Abstract
In cochlear development, the Notch signaling pathway is required for both the early prosensory phase and a later lateral inhibition phase. While it is known that Hes genes are important downstream mediators of Notch function in lateral inhibition, it is not known what genes function as mediators of the early prosensory function of Notch. We report that two members of the Hes-related gene family, Hesr1 and Hesr2, are expressed in the developing cochlea at a time and place that makes them excellent candidates as downstream mediators of Notch during prosensory specification. We also show that treatment of cochlear explant cultures at the time of prosensory specification with a small-molecule inhibitor of the Notch pathway mimics the results of conditional Jag1 deletion. This treatment also reduces Hesr1 and Hesr2 expression by as much as 80%. These results support the hypothesis that Hesr1 and Hesr2 are the downstream mediators of the prosensory function of Notch in early cochlear development.
Keywords: Hey1, Hey2, Hesr3, Hey3, Notch, inner ear, hair cell, cochlea, prosensory
Introduction
The cochlea of the mammalian inner ear has a specialized sensory epithelium, the organ of Corti that is responsible for the perception of sound. During the development of the organ of Corti, sensory hair cells, and adjacent supporting cells, develop from a "prosensory” domain. The highly ordered array of hair cells and supporting cells has served as an excellent model system to analyze the role of Notch in lateral inhibition in the vertebrate nervous system (Lai, 2004; Lewis et al., 1998; Louvi and Artavanis-Tsakonas, 2006).
The development of the organ of Corti can be divided into two phases. In the early, or "prosensory" phase, the sensory epithelial domain of the cochlear duct is specified. In the second phase, the hair cells and their accompanying supporting cells differentiate from the sensory epithelial domain (Kelley, 2006). Several lines of evidence indicate that Notch is required during both phases of inner ear development. The most well studied role for Notch in cochlear development is in the second phase, during the process of lateral inhibition. During lateral inhibition, Delta1 (Dll1), Delta3 (Dll3) and Jagged2 (Jag2) are expressed in the differentiating hair cells (Hartman et al., 2007; Morrison et al., 1999) and signal to Notch-expressing supporting cells to inhibit them from differentiating as hair cells (Lanford et al., 1999). Conditional deletions in either the Jag2 or Dll1 genes in the developing cochlea lead to overproduction of hair cells, through a fate switch of supporting cells (Brooker et al., 2006; Kiernan et al., 2005).
However, in addition to this lateral inhibitory function, recent evidence supports a role for Notch signaling earlier in cochlear development, during the prosensory phase. The Notch ligand Jagged1 (Jag1) is expressed in the prosensory epithelium prior to hair cell differentiation; Jag1 mutants and conditional Jag1 knockout mice have a loss of most of the hair cells and supporting cells (Brooker et al., 2006; Morrison et al., 1999; Tsai et al., 2001 {Kiernan, 2006 #1651). Forced activation of Notch signaling using a Notch intracellular domain (Notch-ICD) expressing construct has two distinct, and contrasting effects, on the development of the cochlea in chick embryos: Notch-ICD expressed in the sensory patch inhibits the differentiation of hair cells, while expression of Notch-ICD outside the normal sensory epithelium causes ectopic patches of hair cells (Daudet and Lewis, 2005).
The mechanism by which Notch activation can have these two distinct effects is not clear. Presumably, these two distinct functions are likely to require separable downstream effectors to translate Notch activation into different transcriptional responses. Once activated by one of its ligands, the intracellular domain of Notch associates with RBPjK/SuH in the nucleus and activates transcription of genes in the hairy/enhancer (Hes) of split family (Bray, 2006). The gene products of the Hes family are bHLH proteins that act as transcriptional repressors at specific DNA sequences in the promoters of target genes. There are three main subtypes in this family, the Hes genes (Hes1 – Hes7), the Hes related genes, Hesr1, Hesr2 and Hesr3 (also known as Hey, Herp, Hrt, Chf and Gridlock), and the Dec genes (Dec1 and Dec2) (Iso et al., 2003 {NOTE:for review). The Hes genes, Hes1 and Hes5, have been previously shown to have a role as critical transcriptional targets of the Notch pathway in the lateral inhibitory phase of cochlear development. Deletions in either gene produce additional rows of hair cells in the cochlea (Zine et al., 2001). However, analogous target genes for the Notch pathway during the earlier, prosensory phase of cochlear development have not been identified.
To investigate this question, we analyzed the expression of the Hesr genes, Hesr1, Hesr2 and Hesr3 in the developing cochlea and we show that Hesr1 and Hesr2 are expressed at the right time and place to act downstream of Notch for its prosensory actions. By inhibiting Notch at specific phases of cochlear development, we were able to experimentally dissect the distinct prosensory and lateral inhibitory functions. We find that both Hesr1 and Hesr2 are regulated during the prosensory phase, supporting a role for these molecules in prosensory specification.
Materials and Methods
Mice
Timed pregnant matings of Swiss-Webster mice purchased from Harlan (Indianapolis, IN) and were housed in the Department of Comparative Medicine; all procedures were carried out in accordance with the guidelines of the animal care and use committee at the University of Washington. We used the staging system of Theiler (Theiler, 1989) to accurately stage the embryos at the time of harvest (http://genex.hgu.mrc.ac.uk/Atlas/intro.html). For the postnatal animals, P0 is defined as the day of birth. Hesr1, Hesr2-knockout mice were housed in the Division of Mammalian Development and procedures were carried out in accordance with the guidelines of the animal care committee at the National Institute of Genetics (Mishima, Japan). The generation and characterization of these mice has been previously described (Kokubo et al., 2005a; Kokubo et al., 2004). Whole heads of P3 pups were fixed with 4% paraformaldehyde (PFA) and then the cochlear ducts were dissected. To expose the organ of Corti, the anlage of stria vascularis was removed using a fine forceps. Some cochlear ducts were embedded in paraffin for sectioning and Hematoxylin-Eosin (HE) staining.
Histology/ In situ hybridization
Whole heads (E12.5–16.5 and E18.5) were fixed in a modified Carnoy's solution for 6 hours at room temperature. Inner ear tissues were also harvested from P0 mice. The samples were washed and dehydrated in 100% ethanol overnight at 4°C, and then were embedded in paraffin and 6 µm sections were collected. At least three animals were examined at each time point. Mouse Hesr1, Hesr2, Hesr3, Math1, Jag1 and Hes5 cDNAs were obtained from Open Biosystems Inc. (Huntsville, AL), and cDNA coding for mouse Sox2 was a gift from Hisato Kondoh (Osaka University, Osaka, Japan). Digoxigenin (DIG)-labeled probes were prepared according to the manufacturer’s manual for DIG-11-UTP (Roche, Indianapolis, IN) and the hybridization was carried out according to Hayashi et. al. (Hayashi et al., 2007) The in situ product was visualized using anti-DIG alkaline phosphatase conjugated secondary antibody (Roche) and NBT/ BCIP.
Immunofluorescence
After in situ hybridization, the slides were fixed with 4% PFA for 1 hour and washed in PBS. The slides were then incubated with 10% fetal bovine serum and 2% nonfat dry milk in PBS/0.1% Triton X-100 (PBST) for 30 minutes. After an overnight incubation with the primary antibody at 4°C, the sections were rinsed with PBST, incubated for 90 minutes with a fluorescent-conjugated secondary antibody, rinsed with PBST, and coverslipped in Fluoromount G (Southern Biotechnology, Birmingham, AL). Whole mount staining of cochleas was carried out according to Hayashi et. al. (2006). The primary antibodies used in this study were as follows: rabbit anti-Prox1 (Chemicon, Temecula, CA) used at 1:300 (1:1000 for whole mount) dilution; mouse anti-p27kip1 (BD Transduction Laboratories, San Diego, CA) at 1:300 dilution; rabbit anti-Myosin6 (Myo6) at 1:1000 dilution; goat anti-Sox2 (Santa Cruz, San Diego, CA) at 1:1000 dilution; biotinylated Griffonia Simplicifolia (GS)-lectin (Vector Laboratories, Burlingame, CA) was used at 1:100 dilution. The secondary antibodies used were goat anti-mouse Alexa 594, chicken anti-rabbit Alexa 594, donkey anti-goat Alexa 594 and streptavidin conjugated Alexa 488 all from Molecular Probes (Eugene, OR) and used at 1:750. Images were captured on a Zeiss Axioplan microscope using a SPOT CCD camera and processed using Adobe Photoshop.
Organ cultures of embryonic cochlea
Inner ear tissue was isolated from E12.5–E13.0 mice and separated into cochlear and vestibular parts. The cochlea was treated with 0.1% Dispase (GIBCO, Carlsbad, CA), 0.1% collagenase (GIBCO) and 0.001% DNase (Sigma) for 15 minutes at 37°C. The cochlear capsule was opened using forceps to expose the cochlear duct. The cochlear ducts were placed on a collagen/Matrigel substrate, along with the mesenchyme surrounding the cochlea. Cochleas were cultured in modified DMEM: F12 media [DMEM: F12 (GIBCO), 0.6% glucose, 5mM HEPES, 0.13% NaHCO3, 800 nM L-glutamine, 100u/ml penicillin (Sigma, St. Louis, MO), N2 supplement and 20% fetal bovine serum], 5% CO2, at 37°C and the all media was replaced each day. To inhibit Notch signaling, 5–50 µM of N-[N-(3,5-difluorophenacetyl)-L-alanyl]-S-phenylglycine t-butyl ester (DAPT; Calbiochem, San Diego, CA) or 20–100 µM of N-(R)-[2-(hydroxyaminocarbonyl)methyl]-4-methylpentanoyl-L-naphthylalanyl-L-alanine-2-aminoethyl amide (TAPI-1; EMD Biosciences) were added to the culture medium as a γ-secretase inhibitor (DAPT) or TNFa-converting enzyme (TACE) inhibitor (TAPI-1). The cultured cochleas were fixed in 4% PFA, and the hair cells were labeled by immunostaining using rabbit anti-Myo6 antibody. The cochlear ducts shown in Fig. 7–Fig. 9 were outlined using DAPI and Normarski images.
Fig. 7. DAPT treatment down-regulated Hesr1/2 expression, and inhibited sensory epithelium development.
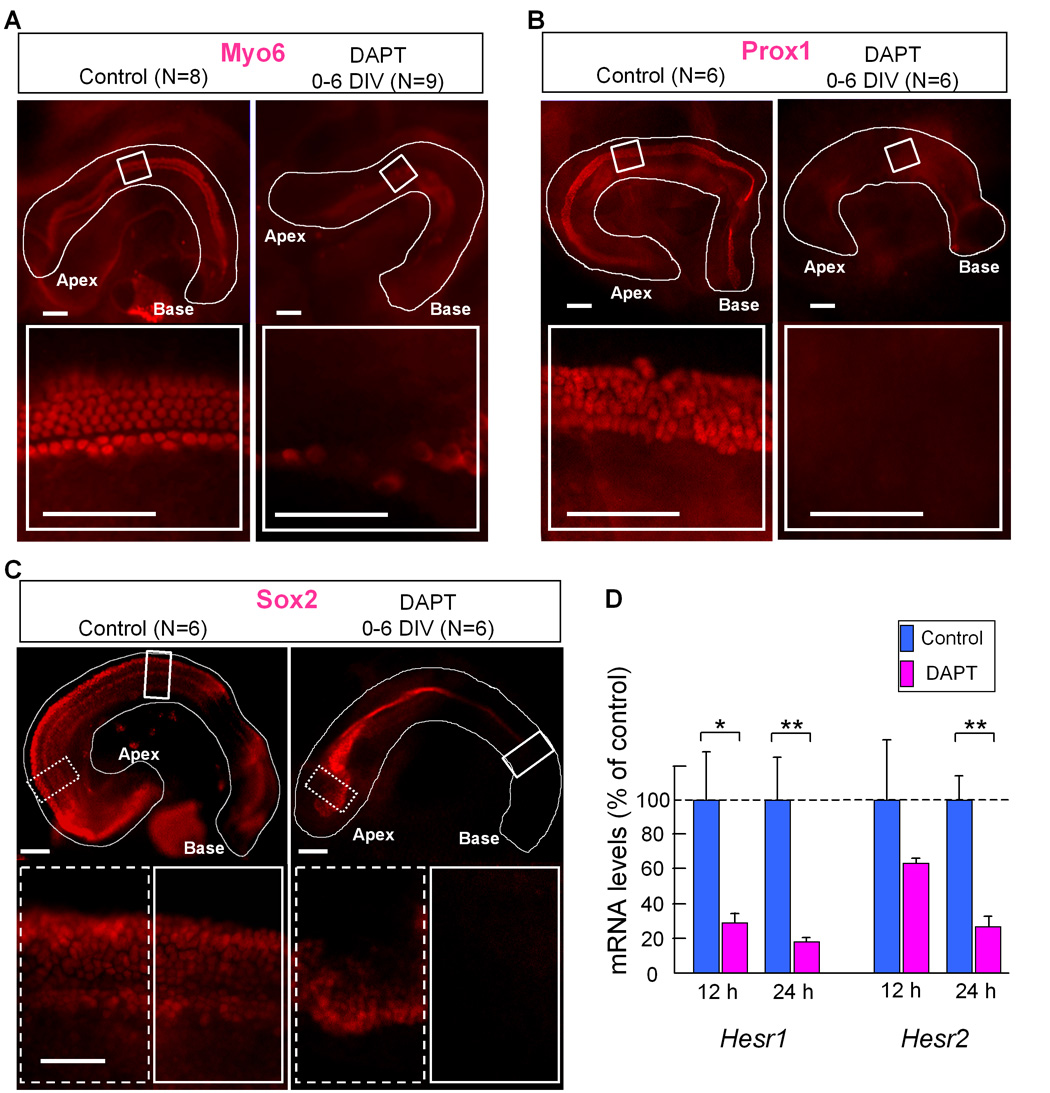
A–C: Explant cultures treated with DAPT for 6 days in vitro (see Fig. 7A), and stained with anti-Myo6 (A), Prox1 (B) and Sox2 (C). Basal end is to the right the apex is to the left. D: Hesr1 and Hesr2 mRNA levels of cochleas treated with DAPT (magenta bars) for 12 or 24 hours were compared with control cultures (no DAPT, blue bars). Error bars indicate standard deviations of the means¨ Asterisk indicates P< 0.05, double asterisk indicate P< 0.005 compared to the control with a Student's T-test. The number of explants (N) is indicated above each panel. Scale bar= 100 µm.
Fig. 9. Change in the number of hair cells that develop in the cochleae cultured with DAPT for different durations.
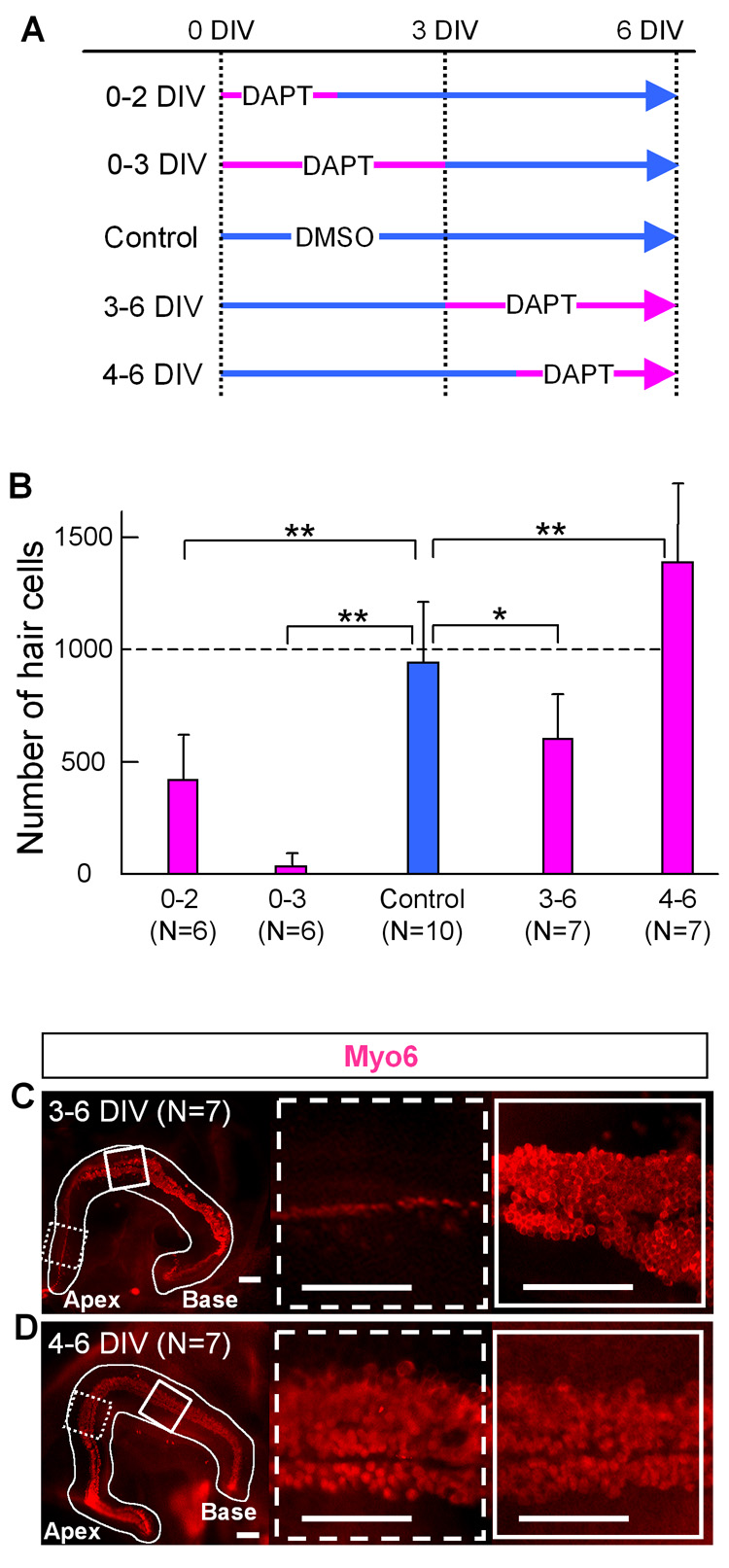
A: Experimental design of the DAPT treatment of cochlear culture. The cochleas were separated into control and 4 different treatment groups. DIV: days in vitro. B: Number of hair cells in a cochlea cultured for 6 days with or without DAPT according to the time schedule in A. Error bars indicate standard deviations of the means. N= number of cochleas. Asterisk indicates P< 0.05, double asterisk indicate P< 0.005 compared to the control with a Student's T-test. C, D: anti-Myo6 staining of cochlea in middle (C) or late (D) treatment group. The basal end of the ducts are to the right, the apex is to the left. White box shows the sensory epithelium from the basal send of the explant and dashed box shows the apical region. Scale bar= 100 µm.
Quantitative PCR (Q-PCR) analysis of cochlea RNA
Total RNA was extracted from pools of 6–8 E12.5–E13.0 cochleas cultured with/without DAPT using TRIzol (Invitrogen, Carlsbad, CA), and then the RNA was quantified using RiboGreen (Molecular Probes). Three independent RNA pools were prepared for each experiment. cDNA was synthesized by Superscript III reverse transcriptase (Invitrogen) using random primers and 2.5 µg total RNA as template, and RT-PCR reactions were carried out using the cDNAs and the following primer sets (forward vs. reverse): Hesr1 (5′-TGAGCTGAGAAGGCTGGTAC-3′, 5′-ACCCCAAACTCCGATAGTCC-3′), Hesr2 (5′-TGAGAAGACTAGTGCCAACAGC-3′, 5′-TGGGCATCAAAGTAGCCTTTA-3′), GAPDH(5′-CGGAAGCCCGGGTCTTCTCAC-3′, 5′-CGAACCGCGTCTCTTCTGCAGTG-3′). The PCR products were separated by electrophoresis and stained with SYBR Safe (Molecular Probes). Quantitative PCR was performed by using an Opticon monitor (Genetic Technologies, Inc., Miami, FL), and the cycle in which log phase was attained was recorded. A SYBR Green-based mastermix (Applied Biosystems, Foster City, CA) was used for the PCR reactions. All samples were normalized to expression of glyceraldehyde-3-phosphate dehydrogenase (GAPDH)
Electroporation of organ cultures
Explants, prepared as above (the cochlear duct was opened to allow the DNA solution to reach the surface of the sensory epithelium), were incubated for 4 hours at 37°C to allow attachment to the membrane coated with Collagen-Matrigel. The explants were transferred to agarose plate filled with 0.6 ml of HBSS. DNA solutions (10µg/ µ l) were mixed with a equal amount of 50% glycerol/ 2x PBS loading buffer to increase the density. Explants were positioned sensory epithelium surface-up, and approx. 10 µl of DNA solution was pipette onto the explant such that the solution flowed over the surface of the sensory epithelium. The cochlear explant was oriented perpendicular to the surface of the well and the two electrodes were placed on opposite sides of the explant. Electroporation conditions were six pulses, 35 V, 75 ms, and applied with a T820 square-wave electroporator (BTX, San Diego, CA). Transfected explants were transferred into fresh media and cultured for 6 days at 37°C. The dominant negative MAML (human MAML1, aa 13–74 : (Nam et al., 2003; Weng et al., 2003)) was driven by combined cytomegalovirus immediate early enhancer (CMV IE) and chicken beta-actin promoter, and IRES2-EGFP sequence was inserted the downstream of dnMAML to identify transfected cells.
Results
Expression patterns of Hesr genes in the developing cochleas
We analyzed the expression of Hesr genes at several key developmental stages, and compared their expression to that of other markers of cochlear development. Both Hesr1 and Hesr2 are expressed in E12.5 and E13.5 cochlea. Hesr1 is expressed as a broad band of labeled cells in the ventral side of the developing cochlear duct (Fig. 1A, D), while Hesr2 is expressed as a narrower band of labeled cells, nested in the Hesr1 expressing region (Fig. 1B, E). At E14.5, in basal regions of the cochlea, the area of Hesr1 expression is divided into a robust band (Fig. 1F, H brackets) and a region of faint expression in the basal greater epithelial ridge (GER; Fig. 1F, H arrows). By E14.5 the Hesr2 expression becomes more intense at all turns of the cochlea and overlaps almost entirely with the Hesr1 expression domain (Fig. 1G). By E16.5, the expression of Hesr2 is undetectable in the basal turn (Fig. 1I, open arrowheads), but is still expressed in the apex. Hesr2 expression continues to be expressed at the apex to E18.5 (Fig. 1K, closed arrowhead), but is not detected in the postnatal cochlea. By contrast, Hesr1 expression is detectable in all turns of the cochlea at E16.5 (Fig. 1H) E18.5 (data not shown) and P0, where it appears to be selectively expressed in the Deiters' cells (Fig. 1J).
Fig. 1. Expression patterns of Hesr1 and Hesr2 in the developing cochlea.
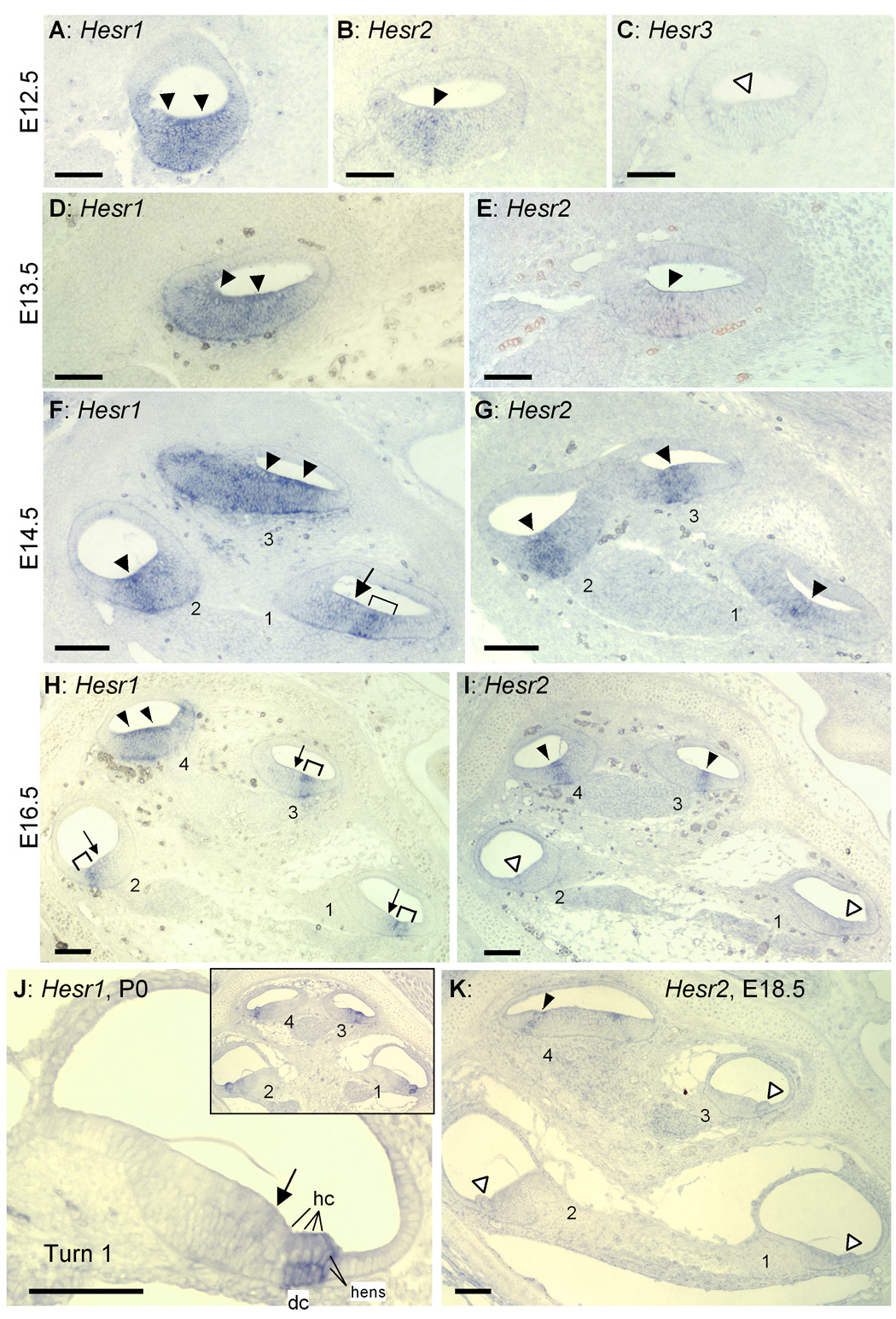
A–E: Adjacent sections of the middle part of the E12.5 (A–C) and E13.5 (D,E) cochlear duct. Expression of Hesr1 or Hesr2 are indicated by solid arrowheads. No expression in the prosensory domain was detected by Hesr3 probes at this stage (open arrowhead in C). Adjacent sections of E14.5 (F, G), E16.5 (H, I) and E18.5 (K) cochlea. Levels (half-turns) of cochlear duct are numbered from base (turn 1) to apex (turn 3 or turn 4). Open arrowheads in I and K indicate no expression. J: Higher magnification micrograph of the basal turn (turn1) of P0 cochlea. Inset shows entire cochlea. Arrowheads, arrow and brackets indicate expression domains. dc: Deiters’ cells. hc: Hair cells. hens: Hensen's cells. Scale bar= 100 µm.
Hesr3 mRNA was not detected at E12.5 in the cochlear duct (white arrowheads in Fig. 1C). In addition, Hesr3 was not detected at E14.5 in the area of the developing sensory epithelium delineated by immunostaining for p27kip1, a protein that marks the area of the duct that gives rise to the sensory epithelium (Chen and Segil, 1999; Lee et al., 2006) (Fig. 2A). However, the expression of Hesr3 in the developing sensory epithelium was clearly detected from E16.5 (Fig. 2,B) and interestingly, as Hesr2 expression is down-regulated at E16.5 and E18.5 (Fig. 1 I and K), Hesr3 is expressed in the sensory epithelium and GER (Fig. 2B and C). After birth, Hesr3 was detected in the Deiters’ cells, and the area of the inner hair cells, inner phalangeal cells and cells in the GER, but not in pillar cells (Fig. 2D).
Fig. 2. Expression patterns of Hesr3 in the development of cochlea.
E14.5 cochlea (A) did not show Hesr3 signal in the developing sensory epithelium (white arrowheads); however, lateral side of cochlea duct showed some expression (bracket). Inset in A shows immunostaining of the same section with anti-p27kip1. E16.5 (B) and E18.5 (C) samples clearly showed the expression of Hesr3 in the sensory epithelium (black arrowheads in B, C). D shows P0 cochlea. Hesr3 mRNA was detected in the sensory epithelium and GER. Inset in D shows immunostaining of the same section with anti-Myo6. Arrow indicates inner hair cell. dc: Deiters’ cells. pc: Pillar cells. Scale bar= 50 µm.
Both Hesr1 mRNA and Hesr3 mRNA were detected in the developing vestibular organs as early as E12.5 (See Supplementary Fig. 1A,C). Hesr1 and Hesr3 were detected in all of the sensory patches in the vestibular epithelium throughout embryonic development and were largely overlapping in their expression (Fig. S1D, F, G, I and data not shown). By contrast, Hesr2 mRNA was not detected in the vestibular system at any age examined (Fig. S 1B, E, H and data not shown).
Hesr1/2 expression precedes hair cell differentiation
We compared the expression of Hesr2 and markers of the prosensory domain (Sox2), differentiating hair cells (Math1) or Notch-signaling components (Jag1, Hes5) in E15.5 cochlea. The Hesr2 expressing region (Fig. 3A) overlapped with the prosensory domain marker Sox2 (Fig. 3B) and Math1 (Fig. 3C), except in the most apical turn, where there was no detection of Math1 mRNA at this age. Hesr2 is expressed in all turns of the cochlea at E15.5 similar to Jag1 but Jag1 is also expressed in cells medial to the Hesr2 expressing cells and the domain is wider than that of Hesr2 in the basal turns (Fig. 3D). This expression pattern is consistent with a role for Hesr2 downstream of Jag1 at the time of prosensory specification. By contrast, neither Hes1 (Zheng et al., 2000) nor Hes5 (Fig. 3E) are expressed in the apical turns at this stage, and they are just beginning to be expressed in the basal turns. Hes1 and Hes5 are expressed after Math1, whereas Hesr1 and Hesr2 are expressed prior to the onset of Math1 expression.
Fig. 3. Timing of Hesr2 relative to other marker genes expressed in the sensory epithelium.
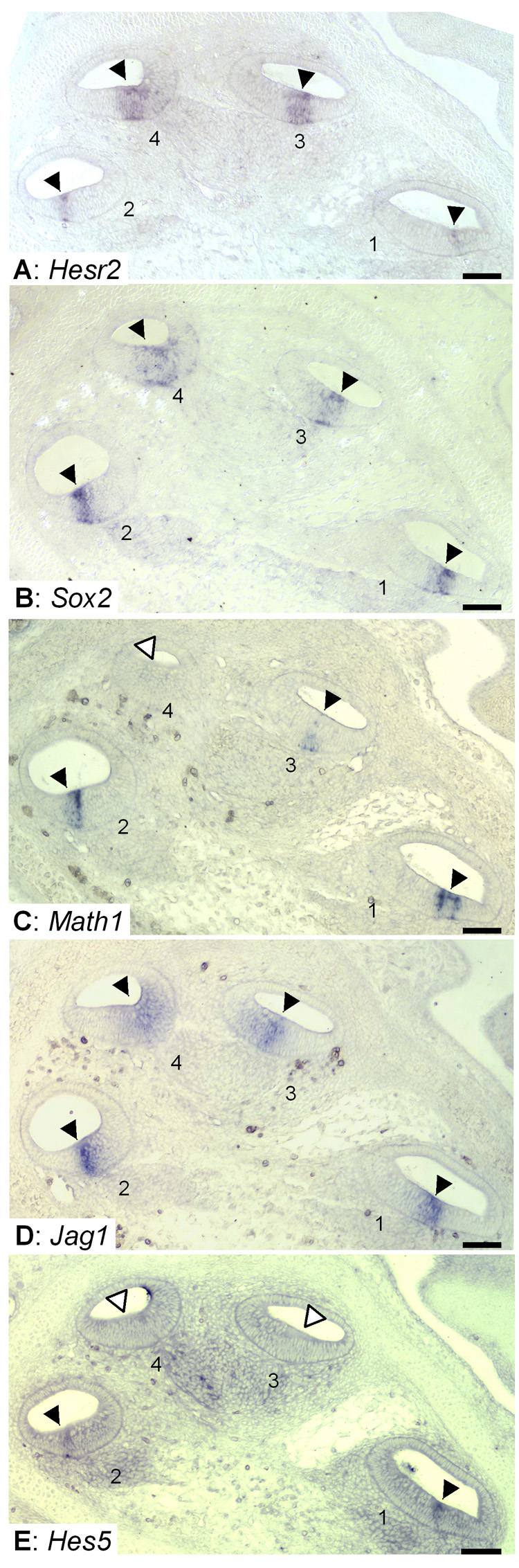
Hesr2 (A) Sox2 (B), Math1 (C) and Notch signaling components, Jag1 (D), Hes5 (E) in the E15.5 cochlea. Levels (half-turns) of the cochlear duct are numbered from base (1) to apex (4). Hesr2, Sox2 and Jag1 were expressed throughout (turn 1 to 4) the cochlea (arrowheads in A, B and D). Math1 and Hes5 were also expressed in the basal turns (arrowheads in C, E) but not in the apical turns (open arrowheads in C, E). Scale bar= 100 µm.
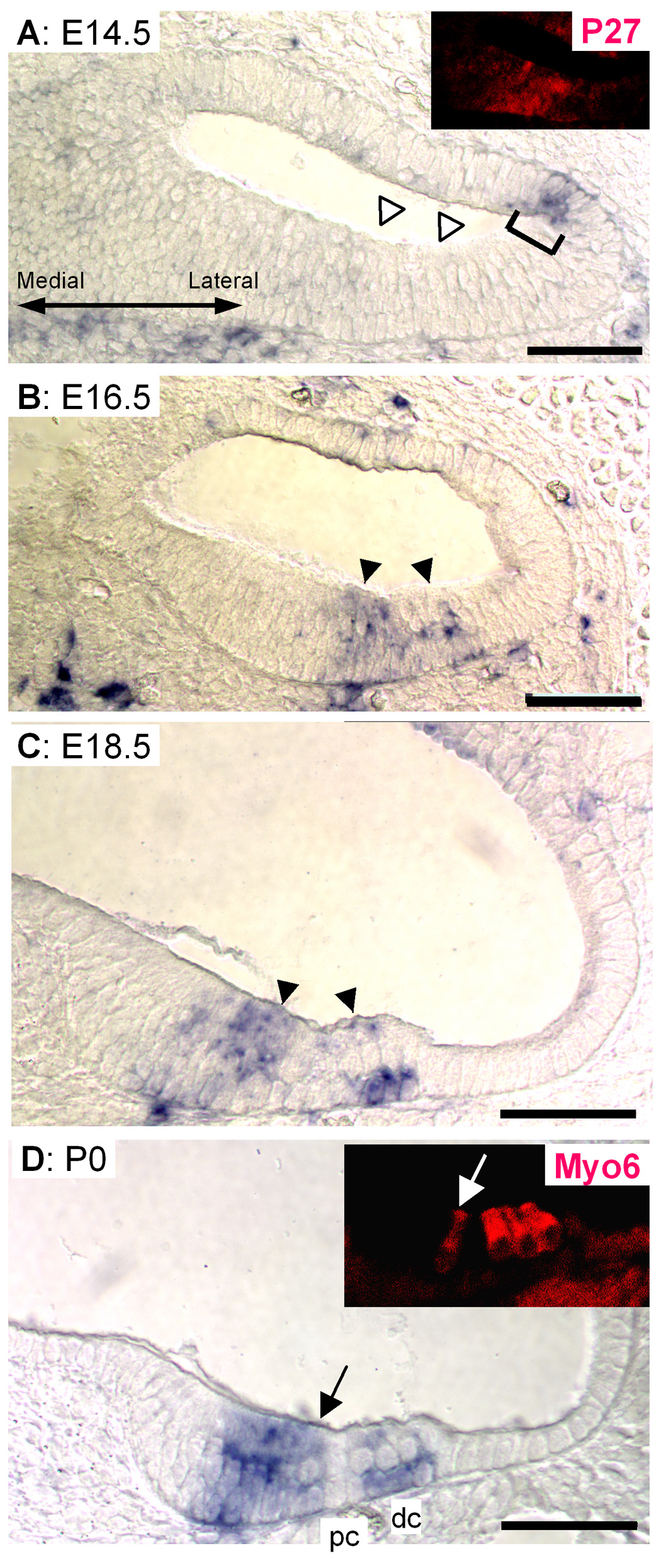
To more precisely define the region of Hesr1 and Hesr2 expression, we post-labeled the in situ sections with antibodies specific for p27kip1 an early marker of the sensory epithelium (Chen and Segil, 1999). In the E13.5 cochlea, Hesr2, Jag1 and Sox2 were expressed in a domain that included the domain of p27kip1 labeling but extends more medially (Fig. 4E,G and I). In addition, it appears that the Hesr1 expression domain extends over a somewhat broader area both medially and laterally. The expression of these molecules occurs before the onset of expression of Math1 (Fig. 4K). At E15.5, the expression of Hesr1 is more restricted, now largely overlapping with the expression of p27kip1 (Fig. 4E) but with some expression medial to p27kip1. Hesr1 expression overlaps with that of Jag1 and Sox2 (Fig. 4H and J). At E15.5 Hesr2 has a more restricted pattern of expression than Hesr1 and is completely contained within the p27kip1 domain and at this age Math1 expression is clear (Fig. 4F and L). The expression of Hesr2 partly overlaps with Jag1, but Jag1 has a somewhat broader domain of expression and is expressed more medially than Hesr2. In addition this pattern of expression for Hesr2 looks similar to the pattern of activated Notch ICD described by Murata et al. (Murato et al. 2006, see Figure 3L).
Fig. 4. Hesr1/Hesr2 expression overlaps with p27kip1.
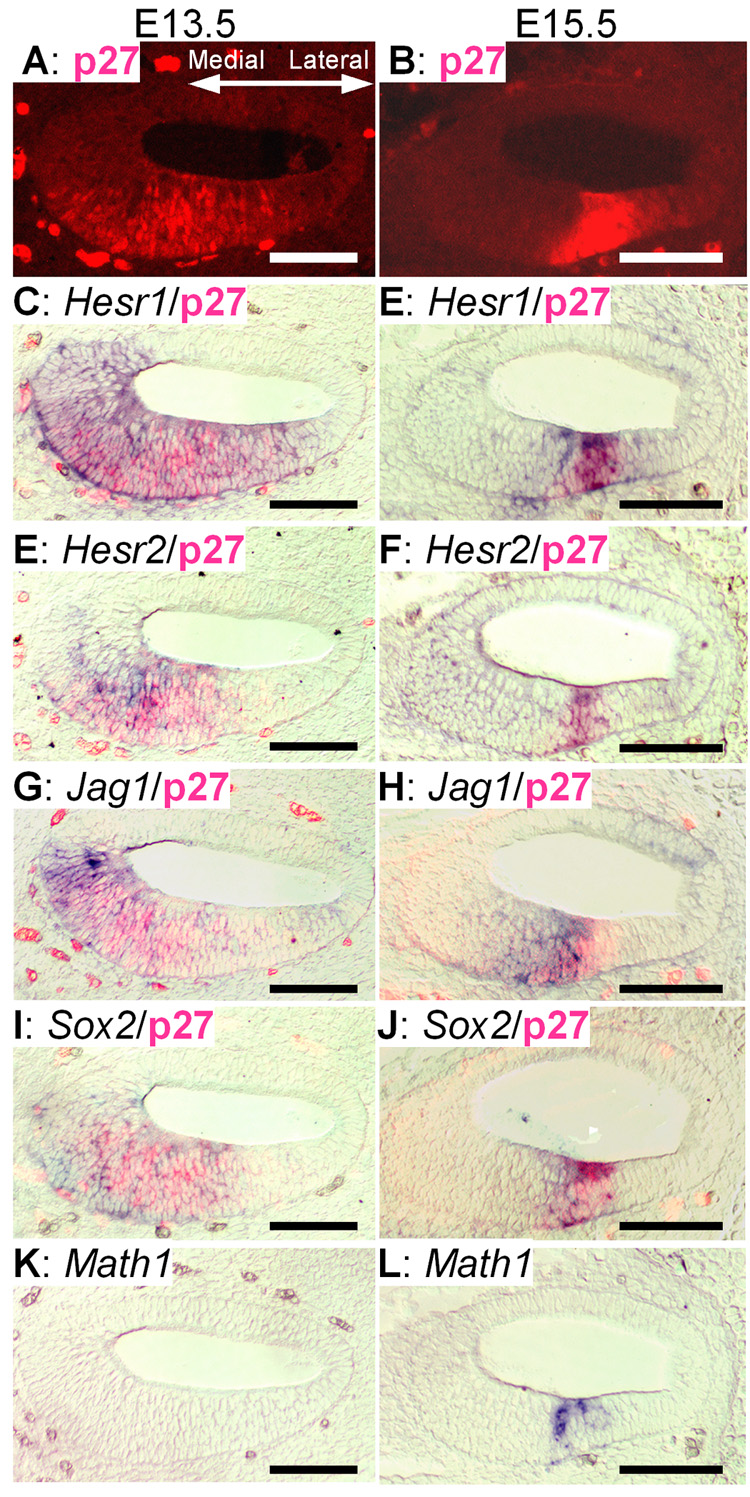
In situ hybridization of Hesr1 or Hesr2 and other genes expressed in the sensory epithelium were counterstained with p27kip1 (C–I, E–J). In situ signal was detected in purple, p27kip1 protein was in red fluorescence. A, C, E, G, I and K: adjacent sections of E13.5 cochlear duct (middle part). Math1 was not yet expressed in this region (K). B, D, F, H, J and L: Adjacent sections of the basal turn (turn1) of an E15.5 cochlea. Scale bar= 100 µm.
Single deletions of either Hesr1 or Hesr2 do not disrupt cochlear development
To determine whether the Hesr genes are the critical downstream mediators of the prosensory function of the Notch pathway in the development of the auditory sensory epithelia, we investigated the structure of the organ of Corti of mice deficient in either Hesr1 or Hesr2. We harvested the cochlea at postnatal day 3 from mice with targeted deletions to these genes and their littermate controls (wild type). These animals are on a C57bl/6,129sv mixed background (Kokubo et al., 2005b). We labeled the organ of Corti for both supporting cells with Prox1 (Bermingham-McDonogh et al., 2006) and hair cells with GS-lectin (Kiernan et al., 2006). We found that the organ of Corti of P3 Hesr1 null or Hesr2 null mice and wild type mice had a single row of inner hair cells (Fig. 5A–C arrows) and three rows of outer hair cells (Fig. 5A–C brackets) along the entire length of the cochlear duct. The differentiation and cellular patterning of supporting cells was also normal as revealed by anti-Prox1 immunostaining and HE-staining sections (Fig. 5). The absence of a phenotype could be explained by the redundancy of Hesr1 and Hesr2 (also Hesr3 in the later stage), unfortunately, mice deficient in both Hesr1 and Hesr2 die between E9.5–E11.5 (Fischer et al., 2004; Kokubo et al., 2005a) due to cardiac and vascular defects, precluding the analysis of the inner ear phenotype in these animals.
Fig. 5. Hesr1 or Hesr2 null mice have normal organs of Corti at postnatal day 3.
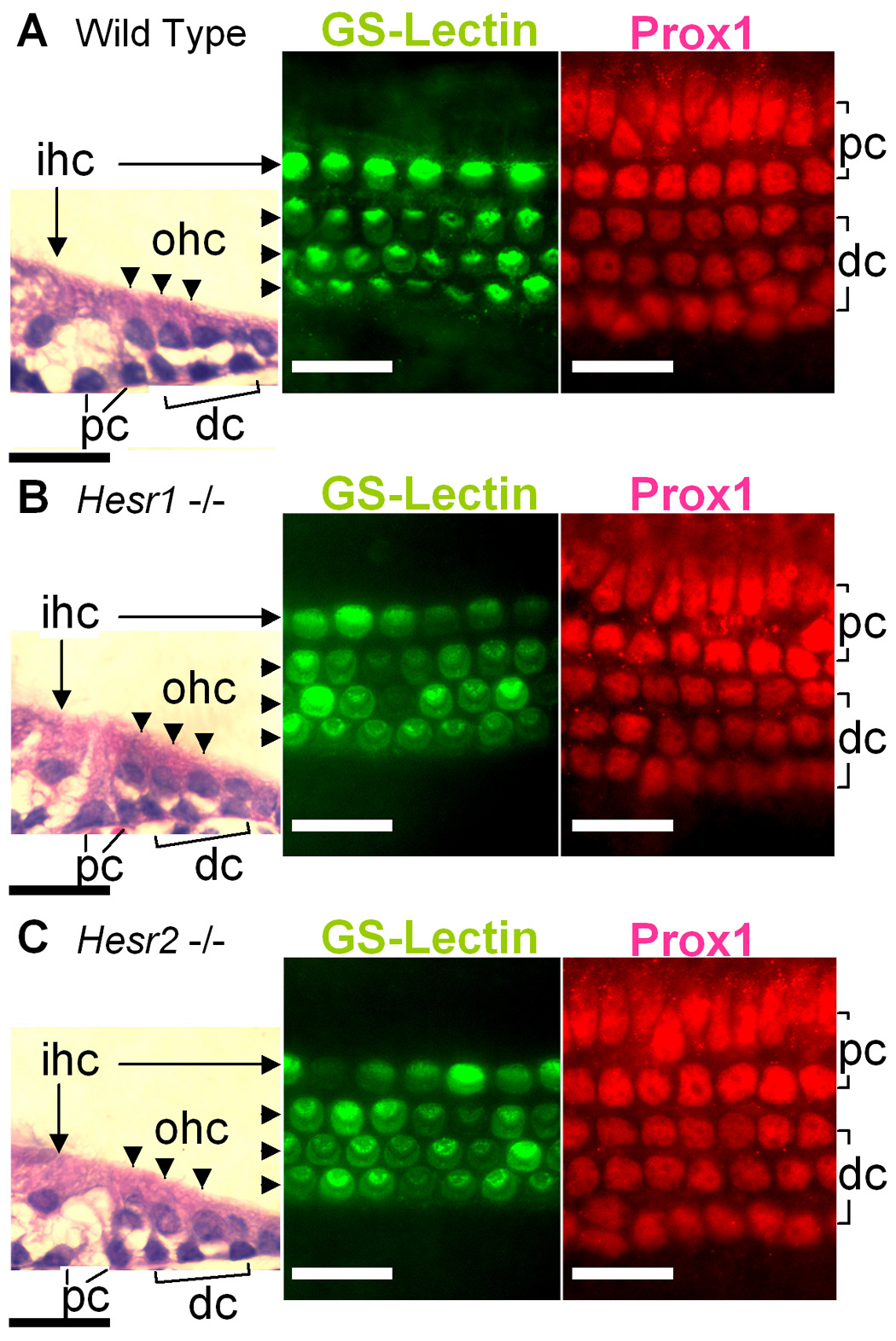
The sensory epithelia from wild type (A), Hesr1 −/− (B), and Hesr2 −/− (C) stained with Hematoxylin-Eosin (HE), GS-lectin (hair cells) and anti-Prox1 antibody (supporting cells). The organ of Corti of Hesr1 or Hesr2 knockout mouse showed 4 rows of hair cells (one of inner and 3 of outer hair cells) and 5 rows of supporting cells (2 pillar and 3 Deiters’ cells) similar to the wild type littermates (A). ihc: Inner hair cells (arrows). ohc: Outer hair cells (arrowheads). pc: Pillar cells. dc: Deiters’ cells. Scale bar= 20 µm.
Inhibition of Notch signaling at E12.5 suppresses sensory epithelial development
To investigate the effect of inhibition of Notch-signaling during the prosensory phase of cochlear development, we prepared explant cultures of E12.5–E13.0 cochlear ducts, as outlined in Fig. 6A. The cultures were then treated with a γ-secretase inhibitor, DAPT or TACE inhibitor, TAPI-1 (Brou et al., 2000; Takebayashi et al., 2007) at varying concentrations and for different time periods (Fig. 6–Fig. 9). Both TACE and γ-secretase are required for the generation of the active Notch intracellular cleavage product that associates with RBPjK/SuH to activate transcription of target genes.
Fig. 6. DAPT treatment inhibits hair cell differentiation.
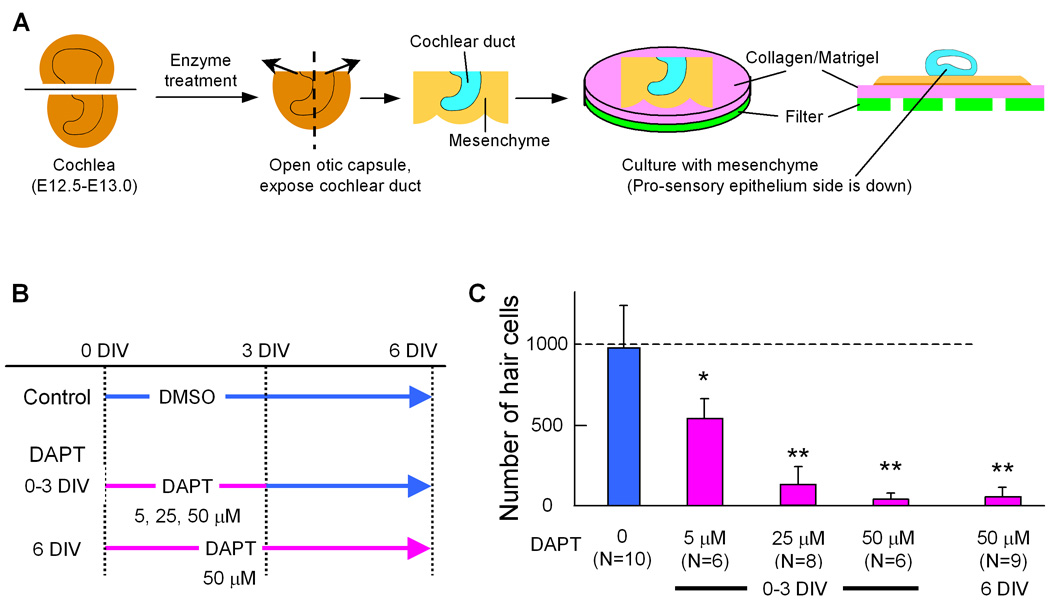
Schematic of culture method and experimental design of DAPT treatments (A, B). E12.5–13.0 cochlear ducts were cultured with surrounding mesenchyme on collagen/Matrigel (A). The cochleas were separated into control and incubated with 3 different concentrations of DAPT for 2 different durations (B), and hair cells were counted after 6 days in vitro (DIV). C: Number of hair cells in a cochlea cultured for 6 days with or without DAPT according the time schedule in A. Error bars indicate standard deviations of the means. N= number of cochleas. Asterisk indicates P< 0.05, double asterisk indicate P< 0.005 compared to the control with a Student's T-test.
The effects of Notch inhibition on sensory epithelial development were assayed with antibodies to Myo6 (hair cell marker), Sox2 (prosensory marker) and Prox1 (supporting cell marker). Cultures treated with 25 µM DAPT for 3 days and harvested at 6 days showed an 85% reduction in the number of hair cells (Fig. 6B,C). When 50 µM DAPT was included in the media for 3 days, or the entire culture period, most hair cell development was suppressed (Fig. 6B,C and Fig. 7A). Supporting cell development was also inhibited with DAPT treatment, as indicated by the lack of Prox1 immunolabeled cells (Fig. 7B). Interestingly, Sox2 was still expressed in the apex of the presumptive sensory epithelial domain in the DAPT treated cultures (Fig. 7C), but the number of Sox2 labeled cells was reduced about 50% compared with the untreated cultures (176±12/100 µm2 in control Vs. 96±27 in DAPT treated). In addition we saw an even more dramatic decrease in Sox2 cells in the base of the explants (150±21 in control Vs. 12 ± 19 in DAPT treated) (Fig. 7C). The reduction in hair and support cells was not due to an effect on cell survival, since we found very little cell death within the developing sensory epithelial region in either the DAPT or control explants when we labeled apoptotic cells using a TUNEL assay (7+/− 4.6 apoptotic cells in control and 8 +/− 5.3 in DAPT treated cultures). We also treated cultures with the TACE inhibitor, TAPI-1. We found that TAPI-1 treatment caused a similar reduction in hair cells in a dose dependent manner (Fig. 8B,C). At a low concentration of TAPI-1 (20 µM), inhibition of hair cell development was moderate resulting in a 50% decrease; however, when TAPI-1 was combined with a low concentration (5 µM) of DAPT (which alone reduces the hair number by 50%), we observed a synergistic effect, resulting in a more than 90% reduction in hair cells (Fig. 8B,D). These results confirm earlier studies showing a role for Notch signaling in the prosensory specification step of cochlear development (Kiernan et al., 2006).
Fig. 8. Inhibition of TACE also inhibits hair cell development.
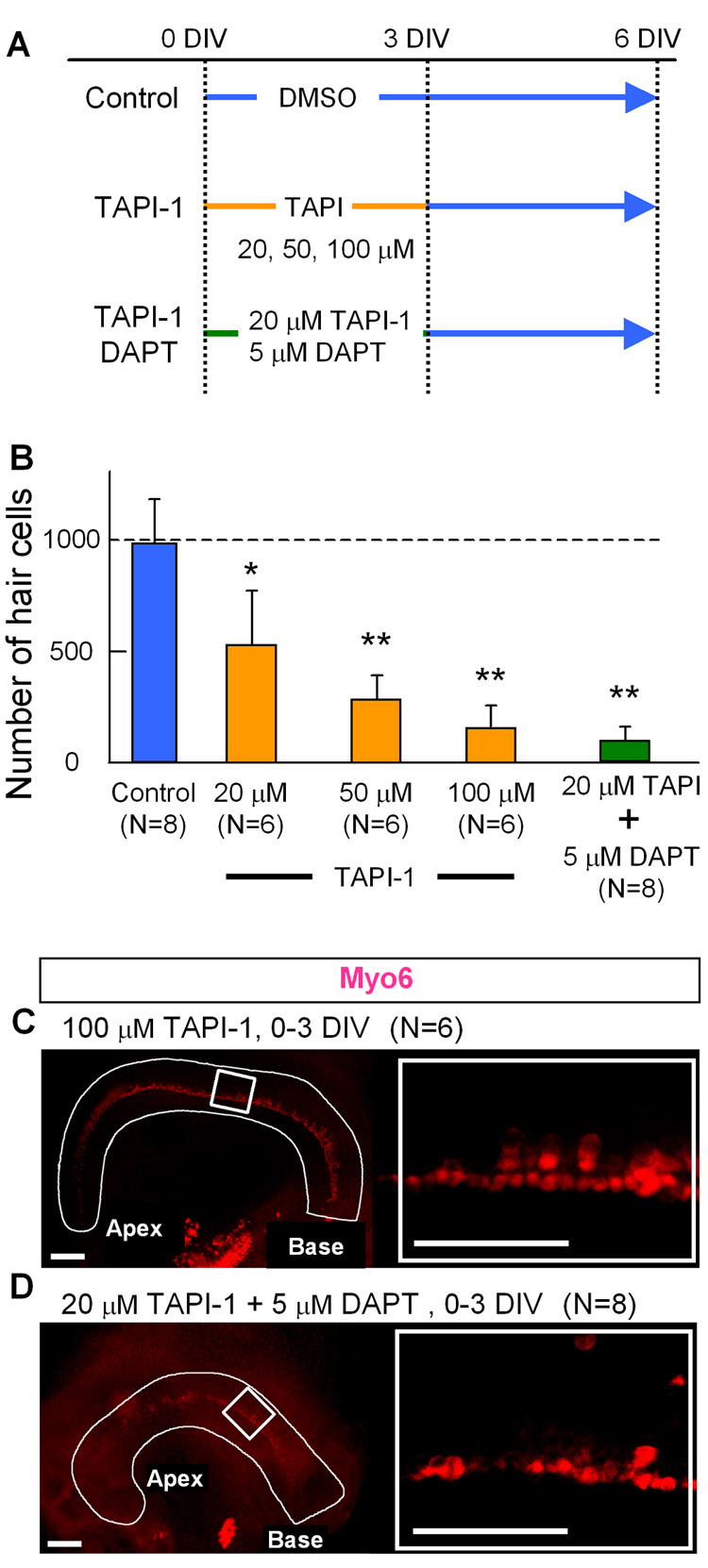
A: Experimental design of the TAPI-1 treatment of cochlear cultures. The explants were separated into control and 4 different treatment groups. DIV: days in vitro. B: Hair cell counts after treatment with various concentrations of TAPI-1 C, D: Explant cultures were treated with 100 µM TAPI-1 (C) or 20 µM TAPI-1 plus 5 µM DAPT (D), and stained with anti-Myo6. Error bars indicate standard deviations of the means. N= number of cochleas. Asterisk indicates P< 0.05, double asterisk indicate P< 0.005 compared to the control with a Student's T-test.
To test whether Hesr1 and/or Hesr2 were regulated by Notch during the prosensory phase of cochlear development, E12.5–E13.0 cochlea ducts were cultured with or without DAPT for 12 or 24 hours and then mRNA levels of Hesr1 and Hesr2 were compared using Q-PCR. We found that both Hesr1 and Hesr2 were down-regulated after 12–24 hours; however, Hesr1 showed a greater change (Fig. 7D). These results support the possibility that Hesr1 and/or Hesr2 may act as effectors of the prosensory function of Notch during cochlear development.
The early treatment with Notch inhibitors allowed us to see the prosensory function in mouse cochlea. However, it has been previously shown that inhibition of Notch later in development results in an overproduction of hair cells, due to the lack of lateral inhibition. We therefore designed a series of experiments to allow us to identify the prosensory period. For these experiments, we separated the E12.5/13 cochlear explants into four DAPT treatment groups, 0–2 DIV, 0–3 DIV 3–6 DIV and 4–6 DIV (Fig. 9A). Treatment of the cultures with DAPT from 0–2 or 0–3 days reduced the numbers of developing hair cells, with the greatest loss occurring when the cultures were treated for 3 days (Fig. 6C and Fig. 9B). These results show that the prosensory period extends to at least E15.5/16.
We also analyzed the effects of Notch inhibition at later stages of cochlear development to confirm its role in lateral inhibition. When E12.5–13.0 cochlear explants were cultured for 4 days prior to addition of DAPT (4–6 DIV group, Fig. 9A), we found a significant increase in the number of hair cells: with 1387 ± 341 hair cells/cochlear explant (Fig. 9B, D) when we treated the explants with 50 uM DAPT, and 1343±235 hair cells (N=6) in explants treated with 5 uM DAPT compared to controls: 944±273. These results confirm previous reports and suggest that a lower concentration of DAPT is required to inhibit Notch dependent signaling during the lateral inhibition phase when compared with the prosensory phase.
In the course of these experiments, we also noticed that explants cultured at intermediate times of development displayed aspects of both Notch-dependent phenotypes. In cultures treated with DAPT from 3 to 6 DIV, we found an increase in hair cells in the base of the explant (83±15.3/100 µm2) and a decrease in hair cells in the apex (11±6.6/100 µm2) relative to untreated cultures (base, 66±13.0/100 µm2: apex, 41±14.3/100 µm2). Thus, both the prosensory function and the lateral inhibitory function of Notch could be blocked in a single explant in different regions of the developing cochlea (Fig. 9C). These results suggest that the prosensory function of Notch proceeds from base to apex, and may still be active in the apex as late as E16.
Since TACE and γ-secretase are necessary for the processing of other cell surface proteins we devised a method of inhibiting notch signaling that was independent of processing. Mastermind (Mam) was identified as a regulator of the Notch signaling pathway by genetic studies in Drosophila (Schweisguth and Posakony, 1992). Homologous proteins, called Mastermind-like (MAML) have been shown in vertebrates to stabilize the DNA binding complex of the Notch ICD and CSL (Wu et al., 2000). Truncated versions of hMam-1 that retain the N-terminal necessary for interaction with NICD and CSL in a dominant negative fashion and inhibit Notch signaling (Kitagawa et al., 2001). We have used such a truncated version of MAML-1 (DN-MAML-1) to determine whether the effects we have observed with the γ-secretase and TACE inhibitors are specific to the Notch pathway. We expressed the DN-MAML-1 (Maillard et al., 2006) in a construct with IRES-GFP to visualize the transfected cells. Cochlear explants were prepared from embryos at E13.5 and the DN-MAML-1 and GFP control constructs were transfected by electroporation. Following electroporation, the explants were cultured for 3 days to allow time for the expression of the constructs and development of the sensory epithelium. The explants were then processed for immunofluorescence using antibodies to Sox2 and MyoVI. As shown in Supplemental Figure 2A–D and Figure 3A–D, we found that regions of the presumptive sensory epithelium that were efficiently transfected with DN-MAML-1 showed clear disruptions in the pattern of hair cells (MyoVI+). In some cases, we found that the hair cells in these regions formed rosette-like patterns, while in other cases there was a clear lack of hair cells in the transfected region. We also found disruptions in Sox2+ cells (not shown); in regions of effective transfection, there were gaps in the Sox2 labeling. In all cases (8/8) we found disruptions and defects in sensory epithelial development in explants transfected with DN-MAML-1. These disruptions in hair cell development or Sox2 patterning were not observed in the pMes-GFP-control transfected explants (Suppl Figure 4A,B). These results are consistent with a prosensory role of Notch signaling in mouse cochlear development and confirm the observations with inhibitors DAPT and TAPI-1.
Discussion
We have found that Hesr1, Hesr2 and Hesr3 are expressed within the developing organ of Corti. A previous analysis of Hesr expression during mouse embryogenesis reported that Hesr1 and Hesr2 are expressed in the otic vesicle at E9.5 and later in the cochlear epithelium at E17.5 (Leimeister et al., 1999). Our study confirms and extends this earlier report by providing a more detailed analysis of the expression pattern and identifying a possible role for these gene products as potential downstream mediators of prosensory Notch signaling. In addition, we have found that inhibition of the Notch signaling pathway with either a γ-secretase inhibitor (DAPT) or a TACE inhibitor (TAPI-1) at different stages of cochlear development, clearly separates the two distinct functions of Notch. This allows dissection of the spatio-temporal gradient in Notch function: at late stages of development, inhibition of Notch promotes hair cell differentiation, confirming earlier reports (Tang et al., 2005; Yamamoto et al., 2006), while at early stages of development, we report for the first time that small molecule inhibitors of the Notch pathway can inhibit the prosensory phase of development in the mammalian cochlea.
The Notch signaling system is required at several distinct phases of cochlear development (Fig. 10). Our results suggest that different downstream effectors may mediate the distinct developmental effects. The earliest "prosensory phase" requires the Notch ligand Jag1 (Brooker et al., 2006; Kiernan et al., 2006; Morrison et al., 1999; Tsai et al., 2001 {Kiernan, 2006 #1651). Hesr1 and Hesr2 are good candidates for downstream effectors of Jag1/Notch signaling during this early prosensory period, while Hes1 and Hes5 appear to be involved only at later stages of cochlear development. While we did not detect Hes1 or Hes5 at this early stage of Notch signaling, the expression of both Hesr1 and Hesr2 in the developing cochlear duct coincides with the timing of an early prosensory function for these genes. The expression pattern of Hesr1 and Hesr2 can be compared spatially and temporally with Jag1 expression and the zone of active Notch signaling as shown by immunolabeling for the NotchICD (Del Monte et al., 2007; Murata et al., 2006). The expression of Hesr2 corresponds closely with that of ActN-ICD at early stages of cochlear development (E13.5 – E15.5), as reported by Murata et al (2006). However, the expression of Jag1 is more medial and only partly overlaps with the domain of Hesr2 and ActN-ICD. By contrast, Hesr1 expression more closely corresponds with that of Jag1, and thus not entirely overlapping with the reported expression of ActN-ICD. Interestingly, Hesr1 responds more rapidly to Notch inhibition than Hesr2 (Fig. 7D), and so it is possible that a lower level of Jag1/Notch signaling is required for Hesr1 expression and my not be detected by the ActN-ICD antibody. It is also possible that Jag1 also acts through another Notch receptor to activate Hesr1, and the ActN1-ICD antibody does not recognize the cleavage site of the other Notch receptors (del Monte et al, 2007). Thus, Jag1/Notch2 signaling might regulate expression of Hesr1, while Jag1/Notch1 signaling might regulate Hesr2. These partly redundant signals might thus explain why conditional deletion of Notch1 failed to show a prosensory phenotype (Kiernan et al, 2005). Thus, although we have shown that both of these genes are potential downstream targets of Notch signaling in early cochlear development, they appear to have slightly different expression patterns.
Fig. 10. Schematic of development of sensory epithelium in cochlea.
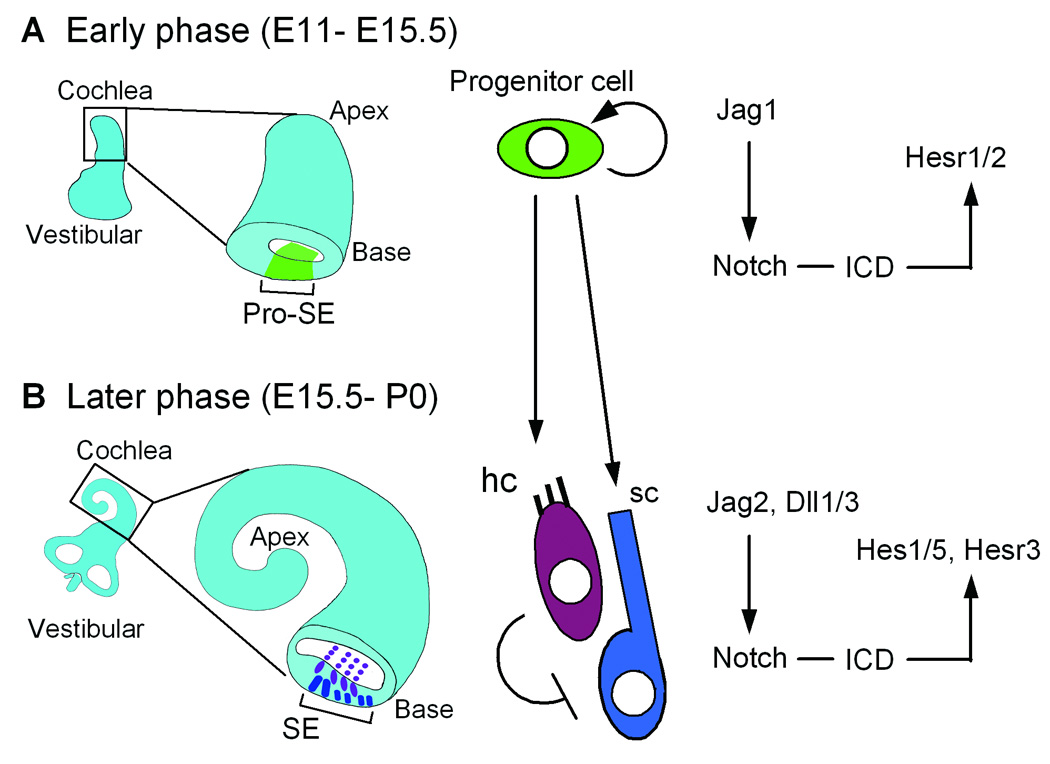
The development of sensory epithelium is separated into early (A) and later (B) phase. In the early phase, Notch has a prosensory role allowing the specification of the sensory domain (A). After hair cell specification, Notch ligand expressing hair cells inhibit surrounding cells to differentiate hair cells (B). Pro-SE: Prosensory epithelium. SE: Sensory epithelium. hc: Hair cells. sc: Supporting cells.
The effectors of the lateral inhibitory phase of cochlear development (Fig. 10) are more well-established. The Hes genes are expressed only at later stages of development (Lanford et al., 2000; Zheng et al., 2000; Zine et al., 2001) and loss of function mutations result in increased numbers of hair cells, consistent with a role in lateral inhibition. However, we have also found that Hesr3 is expressed in support cells at later stages (E16) of cochlear development, and so this gene may also play a role in lateral inhibition. In fact the relatively minor phenotypes observed in the Hes1 and Hes5 knockout mice (see above) suggests that another Notch effector may be involved in these later stages of cochlear development, and Hesr3 is expressed at the right time and place to serve this role.
We have also found that blocking the Notch signal during the prosensory period, with either the γ-secretase inhibitor, DAPT, or the TACE inhibitor TAPI-1, recapitulates the effects of Jag1 mutations/deletions reported previously. DAPT causes a significant reduction of both Hesr1 and Hesr2, to as little as 20% of the untreated control cultures. Interestingly, Jag1 over-expression has been shown to up-regulate Hesr2 expression in C2C12 myoblasts (Iso et al, 2001). In addition to demonstrating that Hesr1 and Hesr2 are regulated by early Notch signaling our results in explant cultures confirm a role for Notch as a prosensory signal. Two other recent studies have used DAPT to identify distinct temporal phases during cochlear development where Notch is required. In the developing chick otocyst, Daudet et al, (2007) found that continuous DAPT treatment of explant cultures caused a significant reduction in the size of sensory epithelial patches, and concomitant reduction in hair cell numbers, thus confirming a prosensory role for Notch signaling. Takebayashi et al, (2007), used DAPT treatment of mouse cochlear cultures to identify additional roles for Notch; in addition to its role in lateral inhibition, they found that Notch signaling is also required for supporting cell differentiation (Prox1 expression) and the maintenance of their phenotype (Takebayashi et al., 2007). Moreover, they reported that Notch signaling also promotes cell cycle withdrawal and p27kip expression. Our results highlight aspects of both of these studies. We find that blocking Notch in E12.5 mouse cochlear explants inhibits sensory epithelial formation similar to that described in the chick embryo explant cultures (Daudet et al., 2007). With continuous DAPT treatment, both Prox1 and Myo6 expressing cells are almost completely lost (Fig. 6 and 7). We found, as did Takebayashi et al, (2007) that DAPT treatment of mouse cochlear explant cultures at later stages of development results in an overproduction of hair cells (Fig. 8B and C). Although both TACE and γ-secretase are known to be involved in the processing of several other signaling proteins and receptors (Ebinu and Yankner, 2002), our results, along with those of Daudet et al (2007) and the conditional deletion of Jag1 (Kiernan et al, 2006; Brooker et al, 2006) suggest that the inhibitors we used are primarily acting through the Notch pathway.
A key difference between our results and those of Takebayashi et al, (2007) is that we were able to demonstrate the prosensory function of Notch in our cultures and they were not. The explant cultures that we used are quite similar to those described in their study; however, while we found a significant effect of DAPT at 5 µM, a higher dose of DAPT (50 µM) is required to fully inhibit the prosensory function of Notch. By contrast, the lateral inhibitory function of Notch can be fully inhibited with only 5 µM DAPT. In addition, to see the full effect of inhibition during the prosensory phase of development, we needed to treat the cultures for three days (Fig. 9A,B), while Takebayashi et al. (2007) treated cultures at a low concentration of inhibitor (5µM) and for only 2 days. Interestingly, Daudet et al, (2007) used up to 100 µM in their experiments, and this was required to block all Hes expression in the chick embryo cultures. In addition, Abello et al. have shown that in order to obtain consistent results, with DAPT inhibition of notch signaling, they needed to use 100 µM in explant cultures (Abello et al., 2007). This may be due to the difficulty of getting an effective dose into relatively large pieces of tissue.
We also find that Sox2 is reduced in the presumptive sensory epithelial domain following DAPT treatment, consistent with recent reports of conditional Jag1 deletions. Kiernan et al (2006) found that conditional deletion of Jag1 using the FoxG1-cre resulted in a significant reduction in Sox2 expression. The base of the cochlea was more severely affected than the apex, where Sox2 expression was still detected. We have found that DAPT treatment causes a very similar result, with Sox2 largely absent from the base and substantially reduced in the apex. This is consistent with recent models of cochlear development that propose the progression of sensory epithelial development (i.e. hair cells and supporting cells), though not its initial induction (e.g. Sox2 and Jag1/Serrate expression), are dependent on Notch signaling (Daudet et al., 2007; Kiernan et al., 2005; Kiernan et al., 2006).
Taken together, our data supporting a role for Hesr1 and/or Hesr2 as downstream effectors of the prosensory Jag1 signal in the cochlea; however, a preliminary analysis of the Hesr1 and Hesr2 deficient mice failed to show any defects in the development of the hair cells or supporting cells in the organ of Corti. This may be due to a functional redundancy in these genes, since they are expressed in largely overlapping domains during most of cochlear development. This is a difficult hypothesis to test, since animals deficient in both Hesr1 and Hesr2 die early in embryonic development, prior to differentiation of the organ of Corti. Analysis of conditional deletions of these genes will ultimately be necessary. Alternatively, it may be that another member of the Hes family of proteins, such as Hes1 or Hes5 serve a prosensory function when Hesr1 or Hesr2 are deleted. However, there is a key difference between these two classes of repressors. Although both classes function by recruiting co-repressors, Hes proteins recruit Groucho via the WRPW domain in the C-terminus, while Hesr proteins have a YRPW instead and do not appear to interact with the same co-repressors, but instead their bHLH and Orange domains are important for recruiting the Sin3/SMRT co-repression complex. Additional experiments, using conditional mutations, will be needed to test whether the Hesr genes are redundant with one another in the developing cochlea (Fischer and Gessler, 2007).
It is interesting that Hesr1 and Hesr2 (Hesr3 in the later stage) are expressed in the cochlea sensory epithelium, while Hesr1 and Hesr3 are expressed in the vestibular sensory patches. In many tissues, there is a mutual exclusivity of Hesr1 and Hesr2; in the developing heart for example, Hesr1 is expressed in the ventricles while Hesr2 is expressed in the atria (Fischer and Gessler, 2003). This complementary expression pattern has led to the proposal that the Hesr gene products cross-repress one another (Iso et al., 2003). In the inner ear, it may be that Hesr2 and Hesr3 cross-repress one another in the auditory and vestibular epithelium, respectively, and this may lead to their lack of mutual expression. By contrast, Hesr1 can apparently be co-expressed with either Hesr2 or Hesr3.
In conclusion, we have shown that Hesr1 and Hesr2 are expressed at the right time and place to act as mediators of the prosensory function of Notch in the developing cochlea. Moreover, these two genes are regulated by Notch in the early stages of cochlea development, prior to the expression of Hes1 or Hes5. We have also found that the inhibition of Notch over precisely defined periods of cochlear development is a powerful way to dissect the various roles for this signaling system in development. For example, in a single explant, we found a reduction in the hair cells in the apex but an overproduction of hair cells in the base, reflecting the relative state of differentiation of the epithelium at each position, and allowing dissection of the spatiotemporal gradients in Notch function.
Supplementary Material
Supplementary Fig. 1. Expression patterns of Hesr1 (A, D, H), Hesr2 (B, E, I), Hesr3 (C, F, J) in the developing vestibular organ. A–C: Adjacent sections of the posterior otocyst of E12.5 embryo. Arrowheads in A, C point to expression domains of Hesr1 and Hesr3. Hesr2 mRNA was not detected (B). D–J: Expression patterns of Hesr1 (D, H), Hesr2 (E, I) and Hesr3 (F, J) in the vestibular organs in E15.5 embryos. Sections of crista of posterior semicircular canal (D, E, F and G) and saccule (H, I, J and K). Although we were not able to detect Hesr3 in the early development of the sensory epithelium in the cochlea, Hesr3 was robustly expressed in the vestibular sensory epithelium throughout its development. Both Hesr1 mRNA and Hesr3 mRNA were detected in the epithelium in the posterior half of the otocyst of the E12.5 embryo, a region, which will give rise to the vestibular organs (A, C). Hesr1 and Hesr3 were detected in all of the sensory patches in the vestibular epithelium throughout embryonic development and were largely overlapping in their expression (D, F, H, J and data not shown). We identified the sensory regions using a hair cell marker Myo6, arrowheads in panels G and K. By contrast, Hesr2 mRNA was not detected in the vestibular system at any age examined (B, E, I and data not shown). Scale bar = 100 µm.
Supplementary Fig. 2 and 3 Two different examples of E13 explants which has been cultured for 5 days after electroporation with a Dn-MAML-IRES-GFP construct. The green cells in A express the Dn-MAML and there is reduced expression of the hair cell marker Myosin 6 in areas that express a lot of the Dn – MAML (arrow). B, C and D show the area indicated with the arrowhead in A at higher magnification. Areas of the epithelium that express a lot of the Dn-MAM show a reduction in the development of hair cells (MyoVI, red).
Supplementary Fig. 4 Electroporation of E13 explants with control plasmid expressing GFP does not disrupt the development of hair cells as evidenced by the normal appearance of MyoVI staining. The sensory epithelium shows it’s regular array of hair cells and not the disrupted pattern we see in Fig. 2 and Fig. 3.
Acknowledgements
The authors are grateful to Dr. Hisato Kondoh, Osaka University for Sox2 cDNA, Dr. William Pear (University of Pennsylvannia) for a DN-MAML1 construct and Dr. Joe Brezenzski (University of Washington) for preparation of the DN-MAML1–IRES-GFP used in this study. We thank Linda Robinson for excellent technical assistance, Drs D. Raible and B. Nelson for critical comments on the manuscript and acknowledge the support of NIH DC005953 to OBMcD, the Virginia Merrill Bloedel Hearing Research Center Hearing Regeneration Initiative for support of TH and OBMcD and T32 HDO7183 for support of BH. This study was also supported by P30DC004661 and P30HD002274. We also thank two anonymous reviewers for their insightful and helpful comments that have improved this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abello G, et al. Early regionalization of the otic placode and its regulation by the Notch signaling pathway. Mech Dev. 2007;124:631–645. doi: 10.1016/j.mod.2007.04.002. [DOI] [PubMed] [Google Scholar]
- Bermingham-McDonogh O, et al. Expression of Prox1 during mouse cochlear development. J Comp Neurol. 2006;496:172–186. doi: 10.1002/cne.20944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bray SJ. Notch signalling: a simple pathway becomes complex. Nat Rev Mol Cell Biol. 2006;7:678–689. doi: 10.1038/nrm2009. [DOI] [PubMed] [Google Scholar]
- Brooker R, et al. Notch ligands with contrasting functions: Jagged 1 and Delta 1 in the mouse inner ear. Development. 2006;133:1277–1286. doi: 10.1242/dev.02284. [DOI] [PubMed] [Google Scholar]
- Brou C, et al. A novel proteolytic cleavage involved in Notch signaling: the role of the disintegrin-metalloprotease TACE. Mol Cell. 2000;5:207–216. doi: 10.1016/s1097-2765(00)80417-7. [DOI] [PubMed] [Google Scholar]
- Chen P, Segil N. p27(Kip1) links cell proliferation to morphogenesis in the developing organ of Corti. Development. 1999;126:1581–1590. doi: 10.1242/dev.126.8.1581. [DOI] [PubMed] [Google Scholar]
- Daudet N, et al. Notch signalling is needed to maintain, but not to initiate, the formation of prosensory patches in the chick inner ear. Development. 2007;134:2369–2378. doi: 10.1242/dev.001842. [DOI] [PubMed] [Google Scholar]
- Daudet N, Lewis J. Two contrasting roles for Notch activity in chick inner ear development: specification of prosensory patches and lateral inhibition of hair-cell differentiation. Development. 2005;132:541–551. doi: 10.1242/dev.01589. [DOI] [PubMed] [Google Scholar]
- Del Monte G, et al. Monitoring Notch1 activity in development: Evidence for a feedback regulatory loop. Dev Dyn. 2007;236:2594–2614. doi: 10.1002/dvdy.21246. [DOI] [PubMed] [Google Scholar]
- Ebinu JO, Yankner BA. A RIP tide in neuronal signal transduction. Neuron. 2002;34:499–502. doi: 10.1016/s0896-6273(02)00704-3. [DOI] [PubMed] [Google Scholar]
- Fischer A, Gessler M. Hey genes in cardiovascular development. Trends Cardiovasc Med. 2003;13:221–226. doi: 10.1016/s1050-1738(03)00082-3. [DOI] [PubMed] [Google Scholar]
- Fischer A, Gessler M. Delta-Notch--and then? Protein interactions and proposed modes of repression by Hes and Hey bHLH factors. Nucleic Acids Res. 2007;35:4583–4596. doi: 10.1093/nar/gkm477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer A, et al. The Notch target genes Hey1 and Hey2 are required for embryonic vascular development. Genes Dev. 2004;18:901–911. doi: 10.1101/gad.291004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartman BH, et al. Dll3 is expressed in developing hair cells in the mammalian cochlea. Dev Dyn. 2007;236:2875–2883. doi: 10.1002/dvdy.21307. [DOI] [PubMed] [Google Scholar]
- Hayashi T, et al. Loss of Fgfr3 leads to excess hair cell development in the mouse organ of Corti. Dev Dyn. 2007;236:525–533. doi: 10.1002/dvdy.21026. [DOI] [PubMed] [Google Scholar]
- Iso T, et al. HES and HERP families: multiple effectors of the Notch signaling pathway. J Cell Physiol. 2003;194:237–255. doi: 10.1002/jcp.10208. [DOI] [PubMed] [Google Scholar]
- Kelley MW. Regulation of cell fate in the sensory epithelia of the inner ear. Nat Rev Neurosci. 2006;7:837–849. doi: 10.1038/nrn1987. [DOI] [PubMed] [Google Scholar]
- Kiernan AE, et al. The Notch ligands DLL1 and JAG2 act synergistically to regulate hair cell development in the mammalian inner ear. Development. 2005;132:4353–4362. doi: 10.1242/dev.02002. [DOI] [PubMed] [Google Scholar]
- Kiernan AE, et al. The Notch Ligand JAG1 Is Required for Sensory Progenitor Development in the Mammalian Inner Ear. PLoS Genet. 2006;2:e4. doi: 10.1371/journal.pgen.0020004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitagawa M, et al. A human protein with sequence similarity to Drosophila mastermind coordinates the nuclear form of notch and a CSL protein to build a transcriptional activator complex on target promoters. Mol Cell Biol. 2001;21:4337–4346. doi: 10.1128/MCB.21.13.4337-4346.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kokubo H, et al. Hesr, a mediator of the Notch signaling, functions in heart and vessel development. Trends Cardiovasc Med. 2005a;15:190–194. doi: 10.1016/j.tcm.2005.05.005. [DOI] [PubMed] [Google Scholar]
- Kokubo H, et al. Mouse hesr1 and hesr2 genes are redundantly required to mediate Notch signaling in the developing cardiovascular system. Dev Biol. 2005b;278:301–309. doi: 10.1016/j.ydbio.2004.10.025. [DOI] [PubMed] [Google Scholar]
- Kokubo H, et al. Targeted disruption of hesr2 results in atrioventricular valve anomalies that lead to heart dysfunction. Circ Res. 2004;95:540–547. doi: 10.1161/01.RES.0000141136.85194.f0. [DOI] [PubMed] [Google Scholar]
- Lai EC. Notch signaling: control of cell communication and cell fate. Development. 2004;131:965–973. doi: 10.1242/dev.01074. [DOI] [PubMed] [Google Scholar]
- Lanford PJ, et al. Notch signalling pathway mediates hair cell development in mammalian cochlea. Nat Genet. 1999;21:289–292. doi: 10.1038/6804. [DOI] [PubMed] [Google Scholar]
- Lanford PJ, et al. Expression of Math1 and HES5 in the cochleae of wildtype and Jag2 mutant mice. J Assoc Res Otolaryngol. 2000;1:161–171. doi: 10.1007/s101620010023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YS, et al. A morphogenetic wave of p27Kip1 transcription directs cell cycle exit during organ of Corti development. Development. 2006;133:2817–2826. doi: 10.1242/dev.02453. [DOI] [PubMed] [Google Scholar]
- Leimeister C, et al. Hey genes: a novel subfamily of hairy- and Enhancer of split related genes specifically expressed during mouse embryogenesis. Mech Dev. 1999;85:173–177. doi: 10.1016/s0925-4773(99)00080-5. [DOI] [PubMed] [Google Scholar]
- Lewis AK, et al. Distinct expression patterns of notch family receptors and ligands during development of the mammalian inner ear. Mech Dev. 1998;78:159–163. doi: 10.1016/s0925-4773(98)00165-8. [DOI] [PubMed] [Google Scholar]
- Louvi A, Artavanis-Tsakonas S. Notch signalling in vertebrate neural development. Nat Rev Neurosci. 2006;7:93–102. doi: 10.1038/nrn1847. [DOI] [PubMed] [Google Scholar]
- Maillard I, et al. The requirement for Notch signaling at the beta-selection checkpoint in vivo is absolute and independent of the pre-T cell receptor. J Exp Med. 2006;203:2239–2245. doi: 10.1084/jem.20061020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison A, et al. Expression of Delta1 and Serrate1 (Jagged1) in the mouse inner ear. Mech Dev. 1999;84:169–172. doi: 10.1016/s0925-4773(99)00066-0. [DOI] [PubMed] [Google Scholar]
- Murata J, et al. Mapping of notch activation during cochlear development in mice: Implications for determination of prosensory domain and cell fate diversification. J Comp Neurol. 2006;497:502–518. doi: 10.1002/cne.20997. [DOI] [PubMed] [Google Scholar]
- Nam Y, et al. Structural requirements for assembly of the CSL.intracellular Notch1.Mastermind-like 1 transcriptional activation complex. J Biol Chem. 2003;278:21232–21239. doi: 10.1074/jbc.M301567200. [DOI] [PubMed] [Google Scholar]
- Schweisguth F, Posakony JW. Suppressor of Hairless, the Drosophila homolog of the mouse recombination signal-binding protein gene, controls sensory organ cell fates. Cell. 1992;69:1199–1212. doi: 10.1016/0092-8674(92)90641-o. [DOI] [PubMed] [Google Scholar]
- Takebayashi S, et al. Multiple roles of Notch signaling in cochlear. Dev Biol. 2007;307:165–178. doi: 10.1016/j.ydbio.2007.04.035. [DOI] [PubMed] [Google Scholar]
- Tang LS, et al. Dynamic expression of COUP-TFI and COUP-TFII during development and functional maturation of the mouse inner ear. Gene Expr Patterns. 2005;5:587–592. doi: 10.1016/j.modgep.2005.03.012. [DOI] [PubMed] [Google Scholar]
- Theiler K. The House Mouse: Atlas of Mouse Development. New York: Springer-Verlag; 1989. [Google Scholar]
- Tsai H, et al. The mouse slalom mutant demonstrates a role for Jagged1 in neuroepithelial patterning in the organ of Corti. Hum Mol Genet. 2001;10:507–512. doi: 10.1093/hmg/10.5.507. [DOI] [PubMed] [Google Scholar]
- Weng AP, et al. Growth suppression of pre-T acute lymphoblastic leukemia cells by inhibition of notch signaling. Mol Cell Biol. 2003;23:655–664. doi: 10.1128/MCB.23.2.655-664.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu L, et al. MAML1, a human homologue of Drosophila mastermind, is a transcriptional co-activator for NOTCH receptors. Nat Genet. 2000;26:484–489. doi: 10.1038/82644. [DOI] [PubMed] [Google Scholar]
- Yamamoto N, et al. Inhibition of Notch/RBP-J signaling induces hair cell formation in neonate mouse cochleas. J Mol Med. 2006;84:37–45. doi: 10.1007/s00109-005-0706-9. [DOI] [PubMed] [Google Scholar]
- Zheng J, et al. Hes1 is a negative regulator of inner ear hair cell differentiation. Development. 2000;127:4551–4560. doi: 10.1242/dev.127.21.4551. [DOI] [PubMed] [Google Scholar]
- Zine A, et al. Hes1 and Hes5 activities are required for the normal development of the hair cells in the mammalian inner ear. J Neurosci. 2001;21:4712–4720. doi: 10.1523/JNEUROSCI.21-13-04712.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Fig. 1. Expression patterns of Hesr1 (A, D, H), Hesr2 (B, E, I), Hesr3 (C, F, J) in the developing vestibular organ. A–C: Adjacent sections of the posterior otocyst of E12.5 embryo. Arrowheads in A, C point to expression domains of Hesr1 and Hesr3. Hesr2 mRNA was not detected (B). D–J: Expression patterns of Hesr1 (D, H), Hesr2 (E, I) and Hesr3 (F, J) in the vestibular organs in E15.5 embryos. Sections of crista of posterior semicircular canal (D, E, F and G) and saccule (H, I, J and K). Although we were not able to detect Hesr3 in the early development of the sensory epithelium in the cochlea, Hesr3 was robustly expressed in the vestibular sensory epithelium throughout its development. Both Hesr1 mRNA and Hesr3 mRNA were detected in the epithelium in the posterior half of the otocyst of the E12.5 embryo, a region, which will give rise to the vestibular organs (A, C). Hesr1 and Hesr3 were detected in all of the sensory patches in the vestibular epithelium throughout embryonic development and were largely overlapping in their expression (D, F, H, J and data not shown). We identified the sensory regions using a hair cell marker Myo6, arrowheads in panels G and K. By contrast, Hesr2 mRNA was not detected in the vestibular system at any age examined (B, E, I and data not shown). Scale bar = 100 µm.
Supplementary Fig. 2 and 3 Two different examples of E13 explants which has been cultured for 5 days after electroporation with a Dn-MAML-IRES-GFP construct. The green cells in A express the Dn-MAML and there is reduced expression of the hair cell marker Myosin 6 in areas that express a lot of the Dn – MAML (arrow). B, C and D show the area indicated with the arrowhead in A at higher magnification. Areas of the epithelium that express a lot of the Dn-MAM show a reduction in the development of hair cells (MyoVI, red).
Supplementary Fig. 4 Electroporation of E13 explants with control plasmid expressing GFP does not disrupt the development of hair cells as evidenced by the normal appearance of MyoVI staining. The sensory epithelium shows it’s regular array of hair cells and not the disrupted pattern we see in Fig. 2 and Fig. 3.


