beyond 4 years after diagnosis of metastatic disease. A number of studies have reported similar findings, with several long-term survivors with the use of chemotherapy in metastatic NPC (Chan et al, 1997; Fandi et al, 2000). Based on the results of our studies, it is possible for single-arm studies or randomised studies that are inadequately powered or stratified to report spuriously improved survival outcome with a new therapeutic intervention because of biased patient selection alone. As a result of this heterogeneity, it is imperative that a method be developed to better prognosticate and stratify patients for more accurate assessment of efficacy of any new therapeutic interventions.
It is important to note that the new prognostic index score resulted in clear demarcation of three prognostically different groups with nonoverlapping confidence intervals of the median survival durations and this demarcation was replicated in the separate cohort of patients. Another important point to note is that the new prognostic index score incorporates clinical data that are used in routine patient care in most institutions.
There is accumulating evidence for the use of biomarkers in the detection and prognostication of NPC. The biomarkers include circulating markers in the blood, such as Epstein–Barr virus (EBV) DNA (Chien et al, 2001) and CYFRA 21-1 (Ma et al, 2004), as well as tumour immunohistochemical markers such as the expression of multidrug-resistance protein (Hsu et al, 2002), epidermal growth factor receptors (Chua et al, 2004) and signal transducers and activators of transcription factors (Hsiao et al, 2003). Among them, plasma EBV DNA has been the most validated and has been shown to be a predictor of poor survival after radiotherapy for local disease (Chan et al, 2002). However, these biomarkers are not readily available in most institutions and they are yet to be validated as prognostic factors in the metastatic setting.
It is conceivable that similar prognostic classifications can be designed and used for other disseminated solid tumours as well, for instance breast or colorectal cancers. Such a prognostic classification based on combination of patient and laboratory factors may be able to categorise patients with disseminated disease more accurately as shown in NPC in this study and hence will be useful for stratifying patients in randomised studies.
We have validated a new prognostic index score in metastatic NPC patients treated with chemotherapy. This new prognostic index score can stratify patients into three different prognostic groups with significantly different median metastatic survivals. This prognostic classification system will be useful for more accurate prognostication of patients with disseminated NPC. In addition, it can prove useful in the design of clinical trials for metastatic NPC as it can more accurately stratify patients into groups with fairly consistent outcome and thus make the results more comparable and interpretable.
Acknowledgments
This work was presented in part at the 40th annual meeting of the American Society of Clinical Oncology, 5–8 June 2004, New Orleans, LA, USA. The authors have no disclosures of financial interests to declare.
References
- Ahmad A, Stefani S (1986) Distant metastases of nasopharyngeal carcinoma: a study of 256 male patients. J Surg Oncol 33: 194–197 [DOI] [PubMed] [Google Scholar]
- Al-Sarraf M, LeBlanc M, Giri PGS, Fu KK, Cooper J, Vuong T, Forastiere AA, Adams G, Sakr WA, Schuller DE, Ensley JF (1998) Chemoradiotherapy versus radiotherapy in patients with advanced nasopharyngeal cancer: phase III randomised intergroup study 0099. J Clin Oncol 16: 1310–1317 [DOI] [PubMed] [Google Scholar]
- Altun M, Fandi A, Dupuis O, Cvitkovic E, Kranjina Z, Eschwege F (1995) Undifferentiated nasopharyngeal cancer (UCNT): current diagnostic and therapeutic aspects. Int J Radiat Oncol Biol Phys 32: 859–877 [DOI] [PubMed] [Google Scholar]
- Au E, Tan EH, Ang PT (1998) Activity of paclitaxel by three-hour infusion in Asian patients with metastatic undifferentiated nasopharyngeal cancer. Ann Oncol 9: 327–329 [DOI] [PubMed] [Google Scholar]
- Bokemeyer C, Oechsle K, Hartmann JT, Schoffski P, Schleucher N, Metzner B, Schleicher J, Kanz L (2002) Treatment-induced anaemia and its potential clinical impact in patients receiving sequential high dose chemotherapy for metastatic testicular cancer. Br J Cancer 87: 1066–1071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caro JJ, Salas M, Ward A, Goss G (2001) Anaemia as an independent prognostic factor for survival in patients with cancer: a systematic, quantitative review. Cancer 91: 2214–2221 [PubMed] [Google Scholar]
- Chan AT, Lo YM, Zee B, Chan LY, Ma BB, Leung SF, Mo F, Lai M, Ho S, Huang DP, Johnson PJ (2002) Plama Epstein–Barr virus DNA and residual disease after radiotherapy for undifferentiated nasopharyngeal carcinoma. J Natl Cancer Inst 94: 1614–1619 [DOI] [PubMed] [Google Scholar]
- Chan AT, Teo ML, Lee WY, Kwan WH, Choi PH, Johnson PJ (1998) The significance of keratinising squamous cell histology in Chinese patients with nasopharyngeal carcinoma. Clin Oncol (R Coll Radiol) 10: 161–164 [DOI] [PubMed] [Google Scholar]
- Chan ATC, Teo PML, Leung TWT, Johnson PJ (1997) The role of chemotherapy in management of nasopharyngeal carcinoma. Cancer 82: 1003–1012 [DOI] [PubMed] [Google Scholar]
- Chia KS, Seow A, Lee H P, Shanmugaratnam K (2000) Cancer incidence in Singapore 1993–1997: Singapore Cancer Registry Report No. 5
- Chien YC, Chen JY, Liu MY, Yang HI, Hsu MM, Chen CJ, Yang CS (2001) Serologic markers of Epstein–Barr virus infection and nasopharyngeal carcinoma in Taiwanese men. N Engl J Med 345: 1877–1882 [DOI] [PubMed] [Google Scholar]
- Chua DT, Nicholls JM, Sham JS, Au GK (2004) Prognostic value of epidermal growth factor receptor expression in patients with advanced stage nasopharyngeal carcinoma treated with induction chemotherapy and radiotherapy. Int J Radiat Oncol Biol Phys 59: 11–20 [DOI] [PubMed] [Google Scholar]
- Fandi A, Altun M, Azli N, Armand JP, Cvitkovic E (1994) Nasopharyngeal cancer: epidemiology, staging, and treatment. Semin oncol 21: 382–397 [PubMed] [Google Scholar]
- Fandi A, Bachouchi M, Azli N, Taamma A, Boussen H, Wibault P, Eschwege F, Armand JP, Simon J, Cvitkovic E (2000) Long-term disease-free survivors in metastatic undifferentiated carcinoma of nasopharyngeal type. J Clin Oncol 18: 1324–1330 [DOI] [PubMed] [Google Scholar]
- Hong RL, Sheen TS, Ko JY, Hsu MM, Wang CC, Ting LL (1999) Induction with mitomycin C, doxorubicin, cisplatin and maintenance with weekly 5-fluorouracil, leucovorin for treatment of metastatic nasopharyngeal carcinoma: a phase II study. Br J Cancer 80: 1962–1967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsiao JR, Jin YT, Tsai ST, Shiau AL, Wu CL, Su WC (2003) Constitutive activation of STAT3 and STAT5 is present in the majority of nasopharyngeal carcinoma and correlate with better prognosis. Br J Cancer 89: 344–349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu CH, Chen CL, Hong RL, Chen KL, Lin JF, Cheng AL (2002) Prognostic value of multidrug resistance 1, glutathione-S-transferase-pi and p53 in advanced nasopharyngeal carcinoma treated with systemic chemotherapy. Oncology 62: 305–312 [DOI] [PubMed] [Google Scholar]
- Lee AW, Lau WH, Tung SY, Chua D, Chappell R, Siu L, Chia S, Lau J, Law SCK (2004) Prospective randomised study on therapeutic gain achieved by addition of chemotherapy for T1-4N2-3M0 nasopharyngeal carcinoma (NPC). J Clin Oncol 22: 489 (suppl; abstr. 5506) [Google Scholar]
- Lee AWM, Poon YF, Foo W, Law SCK, Cheung FK, Chan DKK, Tung SY, Thaw M, Ho JHC (1992) Retrospective analysis of 5037 patients with nasopharyngeal carcinoma treated during 1976–1985: overall survival and patterns of failure. Int J Radiat Oncol Phys 23: 261–270 [DOI] [PubMed] [Google Scholar]
- Lin J, Jan J, Hsu C, Liang W, Jiang R, Wang W (2003) Phase III study of concurrent chemoradiotherapy versus radiotherapy alone for advanced nasopharyngeal carcinoma: positive effect on overall and progression-free survival. J Clin Oncol 21: 631–637 [DOI] [PubMed] [Google Scholar]
- Ma BBY, Leungm SF, Hui EP, Mo F, Kwan WH, Zee B, Yuen J, Chan ATC (2004) Prospective validation of serum CYFRA 21-1, β-2-microglobulin, and ferritin levels as prognostic markers in patients with nonmetastatic nasopharyngeal carcinoma undergoing radiotherapy. Cancer 101: 776–781 [DOI] [PubMed] [Google Scholar]
- Markman M, Rothman R, Hakes T, Reichman B, Hoskins W, Rubin S, Jones W, Almadrones L, Lewis Jr JL (1991) Second-line platinum therapy in patients with ovarian cancer previously treated with cisplatin. J Clin Oncol 9: 389–393 [DOI] [PubMed] [Google Scholar]
- Micheau C, Boussen H, Klijanienko J, Cvitkovic E, Stosic S, Schwabb G, Eschwege F, Armand JP (1987) Bone marrow biopsies in patients with undifferentiated carcinoma of the nasopharyngeal type. Cancer 60: 2459–2464 [DOI] [PubMed] [Google Scholar]
- Motzer RJ, Bacik J, Schwartz LH, Reuter V, Russo P, Marion S, Mazumdar M (2004) Prognostic factors for survival in previously treated patients with metastatic renal cell carcinoma. J Clin Oncol 22: 454–463 [DOI] [PubMed] [Google Scholar]
- Obermair A, Cheuk R, Horwood K, Janda M, Bachtiary B, Schwanzelberger B, Stoiber A, Nicklin JL, Perrin LC, Crandon AJ (2001) Impact of hemoglobin levels before and during concurrent chemoradiotherapy on the response of treatment in patients with cervical carcinoma: preliminary results. Cancer 92: 903–908 [DOI] [PubMed] [Google Scholar]
- Ong YK, Heng DM, Chung B, Leong SS, Wee J, Fong KW, Tan T, Tan EH (2003) Design of a prognostic index score for metastatic nasopharyngeal carcinoma. Eur J Cancer 39: 1535–1541 [DOI] [PubMed] [Google Scholar]
- Paesmans M, Sculier JP, Libert P, Bureau G, Dabouis G, Thiriaux J, Michel J, Van Cutsem O, Sergysels R, Mommen P (1995) Prognostic factors for survival in advanced non-small cell lung cancer: univariate and multivariate analyses including recursive partitioning and amalgamation algorithms in 1052 patients. J Clin Oncol 13: 1221–1230 [DOI] [PubMed] [Google Scholar]
- Parkin DM (2001) Global cancer statistics in the year 2000. Lancet Oncol 2: 533–543, Erratum in: Lancet Oncol 2: 596. [DOI] [PubMed] [Google Scholar]
- Polee MB, Hop WCJ, Kok TC, Eskens FALM, van der Burg MEL, Splinter TAW, Siersema PD, Tilanus HW, Stoter G, van der Gaast A (2003) Prognostic factors for survival in patients with advanced oesophageal cancer treated with cisplatin-based combination chemotherapy. Br J Cancer 89: 2045–2050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy SP, Raslan WF, Gooneratne S, Kathuria S, Marks JE (1995) Prognostic significance of keratinisation in nasopharyngeal carcinoma. Am J Otolaryngol 16: 103–108 [DOI] [PubMed] [Google Scholar]
- Shanmugaratnam K, Sobin LH (1993) The World Health Organisation histological classification of tumours of the upper respiratory tract and ear. Cancer 71: 2689–2697 [DOI] [PubMed] [Google Scholar]
- Teo PM, Kwan WH, Lee WY, Leung SF, Johnson PJ (1996) Prognosticators determining survival subsequent to distant metastasis from nasopharyngeal carcinoma. Cancer 77: 2423–2431 [DOI] [PubMed] [Google Scholar]
- Wee J, Tan EH, Tai BC, wong BH, Leong SS, Tan T, Chua ET, Lee KM, Yang E, Machin D (2004) Phase III randomised trial of radiotherapy versus concurrent chemo-radiotherapy in patients with AJCC/UICC (1997) stage 3 and 4 nasopharyngeal cancer of the endemic variety. J Clin Oncol 22: 488 (suppl; abstr. 5500) [DOI] [PubMed] [Google Scholar]