Abstract
Proteomics is the study of expressed proteins and has emerged as a complement to genomic research. The major advantage of proteomics over DNA-RNA based technologies is that it more closely relates to phenotype and not the source code. Proteomics thus holds the promise of providing direct insight into the true mechanisms of human disease. Historically, examination of the placenta was the first modality to subclassify pathogenetical entities responsible for preterm birth. Because placenta is a key pathophysiological participant in several major obstetrical syndromes (preterm birth, preeclampsia, intrauterine growth restriction) identification of relevant biomarkers of placental function can profoundly impact on the prediction of fetal outcome and treatment efficacy. Proteomics is a young science and studies that associate proteomic patterns with long-term outcome require follow-up of children up to school age. In the interim, placental pathological footprints of cellular injury can be useful as intermediate outcomes. Furthermore, knowledge of the identity of the dys-regulated proteins may provide the necessary insight into novel pathophysiological pathways and unravel possible targets for therapeutic intervention that could not have been envisioned through hypothesis-driven approaches.
CLINICAL PROTEOMICS AND MOLECULAR THERANOSTICS AS APPROACHES FOR DISEASE CLASSIFICATION IN OBSTETRICS
Proteomics is the field of study that encompasses knowledge of the structure, function and expression of all proteins in the biochemical or biological context of an organism at a given moment.1,2,3 Since first introduced in the late 1990’s, 4 few basic science concepts have evolved as rapidly as proteomics, especially when it became clear after sequencing that the genome itself accounted for only a small percentage of biological processes. In 2005, the March of Dimes Scientific Advisory Committee on Prematurity concluded that it is critical for future research to identify relevant regulatory biomarkers that might impact the prediction of fetal outcome and treatment efficacy. 5 The term biomarker was defined as “an objectively measured characteristic evaluated as an indicator of normal, pathogenic or pharmacologic response of a biological system.” Proteomics has all the necessary attributes to identify the combination of biomarkers which most closely relates to phenotype, since it investigates effector molecules directly and not the source code.
The design of proteomic experiments is the most important limiting factor in obtaining conclusions with biological significance.2,3 For example, the choice of cases and the biological sample where biomarkers are first sought are critical steps in ensuring that the final combination of biomarkers is indeed representative of the disease process and not a confounding event. Furthermore, only when biomarkers perform adequately in a population different than that used for their development can one claim that they have clinical utility.
In terms of choice of proteomic technique for biomarker discovery two opposing views have emerged: diagnostic pattern proteomics and identification-centred proteomics.
Diagnostic pattern proteomics uses high throughput mass spectrometry approaches to generate proteomic profiles (sequence of peaks separated by molecular weight) in different experimental conditions while minimizing the importance of biomarker identity. The advantages of this approach are the ability to derive the final biomarker combination from large numbers of cases, the minimal manipulation of biological samples, and the lack of bias with respect to identities. The diagnosis is thus intrinsic to the pattern and not to the protein nomenclature. Disadvantages are that it often requires customized bioinformatics approaches for data analysis and offers no further clues as to why the particular pattern is present or not. Therefore, diagnostic pattern proteomics cannot by itself further our knowledge of the disease process and this is crucial in identifying novel therapeutic targets.
At the opposite end is identification-centred proteomics which focuses on providing the most comprehensive list of protein identities differentially expressed in the respective biological samples using an arbitrary cut-off. The advantage is that it offers compelling indication of the identities of the proteins differentially expressed among groups. Disadvantages are that it generally involves a more extensive sample manipulation and quantitative relationships, which are at the basis of establishing which protein identities are important, become less reliable. Second, protein identities are derived from algorithms which match peptide sequences into databases and therefore the identity depends heavily on the quality of the match. Third, biomarkers are generally fragments of proteins and the resulting database match to a protein precursor may not have any relationship to the biological role performed by the different fragments in vivo. Fourth, the lists of protein identities are large and need to be filtered down using a biological significance criterion, which is not always the degree of increase or decrease of the signal. However, with all the aforementioned limitations, if correctly designed proteomic experimentation can provide invaluable insight into diagnostic modalities and pathogenetical pathways for preterm birth that could not have been envisioned by any other methodology.
Molecular theranostics is an emerging new concept in which molecular biology tools are used to provide the practitioner with a rapid, accurate and completely informative diagnosis, thus enabling better therapeutic intervention. 17 Bioinformatics, genomics and clinical proteomics are methodological tools essential for the progress of molecular theranostics. Cancer therapy has been the frontrunner of molecular theranostics. For example, Herceptin™, which targets the her2/neu receptor, should not be given to the general population of breast cancer patients, because it has no beneficial impact on tumours that do not express the her2/neu receptor. However, in the her2/neu-expressing breast cancer subpopulation, treatment with Herceptin™ cuts the 4-year recurrence rate in half (from 33% to 15%) and thus extends the life of the many breast cancer patients who are appropriate candidates for this targeted therapy.6
Our group was the first to apply a proteomic approach to preterm delivery.7 Since then, we and others have increasingly used proteomic tools in attempts to distinguish among subclasses of preterm birth and to understand its determinism.8,9,10,11,12,13,14,15,16 The basic paradigm for application of proteomics in our laboratory is presented in Figure 1. The final goal in the short run is to use proteomic derived biomarker patterns at the bedside to improve classification of preterm birth syndrome in the current state of clinical practice (clinical proteomics). In the long run the goal is to identify the best therapeutic targets for future drug design or intervention that can diminish the rate of preterm delivery and make a significant difference in neonatal outcome in a modern theranostic approach.17
Figure 1.
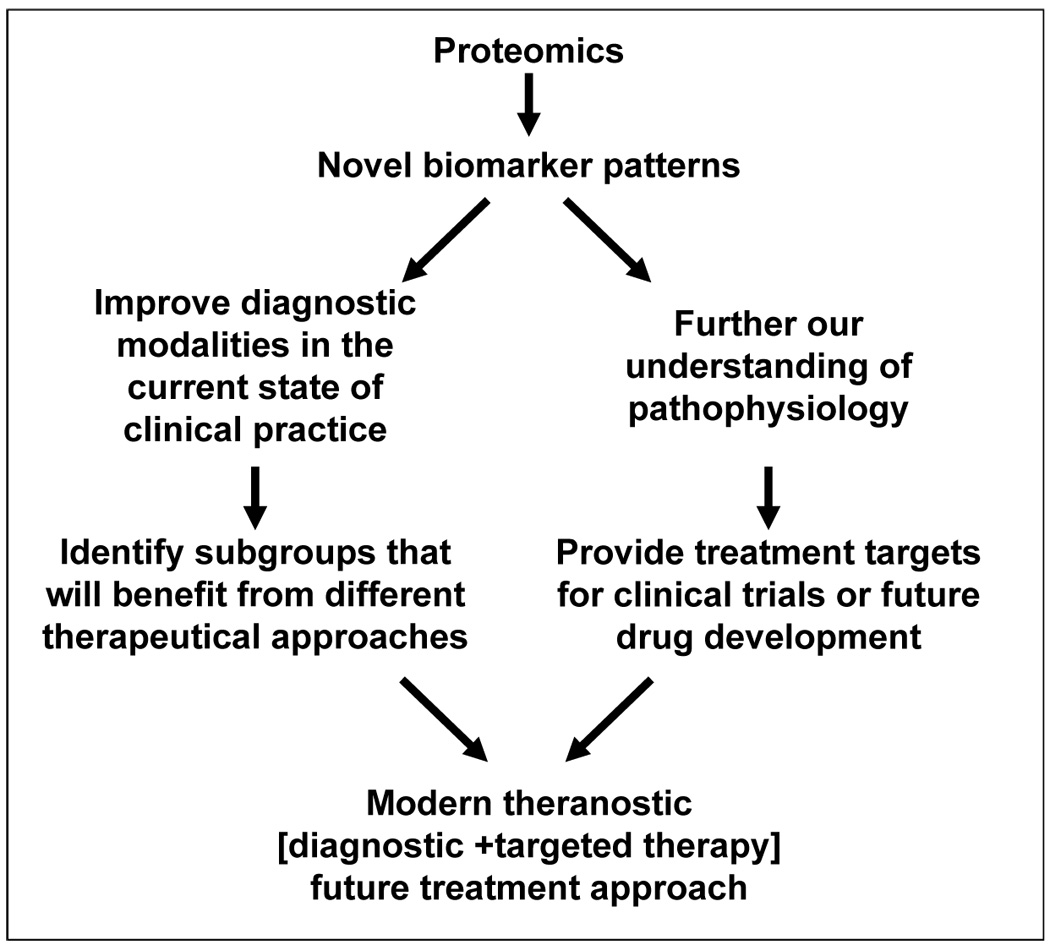
Basic paradigm of proteomic applications in our laboratory.
PATHOLOGICAL EXAMINATION OF THE PLACENTA – A HISTORICAL THERANOSTIC TOOL
Pathological examination of the placenta was the first modality used to subclassify the pathogenetical entities responsible for preterm birth.18,19,20,21 However, histological biomarkers are irrelevant for therapeutic choices aimed to prevent preterm birth or adverse neonatal outcome antenatally, since tissues are available for examination only after birth. For this reason their significance is currently limited to counselling and research. Additionally, placental pathological examination has limitations intrinsic to pathology practice, since it relates to examination of tissues rather than biological fluids. For instance significant heterogeneity in inflammatory responses and poor to moderate intra- and inter-operator variability have been reported.22 Third, the intricacy and redundancy of biological processes in generating cellular and tissue lesions might lead to identical pathological footprints, whereas the initiating triggers might have been distinct.
Given that studies have associated short and long-term follow-up characteristics to distinct placental lesions, we have used placental examination (performed by a perinatal pathologist blinded to proteomic results) as an intermediate outcome variable. 22,21,23,24,25 It is obvious that, since proteomics is a young science, conclusions regarding the clinical utility of proteomic biomarkers based on long-term follow up studies are not yet possible.
PROTEOMIC PROFILING OF AMNIOTIC FLUID TO PROVIDE INSIGHT INTO MECHANISMS OF PRETERM BIRTH
A generalized scheme of the study steps necessary to derive the novel proteomic patterns and quantify their biological significance as performed in our laboratory is presented in Table 1. Often steps 4–6 and 7–8 evolve in parallel. In other words, once the best combination of biomarkers is delineated, experiments aimed to identify the discriminatory proteins are initiated.
Table 1.
Generalized scheme of proteomics study design employed in our laboratory
| Study Steps | Objective |
|---|---|
| Step 1 | Identify an important disease where improved diagnosis would change clinical management and make a measurable difference in outcome. |
| Step 2 | Optimize SELDI conditions for the most relevant biological sample available using select groups of patients that either have or do not have the disease based on very strict clinical and/or laboratory criteria. |
| Step 3 | Learn proteomic profiles and identify the best discriminative combination of biomarkers (PROTEME). |
| Step 4 | Using customized bioinformatics algorithms transform the proteomic information of the PROTEME into a numeric variable (PROTEOMIC SCORE) which can be further manipulated using regular statistics. |
| Step 5 | Evaluate the SCORE by blind testing in the population used for its development and measure intra and inter-rater variability. |
| Step 6 | Evaluate prospectively the SCORE against relevant outcome measures in a population different than that used for its development. |
| Step 7 | Identify component biomarkers using various proteomics methods. |
| Step 8 | Extend pathophysiological understanding of the disease using hypothesis driven approaches stemmed from knowledge of identity of biomarkers or protein precursors. |
When assessing inflammatory lesions in the placenta, pathologists have put considerable emphasis on identifying the histological footprints characteristic of a fetal as opposed to maternal (less relevant to outcome) inflammatory response.26 It is generally accepted that fetal inflammatory infiltrates in preterm placentas first appear in vessels of the chorionic plate (chorionic vasculitis), while in term infants they are first seen in the umbilical vein (umbilical phlebitis). These two patterns are considered to be the initial stage of fetal inflammation. The observation of an inflammatory response in the wall of the umbilical artery (umbilical arteritis) is considered a more advanced stage and has been associated with increased cytokines in the fetal circulation.27,28 Therefore the histological grading of funisitis based on the location and extent of inflammatory infiltrate can be used for semiquantitative assessment of the severity of the fetal inflammatory response.20
In our opinion amniotic fluid offers clear advantages as a test sample for proteomic experimentation. Almost 99% of the neutrophils identified in the amniotic fluid are fetal in origin.29 Thus, amniotic fluid directly reflects the fetal inflammatory response to intra-amniotic infection. In addition amniotic fluid has a rather low complexity compared to blood and plasma, which are among the most difficult protein-containing samples to characterize due to the abundance of proteins and the large number of proteins bound to albumin.
A two dimensional gel electrophoresis map of normal amniotic fluid at 17 weeks of gestation with protein identities has been provided by Liberatori. 30 However, our first methodological choice to generate and decipher the amniotic fluid patterns characteristic for inflammation was SELDI-TOF (surface-enhanced laser desorption ionization time-of-flight) mass spectrometry, a newly developed proteomic technology that combines chromatography with mass spectrometry. 31 SELDI-TOF offers technical advantages such as speed of screening and the ability to use very small amounts of crude biological fluids with minimal manipulation. In conjunction with the use of various ProteinChip arrays® SELDI-TOF performs, in a matter of an hour, a multidimensional protein separation that can be optimized for complex mixtures of proteins so that quantitative relationships are maintained.32 The resulting output is a Cartesian sequence of relative peak intensities on the x-axis separated by their molecular weights on the y-axis. With each Cartesian sequence containing over 40,000 data points per sample the amount of data to be analyzed is enormous.
There are a number of different analysis tools that identify and use multivariate patterns in expression profile data for this purpose, but they can generally be lumped into one of two categories: unsupervised learning in the form of cluster analysis or supervised learning in the form of classification methods. Irrespective of the method, the challenge remains to extract the most amount of information with diagnostic and prognostic value. Our group took a novel approach and devised a stepwise strategy to extract relevant biomarkers utilizing filter preferences based on Boolean logic applied sequentially. This strategy was named “Mass restricted (MR) scoring”.10, 3 The first goal of the MR scoring method was to identify the minimal combination of SELDI peaks (identified by their molecular mass) with discriminatory value and second, to reduce all proteomic information into a numeric variable that characterizes each sample and can be further compared to other diagnostic modalities or tested prospectively against outcome using standard statistical methods.
We named the minimal discriminatory unit of proteomic information a proteme by analogy with phonology and speech structures where a phoneme represents the smallest unit of sound in spoken language that is used to create a new word, in essence the smallest unit of speech that distinguishes meaning between two words.33 Thus a proteme is a group of proteomic biomarkers found optimal for discrimination between pathogenetical processes, such as between controls and cases with intra-amniotic inflammation prone to preterm delivery. 10
After sequential application of our bioinformatics devised filtering strategy, 4 SELDI peaks (P1–P4; Figure 2A) were found discriminatory and sufficient for the presence of intra-amniotic inflammation. We therefore used these 4 peaks (and the proteins they represented) to devise our MR score, which ranged from 0 to 4 depending on the presence or absence of each of these 4 biomarkers. 10 In the original study, an MR score of 3–4 indicated the presence of inflammation, while a score of 0–2 was considered to exclude it. 10 From the stand point of diagnosis, determining the presence or absence of these 4 biomarkers was adequate and sufficient. However, elucidation of the pathophysiology of intra-amniotic inflammation required identification of the corresponding proteins. On-chip immunoassay, and peptide mass fingerprinting confirmed by Western blotting, established that the 4 protein biomarkers were: neutrophil defensin-2 (3.3 kDa), neutrophil defensin-1 (3.4 kDa), calgranulin C (10.4 kDa) and calgranulin A (10.8 kDa), all members of innate immunity arm of antimicrobial defence. 10 In a separate analysis we performed quantitative studies using immunoassays for the defensins 1–3, calgranulin C, calgranulin A, and calprotectin (a complex of calgranulin A with calgranulin B) in amniotic and vaginal fluid of women with preterm birth and preterm premature rupture of the membranes (PPROM). 15 We concluded that, in the absence of isoform-specific immunoassays, mass spectrometry remains the only way to discriminate between specific biomarkers. Recently, Ruetschi et al. applied SELDI-TOF to the analysis of amniotic and cervical fluid samples from patients with clinical signs of preterm labour with or without intra-amniotic inflammation/infection.12 Peptide mass fingerprinting using tandem mass spectrometry confirmed that five of the biomarkers were human neutrophil defensins 1–3, and calgranulin A and B. Furthermore, the authors applied the MR scoring system to evaluate the diagnostic potential of the four-peak panel described by us earlier. All non-inflammatory samples produced an MR score of zero.
Figure 2.
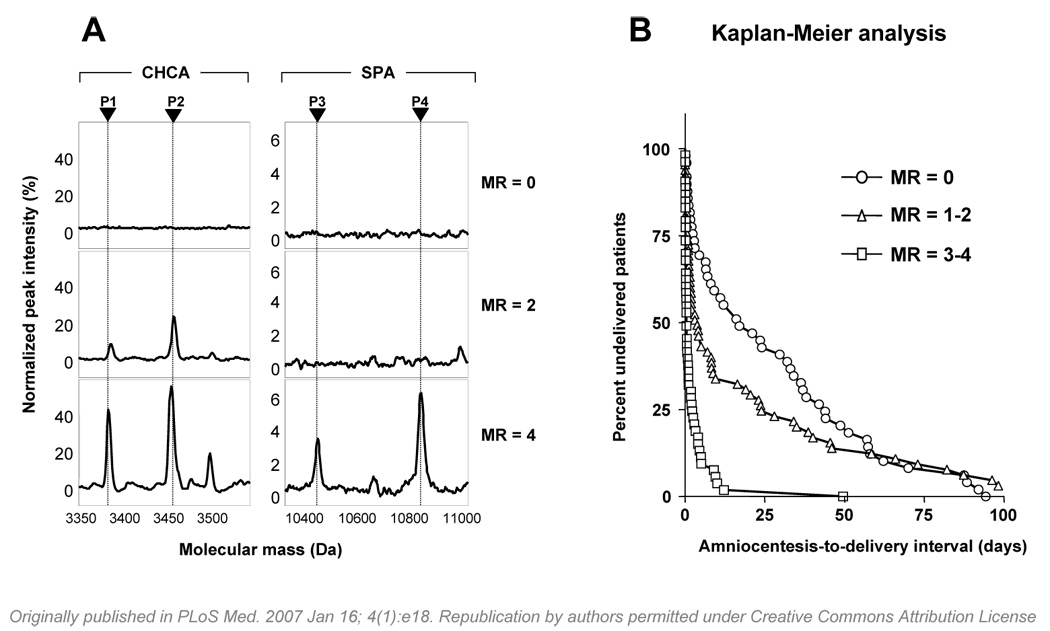
Proteomic profiling of amniotic fluid and risk for preterm birth. A: Representative SELDI-TOF mass spectrometry profiles of amniotic fluid illustrating the biomarkers of the Mass Restricted (MR) score. The MR score enables a classification based on the “severity” of inflammation (MR=0 “no” inflammation; MR=2 “minimal” inflammation; MR=3–4 “severe” inflammation). CHCA and SPA denote the energy absorbing molecules α-cyano-4-hydroxycinnamic acid or sinnapinic acid, respectively. The CHCA chip allows for the identification of defensins 2 (P1: 3377.01 Da) and 1 (P2: 3448.09 Da). The SPA chip allows for identification of the peaks corresponding to calgranulin C (P3: 10,443.85 Da) and calgranulin A (P4: 10,834.51). The x axis of the tracings represents the molecular mass in Daltons; the y axis represents the relative peak intensity. B: Cumulative probability of pregnancy maintenance for the 169 patients illustrating the duration from amniocentesis-to-delivery in women with MR scores of 0 (zero), MR scores of 1–2, and MR scores 3–4.
Because the original enthusiasm for the “omics” discoveries was dampened by concern that these approaches are not truly disease-specific and reproducible, we sought to determine in a prospective and blinded fashion whether in a large cohort of women (n=169), different than that used for its development, our previously identified proteme and MR score is reproducible and maintains its highly accurate “signature” as compared to previously established or proposed markers of intra-amniotic fluid inflammation/infection such as glucose, white blood cell count, lactate dehydrogenase (LDH), Gram stain, and concentrations of the inflammatory mediators interleukin-6 and matrix metalloprotease-8.34 We further tested whether proteomic analysis of amniotic fluid relates to pregnancy outcome, pathological evaluation of the placenta and early-onset neonatal sepsis. In this study the MR score was generated from fresh samples of amniotic fluid and the SELDI-TOF strips analyzed in a blinded fashion.
The most relevant findings of our study were: (a) there was a sequential appearance of the biomarkers in women with intra-amniotic inflammation/infection (defensin 2, defensin 1, calgranulin C, calgranulin A); (b) women with intra-amniotic inflammation have different degrees of inflammation (MR=0 “absent” inflammation; MR=1–2 “minimal” inflammation; MR=3–4 “severe” inflammation; Figure 2A); this classification would not have been apparent without a prospective study design; (c) women with “severe” inflammation (MR 3–4) had shorter amniocentesis-to-delivery intervals than women with “no” (MR 0), or “minimal” (MR 1–2) inflammation (Figure 2B); (d) the presence and the severity of acute inflammation in the chorionic plate, amnion, chorio-decidua and umbilical cord were significantly associated with the occurrence and degree of intra-amniotic inflammation as determined by the MR score; (e) neonates delivered from mothers with MR 3–4 had a significantly higher incidence of suspected and/or confirmed sepsis (OR: 4.4 [95%CI: 1.7–11.6]) compared to neonates from mothers with MR 0 or MR 1–2 after adjusting for gestational age at birth; (f) the accuracy of an MR score 3–4 to detect inflammation was the highest, followed by LDH and glucose; (g) the combination of Gram stain and MR score was identified as best in predicting a positive amniotic fluid culture result (Gram stain: OR=43.9 [9.6-200.5], and MR score: OR=19.6 [6.5-59.0]); (h) results from the 5 laboratory tests were concordant in excluding inflammation in only 64.7% of cases; in contrast, the MR score alone correctly excluded inflammation in 92.4% of cases; (i) when inflammation was present, the 5 tests were concordantly positive in only 20.9%, whereas the MR score confirmed inflammation in 93.0%; (j) the minimum time of incubation required to obtain an accurate spectrum for defensin and calgranulin biomarkers was 15 minutes.
ABILITY OF PROTEOMIC BIOMARKERS TO PREDICT HISTOLOGICAL FUNISITIS ANTENATALLY
Funisitis and early-onset neonatal sepsis are well-recognized risk factors for neonatal mortality and neurodevelopmental impairment.35 In a separate analysis we found that there was significant correlation between the severity of amniotic fluid inflammation as determined by the MR score and the presence and grade of funisitis. Of all component biomarkers of the MR score, the presence of the peak corresponding to calgranulin C (P3) had the highest association with the occurrence of the grade of funisitis associated with early-onset neonatal sepsis (grades 2–4) (OR: 16.7 [95%CI: 5.9–47.2]), while presence of the biomarker peak corresponding to calgranulin A (P4) retained the strongest association with occurrence of neonatal sepsis per se (OR: 4.8 [95%CI: 1.7–13.2]).
ABILITY OF PROTEOMIC BIOMARKERS TO PROVIDE NOVEL INSIGHT INTO MECHANISMS OF PRETERM BIRTH
The sequential appearance of the biomarkers of the MR score during the progress of intra-amniotic inflammation is characterized first by the presence of defensins and later by that of calgranulins.34 Importantly, it is the appearance of the third peak (P3 in Figure 2A) which marks the transition of the MR score 0–2 into 3–4 associated with preterm birth and a shorter duration to delivery. 10 Knowledge of the identity of this SELDI peak as calgranulin C led us to concentrate our attention on the possible molecular mechanisms by which this protein can lead to preterm labour or PPROM. 36
Prior to our discovery it was known that calgranulin C (also known as S100A12) is a member of the low-molecular weight EF-hand S100/calgranulin family. 37 S100A12 is expressed in granulocytes 38, monocytes 39 and select epithelial cells, 40 and it characteristically accumulates at sites of chronic rather than acute inflammation.41 Recently S100A12 has gained increased attention since it has been identified as a potent ligand for RAGE (receptor for advanced glycation end-products), 42 a transmembrane receptor present on numerous cell types including macrophages, neurons, endothelial and smooth muscle cells.43 Due to this property, S100A12 was subsequently named ENRAGE (Extracellular Newly identified RAGE-binding protein).42 Binding of a ligand to RAGE activates key cell signalling pathways such as nuclear factor-kappa-B (NFkB), MAP- kinases and the generation of reactive oxygen species. 43,42,44 A number of studies have shown that RAGE signalling is abrogated by a soluble truncated form of the RAGE receptor named sRAGE, which acts as a “decoy” by binding RAGE ligands, including ENRAGE.42 Therefore we set out to explore whether the ENRAGE–sRAGE–RAGE axis is present in human pregnancy and whether its components are dysregulated in the setting of preterm birth associated with intra-amniotic infection.
We found that: (a) ENRAGE is present in the amniotic fluid of women with intra-amniotic infection in direct relationship to the degree of inflammation and severity of histological chorioamnionitis and funisitis; (b) ENRAGE localizes primarily to infiltrating neutrophils; (c) amniotic fluid contains a high level of sRAGE, the molecular blocker of RAGE signalling; (d) sRAGE levels do not vary with either intra-amniotic infection or with the level of intraamniotic inflammation, but are under a tight gestational age regulation; and (e) amnion epithelial cells well as decidual and extravillous trophoblast cells in the fetal membranes and placenta are potential sources of sRAGE and/or sites of signalling via RAGE receptors.36 Spearheaded by the results of our prior proteomic investigations, our findings were the first to describe expression of ENRAGE and RAGE in reproductive tissues and of sRAGE in amniotic fluid and in the context of chorioamnionitis and preterm birth.
In conclusion, proteomic analysis of amniotic fluid is an important tool to discriminate cases complicated by intra-amniotic inflammation which require different treatment approaches. Proteomics is a young science and studies that associate proteomic patterns with long-term outcome require follow-up of children up to school age. In the interim, placental pathological footprints of cellular injury can be useful as intermediate outcomes. Furthermore, knowledge of the identity of the biomarkers provides insight into involvement of pathophysiological pathways (such as the ENRAGE–sRAGE–RAGE axis) that could not have been envisioned through hypothesis-driven approaches and may in the future provide targets for therapeutic intervention
ROLE OF THE FUNDING SOURCE
The studies described in the article have been partially funded by the Department of Health and Human Services / NIH RO1 HD 047321-01 (IAB). The funding source had any involvement in study design, interpretation of data, writing of the report or decision to submit the paper for publication.
This work was supported from National Institutes of Health Grant RO1 HD 047321 (IAB)
Footnotes
CONFLICT OF INTEREST Dr. Irina Buhimschi is named as inventor on patent applications regarding the use of proteomic profiling of amniotic fluid. The authors have not served as consultants or received any honoraria from any third party as related to the information included in this article.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
LITERATURE CITED
- 1.Lieber CD. Introduction to proteomics. Tools for the New Biology. Totowa, New Jersey: Humana Press Inc.; 2002. [Google Scholar]
- 2.Buhimschi CS, Weiner CP, Buhimschi IA. Clinical proteomics. A novel diagnostic tool for the new biology of preterm labor. Part I: Proteomic tools. Obstet Gynecol Surv. 2006;61:481–486. doi: 10.1097/01.ogx.0000224617.11789.ab. [DOI] [PubMed] [Google Scholar]
- 3.Buhimschi CS, Weiner CP, Buhimschi IA. Clinical proteomics Part II. The emerging role of proteomics over genomics in spontaneous preterm labor/birth. Obstet Gynecol Surv. 2006;61:543–553. doi: 10.1097/01.ogx.0000228779.39568.59. [DOI] [PubMed] [Google Scholar]
- 4.Wilkins MR, Sanchez JC, Gooley AA, Appel RD, Humphery-Smith I, Hochstrasser DF, Williams KL. Progress with proteome projects: why all proteins expressed by a genome should be identified and how to do it. Biotechnol Genet Eng Rev. 1996;13:19–50. doi: 10.1080/02648725.1996.10647923. [DOI] [PubMed] [Google Scholar]
- 5.Green NS, Damus K, Simpson JL, Iams J, Reece EA, Hobel CJ, Merkatz IR, Greene MF, Schwarz RH. March Of Dimes Scientific Advisory Committee On Prematurity. Research agenda for preterm birth: recommendations from the March of Dimes. Am J Obstet Gynecol. 2005;193:626–635. doi: 10.1016/j.ajog.2005.02.106. [DOI] [PubMed] [Google Scholar]
- 6.Pritchard KI, Shepherd LE, O'Malley FP, Andrulis IL, Tu D, Bramwell VH, Levine MN. National Cancer Institute of Canada Clinical Trials Group. HER2 and responsiveness of breast cancer to adjuvant chemotherapy. N Engl J Med. 2006;354:2103–2111. doi: 10.1056/NEJMoa054504. [DOI] [PubMed] [Google Scholar]
- 7.Buhimschi IA, Buhimschi CS, Weiner CP. Acute versus chronic inflammation: What makes the intra-uterine environment "unfriendly" to the fetus? From free radicals to proteomics. Am J Reprod Immunol. 2003;49:328. [abstract] [Google Scholar]
- 8.Vuadens F, Benay C, Crettaz D, Gallot D, Sapin V, Schneider P, Bienvenut WV, Lemery D, Quadroni M, Dastugue B, Tissot JD. Identification of biologic markers of the premature rupture of fetal membranes: proteomic approach. Proteomics. 2003;3:1521–1525. doi: 10.1002/pmic.200300455. [DOI] [PubMed] [Google Scholar]
- 9.Gravett MG, Novy MJ, Rosenfeld RG, Reddy AP, Jacob T, Turner M, McCormack A, Lapidus JA, Hitti J, Eschenbach DA, Roberts CT, Jr, Nagalla SR. Diagnosis of intra-amniotic infection by proteomic profiling and identification of novel biomarkers. JAMA. 2004;292:462–469. doi: 10.1001/jama.292.4.462. [DOI] [PubMed] [Google Scholar]
- 10.Buhimschi IA, Christner R, Buhimschi CS. Proteomic biomarker analysis of amniotic fluid for identification of intra-amniotic inflammation. BJOG. 2005;112:173–181. doi: 10.1111/j.1471-0528.2004.00340.x. [DOI] [PubMed] [Google Scholar]
- 11.Michel PE, Crettaz D, Morier P, Heller M, Gallot D, Tissot JD, Reymond F, Rossier JS. Proteome analysis of human plasma and amniotic fluid by Off-Geltrade mark isoelectric focusing followed by nano-LC-MS/MS. Electrophoresis. 2006;27:1169–1181. doi: 10.1002/elps.200500680. [DOI] [PubMed] [Google Scholar]
- 12.Ruetschi U, Rosen A, Karlsson G, Zetterberg H, Rymo L, Hagberg H, Jacobsson B. Proteomic analysis using protein chips to detect biomarkers in cervical and amniotic fluid in women with intra-amniotic inflammation. J Proteome Res. 2005;4:2236–2242. doi: 10.1021/pr050139e. [DOI] [PubMed] [Google Scholar]
- 13.Buhimschi IA, Buhimschi CS, Weiner CP, Kimura T, Hamar BD, Sfakianaki AK, Norwitz ER, Funai EF, Ratner E. Proteomic but not enzyme-linked immunosorbent assay technology detects amniotic fluid monomeric calgranulins from their complexed calprotectin form. Clin Diagn Lab Immunol. 2005;12:837–844. doi: 10.1128/CDLI.12.7.837-844.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Buhimschi IA, Buhimschi CS, Christner R, Weiner CP. Proteomics technology for the accurate diagnosis of inflammation in twin pregnancies. BJOG. 2005;112:250–255. doi: 10.1111/j.1471-0528.2004.00341.x. [DOI] [PubMed] [Google Scholar]
- 15.Buhimschi CS, Pettker CM, Magloire LK, Martin R, Norwitz E, Funai E, Buhimschi IA. Proteomic technology and delayed interval delivery in multiple pregnancies. Int J Gynaecol Obstet. 2005;90:48–50. doi: 10.1016/j.ijgo.2005.03.022. [DOI] [PubMed] [Google Scholar]
- 16.Weiner CP, Lee KY, Buhimschi CS, Christner R, Buhimschi IA. Proteomic biomarkers that predict the clinical success of rescue cerclage. Am J Obstet Gynecol. 2005;192:710–718. doi: 10.1016/j.ajog.2004.10.588. [DOI] [PubMed] [Google Scholar]
- 17.Warner S. Diagnostics + therapy = theranostics. The Scientist. 2004;18:38. [Google Scholar]
- 18.Benirschke K. Examination of the placenta. Obstet Gynecol. 1961;18:309–333. [Google Scholar]
- 19.Naeye RL. Disorder of the Placenta, Fetus and Neonate: Diagnosis and Clinical Significance. St. Louis: Mosby; 1992. Disorders of the placenta and decidua; pp. 118–247. [Google Scholar]
- 20.Salafia CM, Weigl C, Silberman L. The prevalence and distribution of acute placental inflammation in uncomplicated term pregnancies. Obstet Gynecol. 1989;73:383–389. [PubMed] [Google Scholar]
- 21.Redline RW. Placental pathology and cerebral palsy. Clin Perinatol. 2006;33:503–516. doi: 10.1016/j.clp.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 22.Redline RW, Faye-Petersen O, Heller D, Qureshi F, Savell V, Vogler C. Amniotic infection syndrome: nosology and reproducibility of placental reaction patterns. Pediatr Dev Pathol. 2003;6:435–448. doi: 10.1007/s10024-003-7070-y. [DOI] [PubMed] [Google Scholar]
- 23.Bejar R, Wozniak P, Allard M, et al. Antenatal origin of neurologic damage in newborn infants. I. Preterm infants. Am J Obstet Gynecol. 1988;159:357–363. doi: 10.1016/s0002-9378(88)80084-x. [DOI] [PubMed] [Google Scholar]
- 24.De Felice C, Toti P, Laurini RN, Stumpo M, Picciolini E, Todros T, Tanganelli P, Buonocore G, Bracci R. Early neonatal brain injury in histologic chorioamnionitis. J Pediatr. 2001;138:101–104. doi: 10.1067/mpd.2001.109605. [DOI] [PubMed] [Google Scholar]
- 25.Grafe MR. The correlation of prenatal brain damage with placental pathology. Neuropathol Exp Neurol. 1994;53:407–415. doi: 10.1097/00005072-199407000-00013. [DOI] [PubMed] [Google Scholar]
- 26.Redline RW. Inflammatory responses in the placenta and umbilical cord. Semin Fetal Neonatal Med. 2006;11:296–301. doi: 10.1016/j.siny.2006.02.011. [DOI] [PubMed] [Google Scholar]
- 27.Kim CJ, Yoon BH, Romero R, Moon JB, Kim M, Park SS, Chi JG. Umbilical arteritis and phlebitis mark different stages of the fetal inflammatory response. Am J Obstet Gynecol. 2001;185:496. doi: 10.1067/mob.2001.116689. 500. [DOI] [PubMed] [Google Scholar]
- 28.Rogers BB, Alexander JM, Head J, McIntire D, Leveno KJ. Umbilical vein interleukin-6 levels correlate with the severity of placental inflammation and gestational age. Hum Pathol. 2002;33:335–340. doi: 10.1053/hupa.2002.32214. [DOI] [PubMed] [Google Scholar]
- 29.Sampson JE, Theve RP, Blatman RN, Shipp TD, Bianchi DW, Ward BE, Jack RM. Fetal origin of amniotic fluid polymorphonuclear leukocytes. Am J Obstet Gynecol. 1997;176:77–81. doi: 10.1016/s0002-9378(97)80015-4. [DOI] [PubMed] [Google Scholar]
- 30.Liberatori S, Bini L, De Felice C, Magi B, Marzocchi B, Raggiaschi R, Frutiger S, Sanchez JC, Wilkins MR, Hughes G, Hochstrasser DF, Bracci R, Pallini V. A two-dimensional protein map of human amniotic fluid at 17 weeks' gestation. Electrophoresis. 1997;18:2816–2822. doi: 10.1002/elps.1150181517. [DOI] [PubMed] [Google Scholar]
- 31.Kuwata H, Yip TT, Yip CL, Tomita M, Hutchens TW. Bactericidal domain of lactoferrin: detection, quantitation, and characterization of lactoferricin in serum by SELDI affinity mass spectrometry. Biochem Biophys Res Commun. 1998;245:764–773. doi: 10.1006/bbrc.1998.8466. [DOI] [PubMed] [Google Scholar]
- 32.Fung ET, Yip TT, Lomas L, Wang Z, Yip C, Meng XY, Lin S, Zhang F, Zhang Z, Chan DW, Weinberger SR. Classification of cancer types by measuring variants of host response proteins using SELDI serum assays. Int J Cancer. 2005;115:783–789. doi: 10.1002/ijc.20928. [DOI] [PubMed] [Google Scholar]
- 33.Cook Moats Loiusa. Speech to Print. Baltimore: Paul H. Brooks Publishing Co. Inc.; 2000. pp. 21–58. [Google Scholar]
- 34.Buhimschi CS, Bhandari V, Hamar B, Bahtiyar MO, Zhao G, Sfakianaki AK, Pettker CM, Magloire LK, Norwitz ER, Funai E, Paidas M, Weiner CP, Copel J, Lockwood CJ, Buhimschi IA. Proteomic profiling of the amniotic fluid to detect inflammation, infection, and neonatal sepsis. PLoS Med. 2007;4:e18. doi: 10.1371/journal.pmed.0040018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Buhimschi CS, Buhimschi IA, Abdel-Razeq S, Rosenberg VA, Thung SF, Zhao G, Wang E, Bhandari V. Proteomic biomarkers of intra-amniotic inflammation: relationship with funisitis and early-onset sepsis in the premature neonate. Pediatr Res. 2007;61:318–324. doi: 10.1203/01.pdr.0000252439.48564.37. [DOI] [PubMed] [Google Scholar]
- 36.Buhimschi IA, Zhao G, Pettker CM, Bahtiyar MO, Magloire LK, Thung S, Fairchild T, Buhimschi CS. The receptor for advanced glycation end products (RAGE) system in women with intraamniotic infection and inflammation. Am J Obstet Gynecol. 2007 Feb;196(2):181.e1–181.e13. doi: 10.1016/j.ajog.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 37.Heizmann CW, Fritz G, Schafer BW. S100 proteins: structure, functions and pathology. Front Biosci. 2002;7:1356–1368. doi: 10.2741/A846. [DOI] [PubMed] [Google Scholar]
- 38.Vogl T, Propper C, Hartmann M, Strey A, Strupat K, van den Bos C, Sorg C, Roth J. S100A12 is expressed exclusively by granulocytes and acts independently from MRP8 and MRP14. J Biol Chem. 1999;274:25291–25296. doi: 10.1074/jbc.274.36.25291. [DOI] [PubMed] [Google Scholar]
- 39.Robinson MJ, Hogg N. A comparison of human S100A12 with MRP-14 (S100A9) Biochem Biophys Res Commun. 2000;275:865–870. doi: 10.1006/bbrc.2000.3407. [DOI] [PubMed] [Google Scholar]
- 40.Gottsch JD, Li Q, Ashraf F, O’Brien TP, Stark WJ, Liu SH. Cytokine-induced calgranulin C expression in keratocytes. Clin Immunol. 1999;91:34–40. doi: 10.1006/clim.1998.4681. [DOI] [PubMed] [Google Scholar]
- 41.Foell D, Kucharzik T, Kraft M, Vogl T, Sorg C, Domschke W, Roth J. Neutrophil derived human S100A12 (ENRAGE) is strongly expressed during chronic active inflammatory bowel disease. Gut. 2003;52:847–853. doi: 10.1136/gut.52.6.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hofmann MA, Drury S, Fu C, Qu W, Taguchi A, Lu Y, Avila C, Kambham N, Bierhaus A, Nawroth P, Neurath MF, Slattery T, Beach D, McClary J, Nagashima M, Morser J, Stern D, Schmidt AM. RAGE mediates a novel proinflammatory axis: a central cell surface receptor for S100/calgranulin polypeptides. Cell. 1999;97:889–901. doi: 10.1016/s0092-8674(00)80801-6. [DOI] [PubMed] [Google Scholar]
- 43.Stern D, Yan SD, Yan SF, Schmidt AM. Receptor for advanced glycation endproducts: a multiligand receptor magnifying cell stress in diverse pathologic settings. Adv Drug Deliv Rev. 2002;54:1615–1625. doi: 10.1016/s0169-409x(02)00160-6. [DOI] [PubMed] [Google Scholar]
- 44.Chavakis T, Bierhaus A, Nawroth PP. RAGE (receptor for advanced glycation end products): a central player in the inflammatory response. Microbes Infect. 2004;6:1219–1225. doi: 10.1016/j.micinf.2004.08.004. [DOI] [PubMed] [Google Scholar]


