Abstract
Despite modern medical advances, necrotizing enterocolitis (NEC) remains a significant cause of morbidity and mortality in neonatal intensive care units, affecting 10 percent of premature neonates born weighing less than 1500 grams. Although many advances have been made in the understanding of this disease, the etiology and pathophysiology remain incompletely understood, and treatment is limited to supportive care. In recent years, studies have focused on the role of the inflammatory cascade and its’ impact on the disease process, and investigators are evaluating strategies to attenuate inflammatory signaling that might prevent and/or ameliorate neonatal NEC. In this review, we examine the key points in the signaling pathways involved in NEC, and potential strategies for prevention and treatment of this dreaded disease.
Background
Necrotizing enterocolitis (NEC) is a devastating inflammatory condition of the gastrointestinal tract that afflicts 10% of premature infants born weighing less than 1500 grams. Despite modern medical advances, the etiology remains elusive, and morbidity and mortality is unacceptably high, with as many as 10–30% of affected infants succumbing to the disease.
Although the pathophysiology is incompletely understood, it is known that prematurity, formula feeding, intestinal ischemia, and bacterial colonization are important risk factors.
Based on extensive laboratory and human investigation, it is suggested that these risk factors initiate the activation of the pro-inflammatory response that ultimately leads to bowel necrosis, and in some cases multi-organ dysfunction syndrome, and death. Although a myriad of inflammatory mediators have been identified that might contribute to this final common pathway, the following sections will explore the evidence for some of the most likely participants.
Tumor Necrosis Factor-Alpha (TNF-α)
TNF-α is a pro-inflammatory cytokine that is synthesized and secreted by activated macrophages and other inflammatory cells, and has diverse effects following its’ binding to TNF receptors. Initial reports of TNF-a (cachectin) infusion into animal models describe the occurrence of shock and tissue injury that looked similar to neonatal NEC (Beutler et al). Subsequent studies showed that intravenous administration of TNF-α and LPS concurrently resulted in significant bowel necrosis in animal models (1, 2), and the production of platelet activating factor (PAF), a phospholipid pro-inflammatory mediator also associated with NEC. Confirmatory studies in human neonates with NEC identified significantly elevated plasma levels of TNF-α compared with controls (3). Pentoxifylline, a drug that has multiple effects including inhibition of production of TNF-α, was shown to reduce bowel necrosis in an adult rat ischemia-reperfusion model of bowel injury (4). In addition, pentoxifylline has been found to reduce the incidence of NEC in a neonatal rat model following intraperitoneal injection . Finally, Seitz et al and Halpern et al in two separate studies found a significant reduction in the severity of NEC in neonatal rats after intraperitoneal TNF-α antibody prophylaxis . These studies all suggest a critical role for TNF in this condition, and may warrant evaluation in the human situation.
Platelet Activating Factor (PAF)
PAF is a potent phospholipid inflammatory mediator that is produced by most cells and tissues and can cause epithelial cell injury and programmed cell death (apoptosis), leukocyte and platelet aggregation, vasoconstriction, and increased vascular permeability. Previous work from our laboratory and others has found that PAF plays a significant role in the initiation of the inflammatory response in the intestine as well as the lung, kidney, heart, and other organs. Local and systemic PAF activity is tightly regulated by the synthesizing enzyme phospholipase A2 (PLA2), and the degradation enzyme PAF-acetylhydrolase (PAF-AH). PAF initiates the inflammatory response by binding to, and activating PAF receptors that are present on most tissues, but are found in the highest concentration on intestinal epithelium, thereby suggesting why PAF-induced disease may initially and most potently affect intestine.
The role of PAF in necrotizing enterocolitis has been explored in various animal models and human studies. In adult rats, intravenous PAF administration causes ischemic intestinal necrosis (5), and hypoxia and LPS-induced bowel injury is prevented by PAF-receptor antagonists. In the neonatal rat model of NEC that is accomplished with formula feeding (complete absence of maternal milk) and asphyxia (6), we found that PAF receptor antagonist WEB 2170 prophylaxis decreased the incidence of disease. Furthermore, enteral PAF-acetylhydrolase (PAF-AH) supplementation decreased the incidence of NEC compared with controls (7).
Human studies have confirmed the potential role of PAF in neonatal NEC using several approaches. Stool levels of PAF in preterm infants increase with enteral feeding, and are further elevated in infants who subsequently develop NEC . Similarly, plasma PAF levels are increased in human NEC patients compared to age-matched and respiratory illness-matched controls. Of note, PAF-AH is known to be present in maternal breast milk (8) but not in commercial infant formulas, and breast milk is associated with a reduced incidence of disease (9). Furthermore, our laboratory described a large cohort of human neonates and found dramatically low levels of circulating PAF-AH compared to older children and adults (10). This finding indicates that newborns are vulnerable to high levels of PAF due to their inability to metabolize this inflammatory mediator. In summary, PAF may be one of the early mediators that stimulates the pro-inflammatory response in preterm intestine that ultimately results in the clinical continuum of NEC.
Toll-like receptors
Toll-like receptors (TLRs) are pattern recongnition receptors present on various cells that recognize structurally conserved molecules found on microbes. There are over 10 TLRs now well characterized, and there is significant specificity for particular ligands and TLR. For example, lipopolysaccharide (LPS), a gram-negative bacterial cell wall product, is the main ligand for TLR4. Following activation of TLRs, a complex cascade of signal transduction occurs (see figure x), culminating in the translocation of NFκB (nuclear factor kappa B, a pro-inflammatory transcription factor) to the nucleus and synthesis and release of inflammatory cytokines..
TLR expression varies by tissue, and inflammatory cells typically express high levels of these receptors. Previous studies have found that intestinal epithelial cells normally express low levels of TLR4 and TLR2; this is thought to allow for the hypo-responsiveness of the intestinal environment to commensal bacteria. Thus, in the normal, mature gut lumen with large concentrations of bacterial products, inflammation is suppressed due to the down-regulation of TLRs. Studies have suggested that TLR4 and TLR2 downregulation is developmentally regulated, and that initial perturbations of this response could lead to intestinal inflammation and NEC. Fetal intestinal epithelial cells are responsive to LPS with subsequent activation of chemokines, while cells acquired soon after birth do not respond to LPS (19). Using our established neonatal rat model and a novel mouse model of NEC, we have found that in mother-fed animals, there is a temporal decrease in TLR4 mRNA expression in intestinal epithelium, while in formula-fed and asphyxia-stressed animals, TLR4 expression increases. This abnormal TLR4 upregulation is associated with increased inducible NO synthase (iNOS) expression, a potent pro-inflammatory regulator described in more detail in another chapter, and a downstream marker of TLR activation . In addition, we have found that C3H/HeJ mice which harbor a mutation in TLR4 experience a decreased incidence of NEC compared with C3HeB/FeJ mice, with wild-type TLR4 (reference). A subsequent report found that TLR4 wild-type mice exposed to hypoxia and formula feeding have increased incidence of NEC compared to TLR4 mutant mice, and that the increase in NEC is attributable to increased enterocyte apoptosis as well as decreased capacity for repair of intestinal epithelium (reference) These data highlight the potential importance of TLR signaling in neonatal NEC, and in pilot data, increased TLR4 expression is suggested in human NEC intestinal epithelium. It is hypothesized that upregulation of TLR4 in preterm, stressed intestinal epithelium allows for bacteria-induced activation of TLRs and the initiation of the pro-inflammatory cascade that results in the final common pathway of neonatal NEC.
TLR signaling
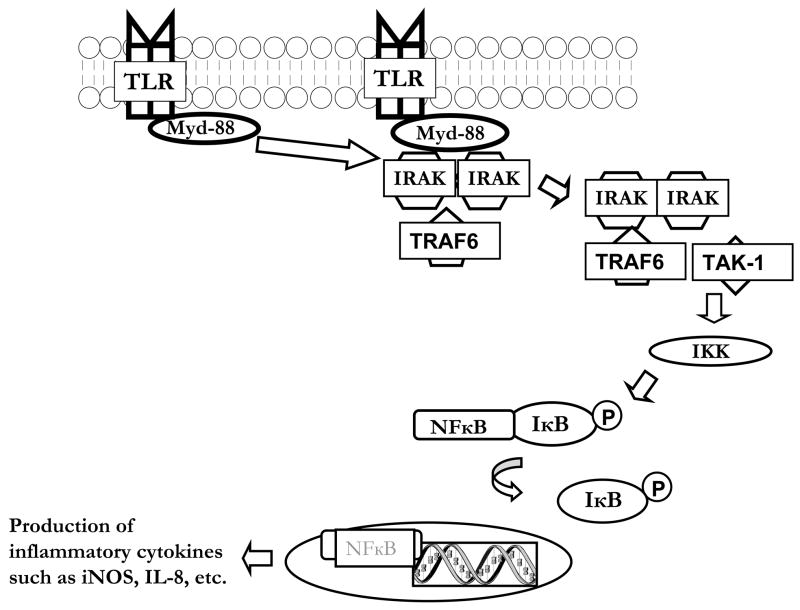
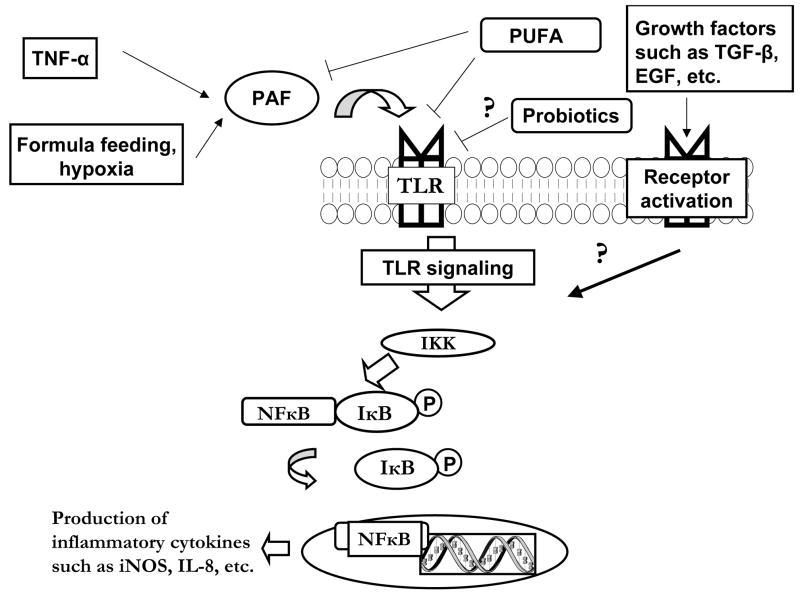
Since TLR activation may play a critical role in the initiation of NEC, a more complete understanding of the complex signaling is warranted. Upon ligand binding to most TLRs (all but TLR3 that signals via a MyD88-indpendent pathway), the adaptor molecule Myd88 is recruited to the receptor complex, with subsequent recruitment of IL-1R associated protein kinases (IRAKs) and tumor necrosis factor receptor-associated factor 6 (TRAF6) (see figure y). Next, IRAK-1 and TRAF6 bind to transforming growth factor-beta-activated kinase (TAK-1) and activate the IκB kinase kinase (IKK) complex which phosphorylates IκB, leading to its degradation and activation of NFκB. Unbound NFκB is then translocated to the nucleus where it upregulates the production of various inflammatory cytokines, including IL-8 and iNOS (Figure 1).
Figure 1.
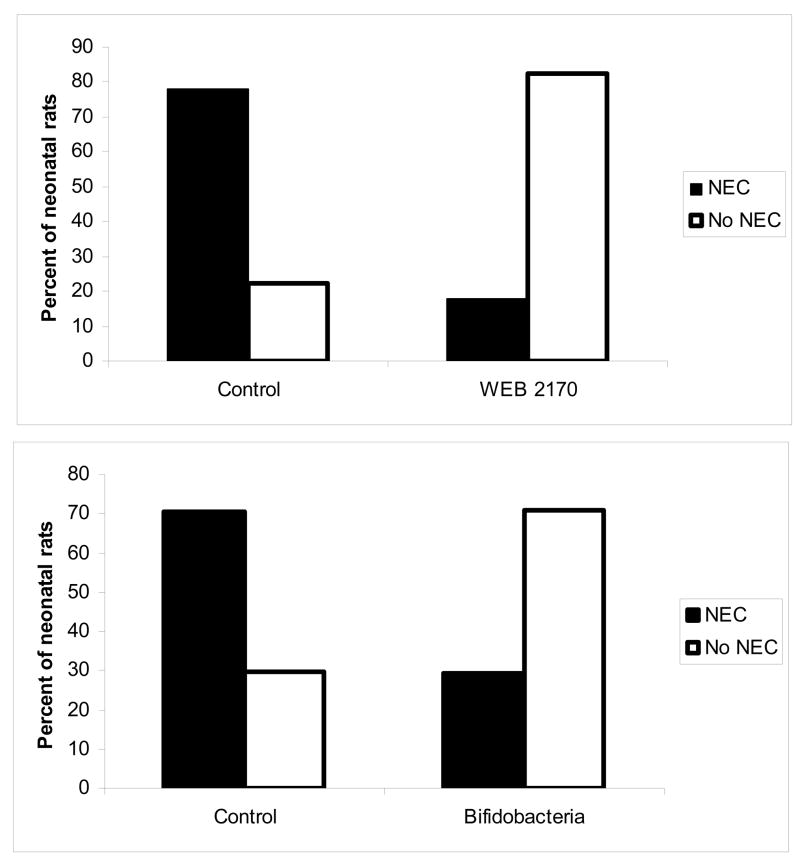
Top panel: Treatment with PAF receptor antagonist WEB 2170 leads to a decrease in the incidence of NEC. Using our established neonatal rat model for NEC, animals were pretreated with WEB 2170, a PAF receptor antagonist, and then exposed to formula feeding, asphyxia, and bacterial colonization. In animals pretreated with WEB 2170, there was a marked reduction in the incidence of NEC (3/17 versus 14/18 in controls, p<0.001).
Bottom panel: Supplementation with Bifidobacterium infantis rats leads to a reduction in the incidence of NEC. Using the same neonatal rat model, animals were treated with B. infantis, E. coli, or saline and then exposed to the NEC protocol. In animals exposed to B. infantis, there was a reduction in NEC to 7/24 versus 19/27 in controls and 16/23 in E. coli-treated animals (p<0.01).
Studies have examined the TLR signaling pathway and its’ implications in the context of NEC. DePlaen et al found that in neonatal rats, intestinal levels of NFκB are elevated at birth, but that activation is downregulated within 24 hours in mother fed pups . This down-regulation of NFκB was not observed at 1 and 2 days in formula fed/hypoxia stressed animals, and was associated with low IκB levels. Of interest, this study demonstrated that inhibition of IKK and subequent NFkB activation with NEMO-binding domain peptide led to decreased bowel necrosis and decreased mortality compared with control animals. Another study by Claud et al found that fetal enterocytes expressed lower levels of IκB compared to mature enterocytes, and that in neonatal rats, expression of IκB slowly increases to that of mature enterocytes following weaning. Additionally, when fetal cells were transiently transfected with IκB, IL-8 production was attenuated in response to flagellin, indicating that a low level of IκB in immature intestinal epithelium may allow for increased NFkB activity and increased expression of pro-inflammatory cytokines, leading to intestinal injury . In sum, the results suggest that developmental alterations in downstream signaling after TLR activation may play a critical role in the balance of the inflammatory response in preterm animals and contribute to the pathophysiology of NEC.
Elaboration of pro- and anti-inflammatory cytokines
The inflammatory response requires appropriate balance between pro- and anti-inflammatory signaling in order to maintain a healthy intestinal homeostatic environment. Several of the pro- and anti-inflammatory molecules have been studied in detail in animal models, in humans, and in vitro, including IL-6, IL-8, and IL-10 as well as nitric oxide, oxygen free radicals, and numerous others.
In an neonatal rat model of necrotizing enterocolitis, Nadler et al found a significant increase in intestinal iNOS mRNA expression in animals exposed to hypoxia and/or formula feeding (commonly employed model of neonatal NEC) versus maternally fed pups who were or were not exposed to hypoxia (11). Furthermore, in a study by Ford et al, 15 neonates with NEC who underwent intestinal resection had NOS-2 immunofluorescent positivity compared with two out of six neonates undergoing resection for other indications such as atresias (12). Additional data from Dr. Ford’s lab and others supports the important role for NO as a key mediator in the pathophysiologic response during NEC, and these studies are described in greater detail in a subsequent chapter.
In addition to nitric oxide, other pro-inflammatory cytokines are elevated in infants affected by NEC. In a study by Ren et al, blood samples from infants affected by stage III NEC were compared with infants with imperforate anus and also with healthy controls. In the NEC patients, they found that levels of macrophage migration inhibitory factor (MIF), a cytokine elaborated by activated macrophages and T-cells, as well as levels of IL-6 and IL-8 were all significantly elevated compared with the other two groups. MIF is known to stimulate the production of IL-6 and IL-8, thus indicating its importance in the inflammatory cascade and a potential target for treatment of neonatal NEC . Although in most instances a mature intestine mounts an appropriate inflammatory response, it is hypothesized that fetal enterocytes are predisposed towards an unbalanced pro-inflammatory response following activation by bacterial ligands. Nanthakumar et al found that in vitro, fetal intestinal cells respond to lipopolysaccharide (LPS) and to IL-Iβ with robust IL-8 production compared with mature enterocytes which have been shown to be hyporesponsive to these common ligands. Organ cultures of small intestine from fetuses display a similar response those from older children or adults. These findings indicate that the inflammatory response of immature neonatal intestine is skewed away from anti-inflammatory protection, and may lead to harmful rather than protective responses (13).
There is minimal evidence from human investigation to confirm the importance of the balanced anti-inflammatory response. A study by Edelson et al evaluated serum levels of cytokines in newborn infants affected with NEC, and found that not only were levels of IL-8 elevated in infants with stage 3 NEC compared with those less severely affected, but they also found that levels of the counter-regulatory cytokines IL-10 and IL-1ra were elevated in the most severely affected infants (14). These data demonstrate that after the acute presentation of disease, systemic anti-inflammatory signaling occurs, at least to some extent. Nonetheless, control of this signaling in the early development of disease and in the local intestinal environment has not been well characterized, and the premature infant may be vulnerable due to the inappropriate regulation of the inflammatory response.
In support of this hypothesis Fituch et al found a lower level of IL-10 in the breast milk from mothers of premature infants who developed NEC compared to those with normal intestinal homeostasis. This study did not find developmental differences in IL-10 breast milk levels, with similar values observed in extremely preterm (23–27 weeks gestation), preterm (32–36 weeks gestation), and term (38–42 weeks gestation) infants. However, in 12 out of 14 women (86%) whose infants developed NEC, the IL-10 level was undetectable, compared with 40% of mothers in the extremely premature group and 27% of mothers in the premature group . In summary, the relationship between pro- and anti-inflammatory cytokine production and signaling in the initiation and propagation of neonatal NEC is complex, and additional information is necessary to better understand this intricate pathophysiology.
Potentially protective factors
If inflammation plays an important role in the pathophysiology of NEC, then regulation of these complex pathways may identify novel preventive approaches. Breast milk confers some protection against NEC, and contains multiple bioactive substances that are not found in commercial infant formulas, and may be the key factors responsible for this epidemiologic benefit. Investigators have identified several growth factors as candidates, such as EGF, HB-EGF, TGF, IGF, and other important antimicrobial and anti-inflammatory molecules including erythropoietin, PUFA, IgA, lactoferrin, and oligosaccharides.
In an in vitro study, Claud et al found that in fetal intestinal epithelial cells, both transforming growth factor-beta (TGF-beta) and erythropoietin decreased tumor necrosis factor-alpha (TNF-alpha)-stimulated IL-8 secretion. Dvorak et al examined the effect of epidermal growth factor (EGF) supplementation in a neonatal rat NEC model, and found that EGF reduced the incidence and severity of NEC, with subsequent studies demonstrating the mechanism to include modulation of TNF and IL-18 synthesis (reference) Supportive evidence was found in premature infants with NEC who had significantly lower salivary and serum levels of EGF compared with age-matched controls (15). Feng et al showed that enteral administration of heparin-binding epidermal growth factor-like growth factor (HB-EGF) reduced the incidence of NEC and intestinal apoptosis in formula fed, hypoxia-stressed neonatal rats. Ledbetter et al found a reduction in NEC in preterm infants receiving recombinant erythropoietin (rEpo) for anemia of prematurity compared with those infants not receiving rEpo (16).
Recent studies have focused on PUFA and probiotics as potential therapies to prevent NEC. Using a neonatal rat model, we found that formula supplementation with PUFA significantly reduced the incidence of NEC and reduced PAF receptor and TLR4 gene expression in intestinal epithelium. Previous studies have shown that PUFA supplementation reduced expression of intestinal phospholipase A2 (PLA2), the rate limiting enzyme in PAF production in this model. Furthermore, Carlson et al demonstrated that in preterm infants, PUFA supplemention decreased the incidence of stage II and III NEC compared with infants fed control formula (2.9% versus 17.6%, p<0.05) (17). Nonetheless, subsequent studies and meta-analyses have failed to confirm this potential effect, and further studies are warranted to clarify the importance of PUFA in NEC.
Probiotics have gained significant recent interest as an approach to reduce the incidence of neonatal NEC, and are discussed in greater detail in a subsequent chapter. Nonetheless, in an animal study, we found that Bifidobacterium infantis prophylaxis in neonatal rats reduced the incidence of NEC compared to controls (18) (Figure 1, bottom panel). Two separate randomized, controlled clinical trials have shown a reduction in the incidence and severity of NEC in neonates fed probiotics enterally, although different probiotic formulations have been used. Hoyos found a reduction in the incidence of NEC in infants fed Lactobacillus acidophilus and Bifidobacterium infantis compared with historical controls (19). Although the mechanisms that confer this possible protection by probiotic therapy are complex, there is substantial evidence that they modulate the intestinal inflammatory response, and these effects may account for the observed beneficial effects on NEC in premature infants.
Conclusions
In conclusion, necrotizing enterocolitis remains a significant cause of morbidity and mortality in neonatal intensive care units despite recent medical advances. As illustrated previously, the pathophysiology of NEC is quite complex and our understanding of this devastating disease is still incomplete. However, we know that following exposure to several risk factors and subsequent up-regulation of PAF production and other mediators, TLRs are activated by bacterial ligands, and an inflammatory cascade ensues that culminates in the production of several pro- and anti-inflammatory cytokines. Within normal physiologic conditions, especially in mature intestinal epithelium, these cytokines are produced and signal in a safe and appropriate balance. Although premature neonates are capable of mounting both pro- and anti-inflammatory responses, they appear to be skewed towards an unbalanced pro-inflammatory pathway. Protective factors such as probiotics or other breast milk components may help to counteract this imbalance, and we may soon have effective preventative and treatment strategies to combat this deadly disease.
Figure 2.
Simplified schematic of TLR signaling. Following binding of ligand to TLR, the adaptor molecule Myd88 is recruited to the receptor complex. IL-1R associated protein kinases (IRAKs) and tumor necrosis factor receptor-associated factor 6 (TRAF6) are next recruited to this complex. IRAK-1 and TRAF6 then bind to transforming growth factor-beta-activated kinase (TAK-1), then the IκB kinase kinase (IKK) complex is activated, which leads to phosphorylation of IκB. IκB is then degraded, and this activates NFκB. NFκB is translocated to the nucleus where it upregulates the production of various inflammatory cytokines.
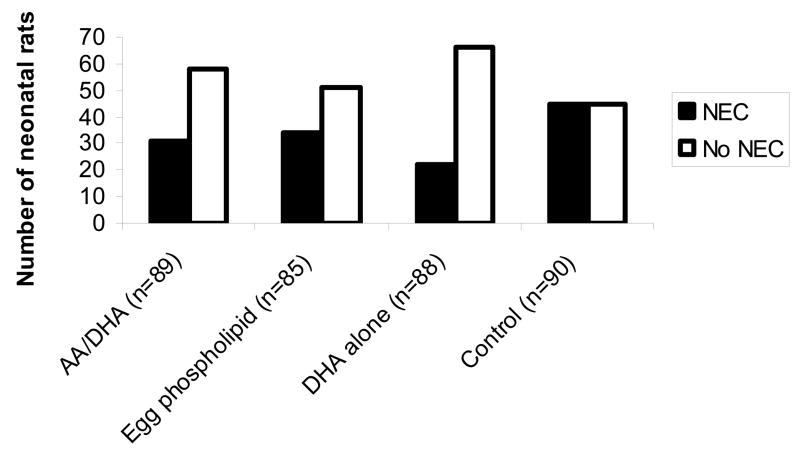
Figure 3.
Formula supplementation with PUFA reduces the incidence of NEC. Using our established neonatal rat model of NEC, we have recently found that PUFA supplementation with AA and DHA, egg phospholipid, or DHA alone all lead to a decreased incidence of NEC compared with controls (p<0.01).
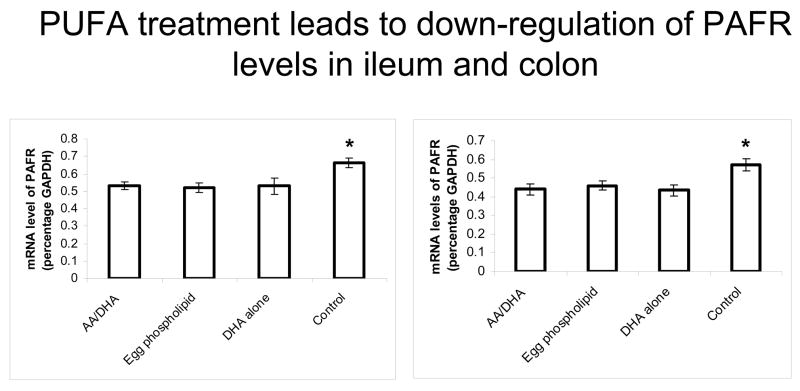
Figure 4.
Formula supplementation with PUFA reduces PAF receptor mRNA expression. Using real-time quantitative RT-PCR, PAFR mRNA levels were measured. We found that within ileum and colon, PUFA supplementation in all groups led to a decrease in PAF receptor expression compared with control group (p<0.05)
Figure 5.
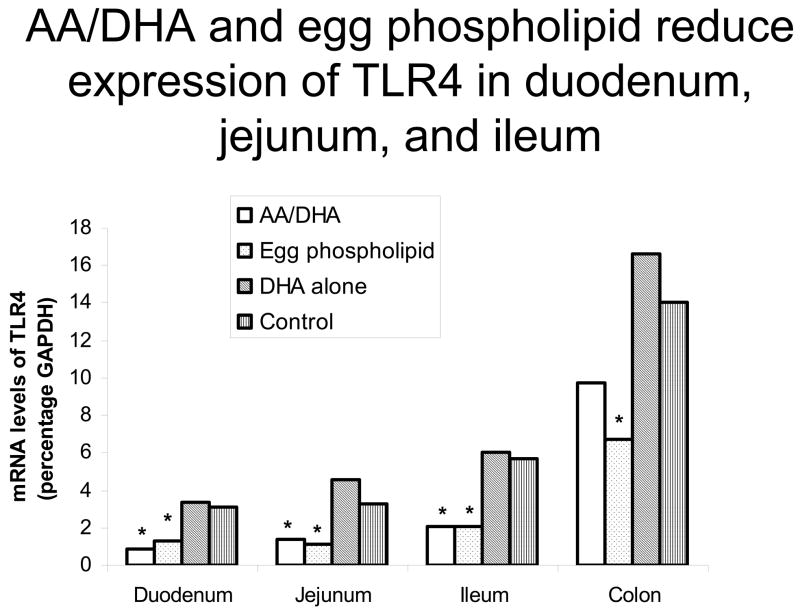
PUFA supplementation reduces TLR4 gene expression in duodenum, jejunum, and ileum. TLR4 mRNA was measured using real-time quantitative RT-PCR. As shown in the figure, in groups supplemented with AA/DHA or egg phospholipid, there was a reduction in TLR4 gene expression in duodenum, jejunum, and ileum compared with controls (p<0.05).
Figure 6.
NEC pathophysiology is a complex series of events leading to a final common pathway of inflammation. This figure demonstrates the proposed pathophysiology of NEC as it is understood currently, as well as potential protective effects of PUFA, probiotics and growth factors. Although much has been elucidated over many years of research, there are still multiple facets of NEC that are incompletely understood.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Sun XM, Hsueh W. Bowel necrosis induced by tumor necrosis factor in rats is mediated by platelet-activating factor. Journal of Clinical Investigation. 1988;81:1328–31. doi: 10.1172/JCI113459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hsueh W, Sun X, Rioja LN, Gonzalez-Crussi F. The role of the complement system in shock and tissue injury induced by tumour necrosis factor and endotoxin. Immunology. 1990;70:309–14. [PMC free article] [PubMed] [Google Scholar]
- 3.Caplan MS, Sun XM, Hseuh W, Hageman JR. Role of platelet activating factor and tumor necrosis factor-alpha in neonatal necrotizing enterocolitis. Journal of Pediatrics. 1990;116:960–4. doi: 10.1016/s0022-3476(05)80661-4. [DOI] [PubMed] [Google Scholar]
- 4.Hammerman C, Goldschmidt D, Caplan MS, Kaplan M, Schimmel MS, Eidelman AI, Branski D, Hochman A. Amelioration of ischemia-reperfusion injury in rat intestine by pentoxifylline-mediated inhibition of xanthine oxidase. J Pediatr Gastroenterol Nutr. 1999;29:69–74. doi: 10.1097/00005176-199907000-00017. [DOI] [PubMed] [Google Scholar]
- 5.Gonzalez-Crussi F, Hsueh W. Experimental model of ischemic bowel necrosis. The role of platelet-activating factor and endotoxin. American Journal of Pathology. 1983;112:127–35. [PMC free article] [PubMed] [Google Scholar]
- 6.Caplan MS, Hedlund E, Adler L, Hsueh W. Role of asphyxia and feeding in a neonatal rat model of necrotizing enterocolitis. Pediatric Pathology. 1994;14:1017–28. doi: 10.3109/15513819409037698. [DOI] [PubMed] [Google Scholar]
- 7.Caplan MS, Lickerman M, Adler L, Dietsch GN, Yu A. The role of recombinant platelet-activating factor acetylhydrolase in a neonatal rat model of necrotizing enterocolitis. Pediatric Research. 1997;42:779–83. doi: 10.1203/00006450-199712000-00010. [DOI] [PubMed] [Google Scholar]
- 8.Moya FR, Eguchi H, Zhao B, Furukawa M, Sfeir J, Osorio M, Ogawa Y, Johnston JM. Platelet-activating factor acetylhydrolase in term and preterm human milk: a preliminary report. Journal of Pediatric Gastroenterology & Nutrition. 1994;19:236–9. doi: 10.1097/00005176-199408000-00015. [DOI] [PubMed] [Google Scholar]
- 9.Lucas A, Cole TJ. Breast milk and neonatal necrotising enterocolitis [see comments] Lancet. 1990;336:1519–23. doi: 10.1016/0140-6736(90)93304-8. [DOI] [PubMed] [Google Scholar]
- 10.Caplan M, Hsueh W, Kelly A, Donovan M. Serum PAF acetylhydrolase increases during neonatal maturation. Prostaglandins. 1990;39:705–14. doi: 10.1016/0090-6980(90)90030-y. [DOI] [PubMed] [Google Scholar]
- 11.Nadler EP, Dickinson E, Knisely A, Zhang XR, Boyle P, Beer-Stolz D, Watkins SC, Ford HR. Expression of inducible nitric oxide synthase and interleukin-12 in experimental necrotizing enterocolitis. J Surg Res. 2000;92:71–7. doi: 10.1006/jsre.2000.5877. [DOI] [PubMed] [Google Scholar]
- 12.Ford H, Watkins S, Reblock K, Rowe M. The role of inflammatory cytokines and nitric oxide in the pathogenesis of necrotizing enterocolitis. Journal of Pediatric Surgery. 1997;32:275–82. doi: 10.1016/s0022-3468(97)90194-9. [DOI] [PubMed] [Google Scholar]
- 13.Nanthakumar NN, Fusunyan RD, Sanderson I, Walker WA. Inflammation in the developing human intestine: A possible pathophysiologic contribution to necrotizing enterocolitis. Proc Natl Acad Sci U S A. 2000;97:6043–8. doi: 10.1073/pnas.97.11.6043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Edelson MB, Bagwell CE, Rozycki HJ. Circulating pro- and counterinflammatory cytokine levels and severity in necrotizing enterocolitis. Pediatrics. 1999;103:766–71. doi: 10.1542/peds.103.4.766. [DOI] [PubMed] [Google Scholar]
- 15.Shin CE, Falcone RA, Jr, Stuart L, Erwin CR, Warner BW. Diminished epidermal growth factor levels in infants with necrotizing enterocolitis. J Pediatr Surg. 2000;35:173–6. 177. doi: 10.1016/s0022-3468(00)90005-8. [DOI] [PubMed] [Google Scholar]
- 16.Ledbetter DJ, Juul SE. Erythropoietin and the incidence of necrotizing enterocolitis in infants with very low birth weight. J Pediatr Surg. 2000;35:178–81. 182. doi: 10.1016/s0022-3468(00)90006-x. [DOI] [PubMed] [Google Scholar]
- 17.Carlson SE, Montalto MB, Ponder DL, Werkman SH, Korones SB. Lower incidence of necrotizing enterocolitis in infants fed a preterm formula with egg phospholipids. Pediatr Res. 1998;44:491–8. doi: 10.1203/00006450-199810000-00005. [DOI] [PubMed] [Google Scholar]
- 18.Caplan MS, Miller-Catchpole R, Kaup S, Russell T, Lickerman M, Amer M, Xiao Y, Thomson R., Jr Bifidobacterial, supplementation reduces the incidence of necrotizing enterocolitis in a neonatal rat model. Gastroenterology. 1999;117:577–83. doi: 10.1016/s0016-5085(99)70450-6. [DOI] [PubMed] [Google Scholar]
- 19.Hoyos AB. Reduced incidence of necrotizing enterocolitis associated with enteral administration of Lactobacillus acidophilus and Bifidobacterium infantis to neonates in an intensive care unit. Int J Infect Dis. 1999;3:197–202. doi: 10.1016/s1201-9712(99)90024-3. [DOI] [PubMed] [Google Scholar]