Abstract
Background
Hepatic de novo lipogenesis (DNL) is markedly stimulated in humans by low-fat diets enriched in simple sugars. However, the dietary responsiveness of the key enzyme controlling DNL in human adipose tissue, fatty acid synthase (FAS), is uncertain.
Hypothesis
Adipose tissue mRNA for FAS is increased in lean and obese subjects when hepatic DNL is elevated by a eucaloric, low-fat, high-sugar diet.
Design
Twelve lean and 7 obese volunteers were given 2 eucaloric diets (10% vs. 30% fat, 75% vs. 55% carbohydrate, sugar/starch 60/40) each for 2 weeks by a random-order, cross-over design. FAS mRNA in abdominal and gluteal adipose tissue was compared to hepatic DNL measured in serum by isotopic and non-isotopic methods. Adipose tissue mRNA for TNF alpha and IL-6, inflammatory cytokines that modulate DNL, were also assayed.
Results
The low-fat, high-sugar diet induced a 4 fold increase in maximum hepatic DNL (P<0.001) but only a 1.3 fold increase in adipose tissue FAS mRNA (P=0.029) and no change in cytokine mRNA. There was a borderline significant positive correlation between changes in FAS mRNA and hepatic DNL (P=0.039). Compared to lean subjects, obese subjects had lower levels of FAS mRNA and higher levels of cytokine mRNA (P<0.001).
Conclusions
The results suggest that key elements of human adipose tissue DNL are less responsive to dietary carbohydrate than is hepatic DNL and may be regulated by diet-independent factors. Irrespective of diet, there is reduced expression of the FAS gene and increased expression of cytokine genes in adipose tissue of obese subjects.
Keywords: lipogenesis, gene expression, VLDL, triacylglycerol, palmitic acid, MIDA
INTRODUCTION
A clear understanding of the metabolic effects of low-fat, high-carbohydrate diets is needed as the public consumption of sugar climbs (1–3). Sugar is implicated as an important nutritional factor in the obesity and diabetes epidemics (2, 3), as well as the metabolic syndrome and atherogenic dyslipidemia (4). Using both stable isotopic and non-isotopic methods, a marked increase in hepatic de novo lipogenesis (DNL) by dietary carbohydrate was demonstrated in vivo from measurements of serum VLDL triacylglycerols (TG) in weight-stable humans (4–9). Lean and obese subjects had similar, large increases in DNL on eucaloric, very low-fat solid food diets enriched in simple sugars, and these increases were not correlated with fasting or 24 h serum insulin, glucagon or glucose concentrations (5). The increases in DNL did not cause weight gain but did positively correlate with increases in serum TG concentrations and the ratio of saturated to unsaturated fatty acids in VLDL-TG- both associated with adverse effects on the cardiovascular system. The importance of the type as well as the amount of dietary carbohydrate was demonstrated by the lack of an increase in hepatic DNL after low-fat diets rich in complex carbohydrates (8, 10).
Although animal studies clearly show that both adipose tissue and liver are major sites of DNL on a high-carbohydrate diet (11–13), the lipogenic responsiveness of human adipose tissue to dietary carbohydrate is uncertain. Previous in vitro studies in adipose tissue of lean subjects overfed carbohydrate showed large increases in DNL (14–16) and mRNA levels of fatty acid synthase (FAS), acetyl CoA carboxylase (ACC1), and sterol response element binding protein 1c (SREBP-1c), a key transcription factor controlling the expression of lipogenic genes (17). In addition, in vivo measurements of hepatic and total body DNL with 13C-acetate labeling and indirect calorimetry in lean subjects after massive intravenous carbohydrate overfeeding suggested that the major site of DNL under these conditions is the adipose tissue, rather than liver (18). In contrast, others have measured little increase in adipose tissue DNL or mRNA levels for lipogenic enzymes in lean subjects after isocaloric or hypercaloric high-carbohydrate diets (19, 20). Less is known about the dietary responsiveness of DNL in adipose tissue from obese subjects. Although several rodent models of obesity show increased DNL in adipose tissue (21), and the development of drugs that inhibit DNL as a treatment for human obesity is underway (22, 23), similar DNL (14, 15, 24, 25) and lower levels of FAS, ACC1, and SREBP-1c mRNA in subcutaneous and visceral adipose tissue have been reported in overweight compared to lean subjects after ad libitum (26, 27) or hypercaloric high-carbohydrate (15, 17) diets.
The objective of the present study was to determine the dietary responsiveness of the gene for the key enzyme controlling rates of DNL, FAS, in adipose tissue from weight-stable lean and obese subjects. We evaluated the effect of the isocaloric substitution of dietary carbohydrate for fat on adipose tissue levels of FAS mRNA and compared it to the large changes in hepatic DNL measured in plasma by an isotopic and nonisotopic method, as previously reported(5). We also determined whether the adipose tissue expression of the FAS gene varied with the expression of the genes for TNF-alpha (TNF) and IL-6, inflammatory cytokines that are produced and secreted by adipose tissue and that suppress the transcription of the FAS gene (28–31).
METHODS AND MATERIALS
Subjects
As previously described (5), 12 lean and 7 obese otherwise healthy volunteers were studied as inpatients at the Rockefeller University Clinical Research Center. The studies were approved by The Rockefeller University Institutional Review Board, and written, informed consent was obtained from each participating subject. To be eligible, the screening fasting LDL cholesterol was <4.14 mmol/L, TG <2.27 mmol/L, and HDL cholesterol >0.91 mmol/L; lipid profiles were similar in lean and obese subjects. Participants were no less than 10% below their maximum weights and were weight-stable within 10% for the previous 6 months. All subjects had normal 3 h oral glucose tolerance tests.
Diets
The details of the diet have been previously reported (5). Two solid food diets that differed in the proportion of carbohydrate and fat were given for 2 wk each according to a random-order, cross-over design. One diet, called the “high-sugar” diet, had 10% of energy as fat, 75% as carbohydrate; the other diet, called the “low-sugar” diet, had 30% of energy as fat, 55% as carbohydrate. Both diets had identical carbohydrate compositions and ratios of sugar to starch (60:40). The total fructose intake (including fructose in sucrose) was 18% energy, or 113 g/10,520 kj, on the high-sugar diet and 13% energy, or 81 g/10,520 kj, on the low-sugar diet. For both diets, the total fiber was 6.5 g/100 g carbohydrate and the soluble to insoluble fiber ratio was 0.25.
The initial total calories were 5,723 kj/m2 and required minimal adjustment across diets to keep weight constant. Each diet had a single-day menu (same food items each day) with 3 meals of similar energy and macronutrient composition. The fatty acid compositions of the 2 diets were identical and matched to each subject’s adipose tissue composition in order to measure fatty acid synthesis by the linoleate dilution method (7).
Real-time RT PCR of Adipose Tissue mRNA
At the end of each dietary period, after a 12 h fast, adipose biopsies were performed at the abdominal and gluteal sites after anaesthetizing each region with 10–20 mL of 1% lidocaine. At least 500 mg of subcutaneous fat was removed through a small incision with multiple passes of a 2.5 mm blunt end liposuction needle attached to a 60 mL syringe partially filled with saline. Within ½ h, the adipose tissue was rinsed with normal saline, blotted dry, weighed, and snap frozen in liquid nitrogen for storage at −70 °C.
RNA was extracted from adipose tissue using RNeasy mini kits as recommended by the manufacturer (Qiagen, Valencia, CA.). 100 mg aliquots were thawed, briefly homogenized in lysis buffer using a Tissue Tearor (Biospec Products, Inc., Bartlesville, OK.), and centrifuged 3 min at room temperature at 8,000 g. Then, 500 µL of the supernatant below the fat layer was removed, diluted with 500 µL of 70% ethanol, and 500 µL passed over an RNeasy silica gel-based mini column that was centrifuged for 15 s at 8,000 g. The eluted solution was discarded and the remaining 500 µL passed over the column, which was centrifuged again and rinsed with RPE and RWI buffers. RNA was eluted from the column by adding 30 µL of RNAase-free water and centrifuging for 1 min at 8,000 g.
The extracted RNA (~5 µg/100 mg tissue) was converted to cDNA in a Gene Amp PCR 9700 thermocycler (Applied Biosystems, Inc., Foster City, CA.) by first denaturing at 70 °C for 10 min in the presence of random hexamers (Invitrogen, Carlsbad, CA.), then chilling for 5 min, leaving at room temperature for 10 min, incubating at 37 °C for 90 min with first strand buffer, dNTP, DTT and reverse transcriptase (Invitrogen), and then denaturing at 95 °C for 5 min. cDNA was amplified using Taqman Master Mix (Applied BioSystems, Inc.), forward and reverse oligonucleotide primers and a Taqman probe. For FAS mRNA, primers and probe were designed from a published gene sequence (GenBank Accession no. U26644.1) using Primer Express, Version 1.0 (Applied Biosystems, Inc.):
Forward sequence 5’ACTTGCAGGAGTTCTGGGACAA 3’, Reverse sequence 5’GAAGGAGGCATCAAACCTAGACA 3’, and probe sequence 5’CGTAGAGCCCAGCCTTCCAGCGA3’. The probe was labeled at the 5’ end with the reporter dye, 6-carboxy-fluorescein (FAM), and at the 3’ end with the quencher, 6-carboxy-tetramethyl-rhodamine (TAMRA). Oligonucleotide primers and Taqman probes for human TNF, IL-6 and beta-actin mRNA were purchased from Applied Biosystems. For each assay, beta-actin mRNA was amplified separately and simultaneously with the target gene(s) to normalize for total RNA. The beta-actin signal per 100 mg adipose tissue was similar irrespective of obesity, anatomical site, and diet.
Real time RT-PCR was carried out in triplicate for each sample on an Applied Biosystems, Inc. 7700 (FAS) or 7900 (TNF, IL-6) sequence detection system. Each reaction well contained cDNA, Taqman PCR Master Mix, 300 nmol/L of each forward and reverse primer, and 100 nmol/L of Taqman probe. Cycling parameters were 50 °C for 2 min, 95 °C for 10 min, then 40 cycles of 95 °C for 15 sec and 60 °C for 1 min. For every run, serial dilutions of a stock cDNA sample from human adipose tissue was used to create standard curves for each gene by plotting Ct (threshold cycle number at which the fluorescence signal is linearly increasing above background) versus log cDNA. The Ct readings for the unknown samples were used to calculate the amount of each target gene relative to beta actin. Non-template controls without cDNA never showed significant DNA contamination.
In Vivo Measurements of Fatty Acid Synthesis
Two independent methods were used simultaneously to measure DNL in vivo: the linoleate dilution (LD) method and the mass isotopomer distribution analysis (MIDA) technique, both described in detail elsewhere (7, 32, 33). Using the LD method, the fraction of total synthesized fatty acids in VLDL-TG was calculated from the decrease, or dilution, of linoleate relative to the concentration in the diet and adipose tissue (made to be identical) by synthesized fatty acids. Using the MIDA method, the fraction of synthesized palmitate in VLDL-TG was measured after the intravenous infusion of [1-13C] acetate by calculating the 13C enrichment of the biosynthetic true precursor pool (hepatic acetyl-CoA), based on the ratio of double- to single-labeled mass isotopomers in VLDL-TG palmitate. The intravenous infusion of sodium [1-13C] acetate (Cambridge Isotope Laboratories, Andover, MA.) was at 4.5 mmol/h, beginning 15 h before breakfast and continuing in most subjects for at least an additional 24 h until the following morning, for a total of 39 h, in order to allow newly labeled palmitate in VLDL-TG and hepatic TG storage pools to reach plateau values (7, 32, 33).
As previously described, at the end of each diet period, blood was sampled at 8a before the 13C-acetate infusion and beginning the morning after the start of the infusion, at 9a, 3p, 9p, 12a, 3a, and 9a. Meals were provided at 9a, 1p, and 5p. Chylomicrons were removed and VLDL was isolated by density gradient ultracentrifugation. The TG were isolated after chloroform:methanol extraction, separated from other lipids by thin layer chromatography, and the fatty acid composition of TG was analyzed by capillary gas chromatography (Hewlett Packard model 5890, Palo Alto, CA.). The fatty acid compositions of the diets and subcutaneous adipose tissue sampled from abdominal and gluteal sites were similarly analyzed. The 13C enrichment of palmitate from VLDL-TG was measured by gas chromatography/mass spectrometry as described (33). Fasting and maximum fed values of DNL were used for statistical analysis.
Analysis of Serum Glucose, Insulin, Glucagon, and Cytokines
At the end of each 2 wk diet period, serum concentrations of glucose, insulin and glucagon were measured at 0, 0.5, 1, 1.5, 2, 3 h after breakfast, then every 2–3 h until the following morning. Glucose was measured by a glucose oxidase assay and insulin was analyzed by EIA (Abbott Laboratories, Santa Clara, CA.). Glucagon was measured in duplicate by double antibody radioimmunoassay of EDTA/aprotinin treated plasma (Diagnostic Products Corp., Los Angeles, CA.). Serum concentrations of IL-6, TNF, and high sensitivity C reactive protein (hsCRP) were measured with chemiluminescent EIA on the Immulite 2000 immunoassay system (Diagnostic Products Corp.) in fasting samples taken just before the adipose biopsy. The intra-sample coefficient of variation was less than 6% for IL-6, TNF and hsCRP. The serum IL-6 results below the detection limit of 0.14 IU/mL were assigned the value of “0.07” (i.e., half-way between 0 and 0.14).
Statistical Methods
Three way analysis of variance (ANOVA) was used to identify factors contributing to the ratio of adipose tissue target mRNA (FAS, TNF or IL-6) to beta-actin mRNA. The dependent variable was the logarithm of the ratio (arbitrary units), fixed factors as diet (high-sugar and low-sugar), site (abdomen, gluteus), and group (lean and obese), and random factor as subjects within a group. Pearson correlation analysis and simple linear regression were used to assess relationships among continuous variables; because of skewing of the distribution, the logarithim of hsCRP was tested. Scatter plots were drawn to check for nonlinear relationships and outliers. To compare the direction of change between diets for FAS mRNA and hepatic DNL, the asymptotic, two-sided sign test for continuous variables was used (34). There was no evidence for period or carry-over effects. Data analysis was performed using Excel XP/2000 (Microsoft, Inc. Redmond, WA), SigmaStat 3.00 (SPSS, Inc., Chicago, IL) and S-PLUS 7 (Insightful, Inc. Seattle, WA) statistical software. Data is shown as mean ± SD except in Figure 3, where SEM is displayed. P values <0.01 were considered statistically significant.
Figure 3.
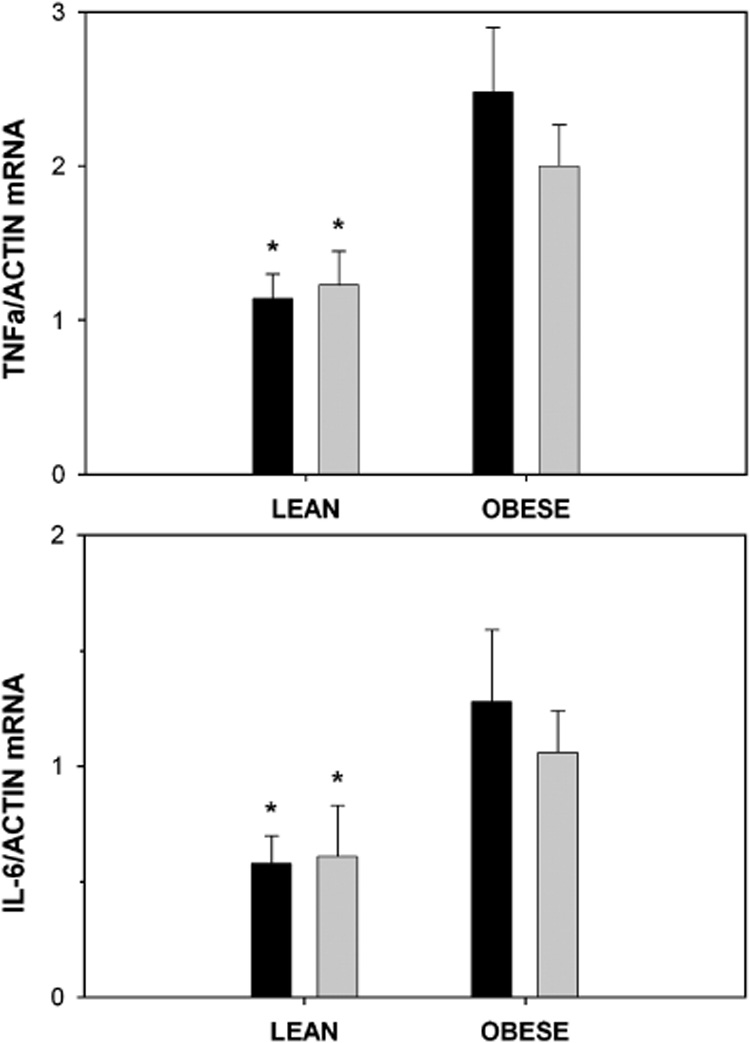
Ratios of mRNA for TNF-alpha to actin (top panel) and IL-6 to actin (bottom panel) in adipose tissue from lean (n=12) and obese (n=7) subjects after a high-sugar diet (dark bar) or low-sugar diet (light bar). Mean ± SEM. 3-way ANOVA: obese > lean for both cytokines (TNF, P=0.004; IL-6, P=0.008, shown by asterisk). High-sugar diet not different from low-sugar diet (P=0.72 and 0.88 for TNF and IL-6, respectively). The values did not differ by site; thus the average of the abdominal and gluteal values per subject were used. There were no significant interactions.
RESULTS
Effect of Diet
Table 1 shows the subject characteristics and average effects of a substitution of dietary carbohydrate enriched in sugar for fat on markers of DNL in the adipose tissue and liver of lean and obese subjects. Obese subjects were similar in age and sex compared to lean subjects but had significantly higher waist-to-hip circumference ratios. Figure 1 shows the effects in individual subjects. The high-sugar diet marginally increased the expression of the FAS gene 1.3 fold in adipose tissue of lean and obese subjects (P=0.029 by ANOVA). In contrast, as we reported previously (5), the same high-sugar diet dramatically increased hepatic DNL to an extent that was not significantly different in lean and obese subjects. By the LD method, the maximum % DNL after meals was 4 fold higher on the high-sugar compared to the low-sugar diet (41 ± 13 vs. 10 ± 11% , P<0.001, between diets). All subjects had higher %DNL on the high-sugar compared to the low-sugar diet. The MIDA method gave qualitatively similar results that were highly correlated with the results by the LD method (R2=0.63, P<0.001).
Table 1.
Changes in adipose tissue, liver and plasma in response to dietary carbohydrate in lean and obese subjects.
| LEAN | OBESE | |||
|---|---|---|---|---|
| Sex | 6 F, 6 M | 4 F, 3 M | ||
| Age (yr) | 31 (18–62) | 42 (22–61) | ||
| Body Mass Index (kg/m2) | 23 (20–27) | 34 (31–38)* | ||
| Waist/Hip circumference | 0.87 (0.76–1.02) | 0.99 (0.80–1.10)* | ||
| High-Sugar | Low-Sugar | High Sugar | Low-Sugar | |
| Adipose Tissue (fasting) | ||||
| ABD mRNA FAS/ACTIN | 2.13 ± 1.21 | 1.57 ± 0.78 | 0.81 ± 0.45* | 0.53 ± 0.23* |
| GLT mRNA FAS/ACTIN | 1.58 ± 0.73 | 1.23 ± 0.61 | 0.56 ± 0.22* | 0.47 ± 0.20* |
| Liver (fed) | ||||
| Max % DNL, LD method | 43 ± 13 | 12 ± 13 ** | 37 ± 15 | 6 ± 6** |
| Max % DNL, MIDA method | 49 ± 7 | 28 ± 12** | 36 ± 10 | 24 ± 10** |
| Liver (fasting) | ||||
| % DNL, LD method | 31 ± 22 | 1 ± 3** | 21 ± 21 | 1 ± 2** |
| % DNL, MIDA method | 37 ± 15 | 19 ± 13** | 27 ± 7 | 16 ± 9** |
| Plasma (fasting) | ||||
| Triacylglycerol (mmol/L) | 1.58 ± 0.59 | 1.02 ± 0.37** | 2.08 ± 1.15 | 1.28 ± 0.43** |
| Glucose (mmol/L) | 4.7 ± 0.3 | 4.6 ± 0.3 | 4.7 ± 0.3 | 4.8 ± 0.2 |
| Insulin (microunits/mL) | 6 ± 2 | 6 ± 1 | 13 ± 6* | 10 ± 2* |
| Glucagon (pmol/mL) | 71 ± 18 | 69 ± 20 | 56 ± 8 | 56 ± 10 |
P<0.01, lean vs. obese
P<0.01, high sugar vs. low sugar diet
Figure 1.
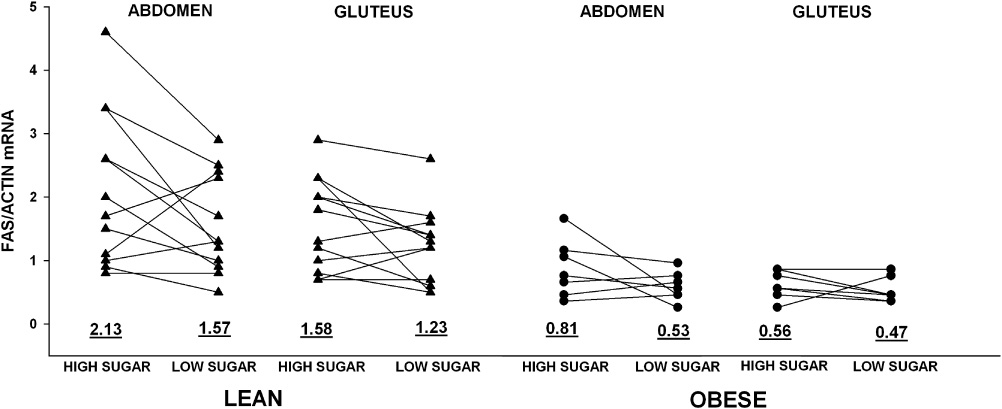
Ratios of mRNA for fatty acid synthase to actin in abdominal and gluteal adipose tissues from lean (n=12) and obese (n=7) subjects after a high-sugar or low-sugar diet. Mean values given below the individual results. 3-way ANOVA: lean > obese, P<0.001; abdomen > gluteus P=0.005; high-sugar > low-sugar diet, P=0.029. There were no significant interactions.
In Figure 2, the diet-induced changes in FAS mRNA, expressed as the mean of the abdominal and gluteal values, are plotted against the changes in maximum % hepatic DNL by the LD (left panel) and MIDA (right panel) methods. The responses of each subject on the two diets are connected. With both methods, in 12 subjects, both FAS mRNA and hepatic DNL increased on the high-sugar compared to the low-sugar diet, but in 5 subjects, FAS mRNA decreased or did not change when hepatic DNL increased. The sign test for the direction of change between diets for the LD and MIDA methods yielded borderline positive trends (P values of 0.039 and 0.066, respectively). On the high-sugar diet, there was a significant positive correlation between mean FAS mRNA and maximum hepatic DNL by MIDA (R2= 0.36, P=0.007), but not by LD (R2= 0.06, P=0.301). This relationship was less strong but in a similar direction for hepatic DNL measured after an overnight fast by either method (data not shown).
Figure 2.
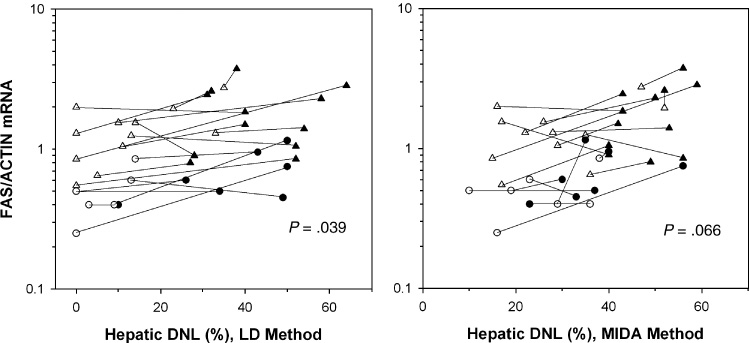
Mean values for FAS/Actin mRNA in abdominal and gluteal adipose tissue are plotted against hepatic DNL measured by LD (left panel) and MIDA (right panel). 12 lean, 7 obese subjects; black= high-sugar diet; white=low-sugar diet; lean=triangles, obese=circles. The responses to the 2 diets per subject are connected. For each plot, the positive relationship between the change in markers of DNL in adipose tissue and liver is of borderline statistical significance (sign test for continuous variables).
Similar to the minimal effect of dietary carbohydrate on FAS mRNA, there was no statistically significant effect of diet on adipose tissue mRNA levels of TNF and IL-6 (Figure 3). Serum levels of TNF, IL-6 and hsCRP were not also affected by diet (data not shown).
Effect of Anatomical Site
Subcutaneous adipose tissue at the abdominal site has a higher percentage of palmitate than the gluteal site (35) and may synthesize more palmitate in response to dietary carbohydrate. Adipose tissue levels of FAS mRNA were slightly but significantly higher at abdominal compared to gluteal sites on both diets (Figure 1). For both diets, the levels of FAS mRNA in abdominal and gluteal sites were strongly positively correlated with each other (R2=0.72, P<0.001). There were no significant site-specific differences in the expressions of the genes for TNF and IL-6.
Effect of Obesity
Adipose tissue levels of FAS mRNA were significantly reduced 2–3 fold in obese compared to lean subjects. This was true on both diets at both abdominal and gluteal sites (Figure 1).
In contrast to the reduced FAS gene expression, expressions of the genes for TNF and IL-6 were significantly elevated in obese compared to lean adipose tissue (Figure 3). Adipose tissue mRNA levels of TNF and IL-6 were strongly correlated with each other. Serum concentrations of hsCRP (but not serum TNF and IL-6) were higher in obese compared to lean subjects (high-sugar diet- 8.4 ± 6.3 vs 1.3 ± 1.6 IU/L, P=0.02; low-sugar diet- 7.0 ± 5.3 vs 0.8 ± 0.7 IU/L, P=0.02) and were significantly positively correlated with BMI, W/H and serum insulin (data not shown). Linear regression analysis, however, did not show significant inverse relations between FAS mRNA and serum hsCRP or serum or mRNA levels of TNF and IL-6. Hepatic DNL and serum triglycerides did not vary with these markers of inflammation.
Relationships with Serum Insulin, Glucose and Glucagon
Other mediators known to directly modulate the expression of the FAS gene are insulin, glucose and glucagon. Fasting levels of insulin, glucose and glucagon on each diet in lean and obese subjects are shown in Table 1. As previously reported (5), meal-stimulated and 24 hour, but not fasting, serum concentrations of insulin were significantly higher on the high-sugar compared to the low-sugar diet. Compared to lean subjects, obese subjects had significantly higher fasting, meal-stimulated and 24 h serum concentrations of insulin, reflecting mild insulin resistance. Glucose levels were higher and glucagon levels lower in the obese compared to the lean (P=0.03 for both comparisons), but were not different between diets. However, as previously reported for hepatic DNL (5), linear regression analysis did not show a significant relation between FAS mRNA and fasting, meal-stimulated or 24 h serum insulin, glucose or glucagon concentrations.
DISCUSSION
It has been established that an increase in dietary carbohydrate can dramatically increase DNL in the human liver, but the dietary responsiveness of DNL in human adipose tissue is more controversial. In our study, a eucaloric, very low-fat, high-carbohydrate diet enriched in sugar markedly induced DNL in the liver but had a blunted effect on the expression of FAS gene in adipose tissue of lean and obese subjects. Thus, in both lean and obese subjects, there are likely large, tissue-specific differences in DNL in response to dietary carbohydrate. The borderline positive relationship between hepatic DNL and levels of FAS mRNA in adipose tissue suggests that DNL is to some extent coordinated in human adipose tissue and liver, but is modified in adipose tissue by factors other than dietary carbohydrate. The large difference in the expression of the gene for FAS in adipose tissue from lean and obese subjects regardless of diet also supports the existence of important diet-independent mediators.
Table 2 summarizes the conflicting literature of studies that measured changes in markers of DNL in adipose tissue after changes in dietary carbohydrate. The results are difficult to compare since the studies vary in the duration and mode of feeding, absolute amount and quality of carbohydrate, and markers of adipose tissue DNL. The only previous study that simultaneously measured the effect of an isocaloric substitution of dietary carbohydrate for fat on DNL in the liver and FAS mRNA in adipose tissue was performed in lean volunteers who consumed two diets each for 21 days as outpatients (20). The authors concluded that dietary carbohydrate stimulated an increase in DNL in liver but not in adipose tissue. The high-carbohydrate diet in our study was lower in fat, higher in carbohydrate and higher in the ratio of sugar to starch, and stimulated hepatic DNL to a greater extent (41 vs. 13%). Even after more marked stimulation of DNL in the liver, our results support their findings that adipose tissue is less diet-responsive than liver in lean subjects, and, for the first time, extend the finding to obese subjects.
Table 2.
Review of the literature: Response of adipose tissue DNL to dietary carbohydrate
| Reference | Subjects | Design | Diets | Method | Results |
|---|---|---|---|---|---|
| Current Study | 12 lean, 7 obese, M & F | 14 days, random order, cross-over, inpt | Isocaloric high vs. low carb meals (10% fat, 75% carb vs. 30% fat, 55% carb, both 60/40 sugar/starch) | ABD and GLT tissue mRNA | Borderline 1.3 fold incr FAS mRNA (fasting) in lean and obese at both sites |
| Hepatic DNL by 13C-acetate/MIDA and linoleate dilution methods | 4 fold incr in hepatic DNL in lean and obese (~40% DNL in fed VLDL TG by both methods) | ||||
| Indirect calorimetry | No net total DNL | ||||
| No Change | |||||
| Letexier, 2003 (20) | 5 lean M & F | 21 days, random order, cross-over, 4 mo washout, outpt | Isocaloric high carb vs. low carb meals (30% fat, 55% carb vs. 45% fat, 40% carb, both 40/60 sugar/starch) | ABD tissue mRNA | No change in FAS mRNA (fasting) |
| Hepatic DNL by 2H2O | 2 fold incr in hepatic DNL (12.9% DNL in TG) | ||||
| Diraison, 2003 (19) | 10 lean M&F | 14 days, single order, parallel (5/group), outpt | Ad lib diet, then hypercaloric meals (35% fat, 50% carb then 23% fat, 61% carb, both 40/60 sugar/starch) | ABD tissue mRNA | No change in FAS mRNA (fasting) |
| ABD tissue DNL by 2H2O | No change in ABD tissue DNL | ||||
| Hepatic DNL by 2H2O | 3 fold incr in hepatic DNL (10.6% DNL in TG) | ||||
| Indirect calorimetry | Low net total (and adipose tissue) DNL (1.5 g/d) | ||||
| Increase | |||||
| Minehira, 2004 (17) | 11 lean, 8 overweight M & F | 4 days, random order, cross-over, 2–4 wk washout, outpt | Hypercaloric, high carb vs. isocaloric, low carb meals (20% fat, 70% carb, 60/40 sugar/starch vs. 35% fat, 50% carb, 40/60 sugar/starch) then oral glucose over 5 h | GLT tissue mRNA | ~1.8 fold incr FAS mRNA (fed) in lean and overweight. |
| Indirect calorimetry | 4.5 fold incr net total DNL in lean, 1.3 fold in overweight | ||||
| Chascione, 1987 (16) | 6 lean M & F, under-nourished | 6–10 days, single order, cross-over, inpt | Hypocaloric, low carb TPN IV (2 days), then hypercaloric glucose IV | ABD, GLT or thorax tissue incubation with 14C glucose + insulin | 79 fold incr DNL (fed, geometric mean) |
| Indirect calorimetry | estimated low % of net total body DNL (~7%) | ||||
| Sjostrom, 1973 (14) | 3 lean, 9 obese F | 23 days, single order, cross-over, outpt | Ad lib, then hypocaloric, high-carb formula meals (1% fat, 90% glucose) | ABD tissue incubation with 14C glucose + insulin | 11 fold incr DNL (fasting) in lean and obese |
| Subcellular incubation with 14C -acetate and -citrate | 3 fold incr DNL (fasting) in lean and obese | ||||
| Sjostrom, 1973 (15) | 6 obese F | 6 days, single order cross-over, inpt | Ad lib, then hypercaloric, high carb formula meals plus insulin (0.1% fat, 90% carb, mostly glucose) | ABD tissue incubation with 14C glucose + insulin | 9 fold incr DNL (NS, variable, fed=fasting) |
| Subcellular incubation with 14C citrate | 3 fold incr DNL (fed=fasting) |
In both adipose tissue and liver, changes in DNL are thought to occur mainly after changes in the transcription rate of the gene for FAS via a variety of mediators. We evaluated the potential autocrine, paracrine and endocrine effects of inflammatory cytokines in the adipose tissue and serum. In vitro and in obese animal models, TNF and IL-6, secreted locally and into the circulation by adipocytes and macrophages that infiltrate adipose tissue depots (28, 36), stimulate lipolysis but inhibit DNL directly (28–31) or indirectly by decreasing insulin sensitivity and glucose transport (37–39). DNL may also be inhibited by leptin, another cytokine-like hormone elevated in obese adipose tissue, directly or after up-regulation of inflammatory cytokines (40, 41). In addition, recent epidemiological findings suggest that an increase in the dietary glycemic load may increase serum or adipose tissue markers of inflammation and contribute to dyslipidemia and insulin resistance (42). In the current study, the high levels of cytokine mRNA and low levels of FAS mRNA in the adipose tissue of obese compared to lean subjects irrespective of diet hint at a diet-independent role for inflammatory cytokines in the suppression of FAS in obese adipose tissue. However, an accurate measurement of the activity (43) as well as mRNA levels of FAS and other lipogenic enzymes in small amounts of adipose tissue of more subjects is required to better understand the regulation of DNL by cytokines.
An increase in dietary carbohydrate and glycemic load are known to produce an increase in the serum levels of insulin and glucose and, in turn, could directly increase the transcription of the FAS gene and rates of DNL (44–46). The increases in serum insulin and glucose in response to dietary carbohydrate are exaggerated in the obese but could increase or decrease FAS in adipose tissue depending on the tissue sensitivity to these mediators. However, as was true for hepatic DNL, we did not find clear evidence for insulin- or glucose-mediated changes in the expression of the FAS gene in adipose tissue. A relationship between FAS mRNA and serum glucagon, a direct inhibitor, was also not apparent. It is possible that different results may be obtained in more insulin-resistant obese subjects with higher insulin levels.
The low levels of FAS mRNA in obese compared to lean subjects are consistent with other reports (20, 26, 27) and may be an adaptation to the higher TG turnover, adipocyte lipid accumulation and total fat stores. Adult obese rodents also have tissue-specific differences in DNL, with high activity in the adipose tissue of young obese rodents (21, 47) that then decline with age to low levels (21, 40, 47, 48), but remain high in the liver irrespective of diet (49). Higher adipose tissue DNL has also been found in human infants and children compared to adults on ad libitum diets (47). The results of this study were obtained after weight maintenance in adults and do not exclude the possibility of significant carbohydrate-induced adipose tissue DNL during weight gain. As shown in Table 2, 3 of the 4 hypercaloric, high carbohydrate feeding regimens significantly increased markers of adipose tissue DNL, albeit to a similar or lower extent in obese compared to lean subjects. Whether less extreme manipulations in dietary carbohydrate and sugar result in an increase in adipose tissue DNL that contributes to obesity requires further study.
In conclusion, very low-fat, high-sugar diets that maintain body weight increase hepatic DNL and serum concentrations of TG but have a slight effect on the gene expression of FAS, and presumably DNL, in lean and obese human adipose tissue. Further studies are required to determine the importance of carbohydrate-induced DNL in the lipid homeostasis of the adipocyte and to define the mediators that regulate the lipogenic pathway in this key tissue.
ACKNOWLEDGEMENTS
The excellent technical assistance of The Rogosin Institute Iris and B. Gerald Cantor Clinical Research Laboratory is much appreciated. We also thank the nursing and dietary staff of the Rockefeller University Hospital GCRC.
This work was supported in part by a General Clinical Research Center grant (M01-RR00102) P30DK26687 and DK40995 from the National Institutes of Health, a Clinical Research Award from the American Diabetes Association (to L. Hudgins), a Grant-in-Aid from the American Heart Association (to L. Hudgins), and a grant from the Nora Eccles Treadwell Foundation (to M. Hellerstein).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Gibney M, Sigman-Grant M, Stanton JL, Keast DR. Consumption of sugars. Am J Clin Nutr. 1995;62 suppl:178S–194S. doi: 10.1093/ajcn/62.1.178S. [DOI] [PubMed] [Google Scholar]
- 2.Schulze MB, Manson JE, Ludwig D, Colditz GA, Stampfer MJ, Willett WC, Hu FB. Sugar-sweetened beverages, weight gain, and incidence of type 2 diabetes in young and middle-aged women. JAMA. 2004;292:927–934. doi: 10.1001/jama.292.8.927. [DOI] [PubMed] [Google Scholar]
- 3.Gross LS, Li L, Ford ES, Liu S. Increased consumption of refined carbohydrates and the epidemic of type 2 diabetes in the United States: an ecologic assessment. Am. J. Clin. Nutr. 2004;79:774–779. doi: 10.1093/ajcn/79.5.774. [DOI] [PubMed] [Google Scholar]
- 4.Parks EJ, Hellerstein MK. Carbohydrate-induced hypertriacylglycerolemia: historical perspective and review of biological mechanisms. Am J Clin Nutr. 2000;71:412–433. doi: 10.1093/ajcn/71.2.412. [DOI] [PubMed] [Google Scholar]
- 5.Hudgins LC, Hellerstein MK, Seidman CE, Neese RA, Tremaroli JD, Hirsch J. Relationship between carbohydrate-induced hypertriglyceridemia and fatty acid synthesis in lean and obese subjects. J Lipid Res. 2000;41:595–604. [PubMed] [Google Scholar]
- 6.Hudgins LC. Effect of high-carbohydrate feeding on triglyceride and saturated fatty acid synthesis. PSEBM. 2000;225:178–183. doi: 10.1046/j.1525-1373.2000.22521.x. [DOI] [PubMed] [Google Scholar]
- 7.Hudgins LC, Hellerstein M, Seidman C, Neese R, Diakun J, Hirsch J. Human fatty acid synthesis is stimulated by a eucaloric low fat, high carbohydrate diet. J Clin Invest. 1996;97:2081–2091. doi: 10.1172/JCI118645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hudgins LC, Seidman CE, Diakun J, Hirsch J. Human fatty acid synthesis is reduced after the substitution of dietary starch for sugar. Am J Clin Nutr. 1998;67:631–639. doi: 10.1093/ajcn/67.4.631. [DOI] [PubMed] [Google Scholar]
- 9.Schwartz JM, Linfoot P, Dare D, Aghajanian K. Hepatic de novo lipogenesis in normoinsulinemic and hyperinsulinemic subjects consuming high-fat, low-carbohydrate and low-fat, high-carbohydrate isoenergetic diets. Am. J. Clin. Nutr. 2003;77:43–50. doi: 10.1093/ajcn/77.1.43. [DOI] [PubMed] [Google Scholar]
- 10.Parks EJ, Krauss RM, Christiansen MP, Neese RA, Hellerstein MK. Effects of low-fat, high-carbohydrate diet on VLDL-triglcyeride assembly, production, and clearance. J Clin Invest. 1999;104:1087–1096. doi: 10.1172/JCI6572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Girard J, Perdereau D, Foufelle F, Prip-Buus C, Ferre P. Regulation of lipogenic enzyme gene expression by nutrients and hormones(review) FASEB J. 1994;8:36–42. doi: 10.1096/fasebj.8.1.7905448. [DOI] [PubMed] [Google Scholar]
- 12.Kim JB, Sarraf P, Wright M, Yao KM, Mueller E, Solanes G, Lowell BB, Spiegelman BM. Nutritional and insulin regulation of fatty acid synthetase and leptin genes expression through ADD1/SREBP1. J Clin Invest. 1998;101:1–9. doi: 10.1172/JCI1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fukuda H, Iritani N, Sugimoto T, Ikeda H. Transcriptional regulation of fatty acid synthase gene by insulin/glucose, polyunsaturated fatty acid and leptin in hepatocytes and adipocytes in normal and genetically obese rats. Eur J Biochem. 1999;260:505–511. doi: 10.1046/j.1432-1327.1999.00183.x. [DOI] [PubMed] [Google Scholar]
- 14.Sjostrom L. Carbohydrate-stimulated fatty acid synthesis de novo in human adipose tissue of different cellular types. Acta Med Scand. 1973;194:387–404. doi: 10.1111/j.0954-6820.1973.tb19464.x. [DOI] [PubMed] [Google Scholar]
- 15.Sjostrom L. Fatty acid synthesis de novo in adipose tissue from obese subjects on hypercaloric high carbohydrate diet. Scand. J. Clin. Invest. 1973;32:339–349. doi: 10.3109/00365517309084357. [DOI] [PubMed] [Google Scholar]
- 16.Chascione C, Elwyn DH, Davila M, Gil KM, Askanazi J, Kinney JM. Effects of carbohydrate intake on de novo lipogenesis in human adipose tissue. Am J Physiol. 1987;253:E664–E669. doi: 10.1152/ajpendo.1987.253.6.E664. [DOI] [PubMed] [Google Scholar]
- 17.Minehira K, Vega N, Vidal H, Acheson K, Tappy L. Effect of carbohydrate overfeeding on whole body macronutrient metabolism and expression of lipogenic enzymes in adipose tissue of lean and overweight humans. Int J Obes. 2004;28:1291–1298. doi: 10.1038/sj.ijo.0802760. [DOI] [PubMed] [Google Scholar]
- 18.Aarsland A, Chinkes D, Wolfe RR. Hepatic and whole-body fat synthesis in humans during carbohydrate overfeeding. Am J Clin Nutr. 1997;65:1774–1782. doi: 10.1093/ajcn/65.6.1774. [DOI] [PubMed] [Google Scholar]
- 19.Diraison F, Yankah V, Letexier D, Dusserre E, Jones P, Beylot M. Differences in the regulation of adipose tissue and liver lipogenesis by carbohydrates in humans. J. Lipid Res. 2003;44:846–853. doi: 10.1194/jlr.M200461-JLR200. [DOI] [PubMed] [Google Scholar]
- 20.Letexier D, Pinteur C, Large V, Frering V, Beylot M. Comparison of the expression and activity of the lipogenic pathway in human and rat adipose tissue. J Lipid Res. 2003;44:2127–2134. doi: 10.1194/jlr.M300235-JLR200. [DOI] [PubMed] [Google Scholar]
- 21.Guichard C, Dugail I, Liepvre XL, Lavau M. Genetic regulation of fatty acid synthetase expression in adipose tissue: overtranscription of the gene in genetically obese rats. J of Lipid Research. 1992;33:679–687. [PubMed] [Google Scholar]
- 22.Hirsch J. The search for new ways to treat obesity. PNAS. 2002;99:9096–9097. doi: 10.1073/pnas.152327099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Thupari JN, Landree LE, Ronnett GV, Kuhajda FP. C75 increases peripheral energy utilization and fatty acid oxidation in diet-induced obesity. PNAS. 2002;99:9498–9502. doi: 10.1073/pnas.132128899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hirsch J, Goldrick RB. Serial studies on the metabolism of human adipose tissue. I. Lipogenesis and free fatty acid uptake and release in small aspirated samples of subcutaneous fat. J Clin Invest. 1964;43:1776–1792. doi: 10.1172/JCI105052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Angel A, Bray GA. Synthesis of fatty acids and cholesterol by liver, adipose tissue and intestinal mucosa from obese and control patients. Eur J of Clin Invest. 1979;9:355–362. doi: 10.1111/j.1365-2362.1979.tb00896.x. [DOI] [PubMed] [Google Scholar]
- 26.Diraison F, Dusserre E, Vidal H, Sothier M, Beylot M. Increased hepatic lipogenesis but decreased expression of lipogenic gene in adipose tissue in human obesity. Am. J. Physiol. Endocrinol. Metab. 2002;282:E46–E51. doi: 10.1152/ajpendo.2002.282.1.E46. [DOI] [PubMed] [Google Scholar]
- 27.Ducluzeau PH, Perretti N, Laville M, Andreelli F, Vega N, Riou JP, Vidal H. Regulation by insulin of gene expression in human skeletal muscle and adipose tissue - Evidence for specific defects in type 2 diabetes. Diabetes. 2001;50:1134–1142. doi: 10.2337/diabetes.50.5.1134. [DOI] [PubMed] [Google Scholar]
- 28.Kershaw EE, Flier JS. Adipose tissue as an endocrine organ. J Clin Endocrinol Metab. 2004;89:2548–2556. doi: 10.1210/jc.2004-0395. [DOI] [PubMed] [Google Scholar]
- 29.Ruan H, Hacohen N, Golub TR, Parijs LV, Lodish HF. Tumor necrosis factor-alpha suppresses adipocyte-specific genes and activates expression of preadipocyte genes in 3T3-L1 adipocytes. Diabetes. 2002;51:1319–1336. doi: 10.2337/diabetes.51.5.1319. [DOI] [PubMed] [Google Scholar]
- 30.Ruan H, Miles PDG, Ladd CM, Ross K, Golub TR, Olefsky JM, Lodish HF. Profiling gene transcription in vivo reveals adipose tissue as an immediate target of tumor necrosis factor-alpha. Diabetes. 2002;51:3176–3188. doi: 10.2337/diabetes.51.11.3176. [DOI] [PubMed] [Google Scholar]
- 31.Doerrier W, Feingold KR, Grunfeld C. Cytokines induce catabolic effects in cultured adipocytes by multiple mechanisms. Cytokine. 1994;6:478–484. doi: 10.1016/1043-4666(94)90074-4. [DOI] [PubMed] [Google Scholar]
- 32.Hellerstein MK. De novo lipogenesis in humans: metabolic and regulatory aspects. Eur J Clin Nutr. 1999;53:S53–S65. doi: 10.1038/sj.ejcn.1600744. [DOI] [PubMed] [Google Scholar]
- 33.Hellerstein MK. Regulation of hepatic de novo lipogenesis in humans. Annu Rev Nutr. 1996;16:523–557. doi: 10.1146/annurev.nu.16.070196.002515. [DOI] [PubMed] [Google Scholar]
- 34.Wittkowski KM. Versions of the sign test in the presence of ties. Biometrics. 1998;54:789–791. [Google Scholar]
- 35.Hudgins LC, Hirsch J. Changes in abdominal and gluteal adipose tissue fatty acid compositions in obese subjects after weight gain and weight loss. Am J Clin Nutr. 1991;53:1372–1377. doi: 10.1093/ajcn/53.6.1372. [DOI] [PubMed] [Google Scholar]
- 36.Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante JAW. Obesity is associated with macrophage accumulation in adipose tissue. J. Clin. Invest. 2003;112:1796–1808. doi: 10.1172/JCI19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wellen KE, Hotamisligil GS. Inflammation, stress and diabetes. J Clin Invest. 2005;115:1111–1119. doi: 10.1172/JCI25102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lofgren P, Harmelen Vv, Reynisdottir S, Naslund E, Ryden M, Rossner S, Arner P. Secretion of tumor necrosis factor-α shows a strong relationship to insulin-stimulated glucose transport in human adipose tissue. Diabetes. 2000;49:688–692. doi: 10.2337/diabetes.49.5.688. [DOI] [PubMed] [Google Scholar]
- 39.You T, Yang R, Lyles MF, Gong D, Nicklas BJ. Abdominal adipose tissue cytokine gene expression: relationship to obesity and metabolic risk factors. Am. J. Physiol. Endocrinol. Metab. 2005;288:E741–E747. doi: 10.1152/ajpendo.00419.2004. [DOI] [PubMed] [Google Scholar]
- 40.Soukas A, Cohen P, Socci ND, Friedman JM. Leptin-specific patterns of gene expression in white adipose tissue. Genes Dev. 2000;14:963–980. [PMC free article] [PubMed] [Google Scholar]
- 41.Mackintosh RM, Hirsch J. The effects of leptin administration in non-obese human subjects. Obesity Res. 2001;9:462–469. doi: 10.1038/oby.2001.60. [DOI] [PubMed] [Google Scholar]
- 42.Liu S, Manson JE, Buring JE, Stampfer MJ, Willett WC, Ridker PM. Relation between a diet with a high glycemic load and plasma concentrations of high-sensitivity C-reactive protein in middle-aged women. Am. J. Clin. Nutr. 2002;75:492–498. doi: 10.1093/ajcn/75.3.492. [DOI] [PubMed] [Google Scholar]
- 43.Strawford A, Antelo F, Christiansen M, Hellerstein MK. Adipose tissue triglyceride turnover, de novo lipogenesis, and cell proliferation in humans measured with 2H20. Am J Physiol. 2004;286:E577–E588. doi: 10.1152/ajpendo.00093.2003. [DOI] [PubMed] [Google Scholar]
- 44.Wang Y, Voy BJ, Urs S, Kim S, Soltani-Bejnood M, Quigley N, Heo Y, Standridge M, Andersen B, Dhar M, Joshi R, Wortman P, Taylor JW, Chun J, Leuze M, Claycombe K, Saxton AM, Moustaid-Moussa N. The human fatty acid synthase gene and de novo lipogenesis are coordinately regulated in human adipose tissue. J. Nutr. 2004;134:1032–1038. doi: 10.1093/jn/134.5.1032. [DOI] [PubMed] [Google Scholar]
- 45.Sul HS, Wang D. Nutritional and hormonal regulation of enzymes in fat synthesis: studies of fatty acid synthase and mitochodrial glycerol-3-phosphate acyltransferase gene transcription. Annu. Rev. Nutr. 1998;18:331–351. doi: 10.1146/annurev.nutr.18.1.331. [DOI] [PubMed] [Google Scholar]
- 46.Semenkovich CF, Coleman T, Fiedorek FT. Human fatty acid synthesis mRNA: tissue distribution, genetic mapping, and kinetics of decay after glucose deprivation. J Lipid Res. 1995;36:1507–1521. [PubMed] [Google Scholar]
- 47.Kamel AF, Norgren S, Strigard K, Thorne A, Fakhrai-Rad H, Galli J, Marcus C. Age-dependent regulation of lipogenesis in human and rat adipocytes. J. Clin. Endocrinol. Metab. 2004;89:4601–4606. doi: 10.1210/jc.2003-030994. [DOI] [PubMed] [Google Scholar]
- 48.Nadler ST, Stoehr JP, Schueler KL, Tanimoto G, Yandell BS, Attie AD. The expression of adipogenic gene is decreased in obesity and diabetes mellitus. PNAS. 2000;97:11371–11376. doi: 10.1073/pnas.97.21.11371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ferrante AW, Thearle M, Liao T, Leibel RL. Effects of leptin deficiency and short-term repletion on hepatic gene expression in genetically obese mice. Diabetes. 2001;50:2268–2278. doi: 10.2337/diabetes.50.10.2268. [DOI] [PubMed] [Google Scholar]


