Abstract
Background
Long-term use of hypnotics is not recommended because of risks of dependency and adverse effects on health. The usual clinical management of benzodiazepine dependency is gradual tapering, but when used alone this method is not highly effective in achieving long-term discontinuation. We compared the efficacy of tapering plus cognitive-behavioural therapy for insomnia with tapering alone in reducing the use of hypnotics by older adults with insomnia.
Methods
People with chronic insomnia who had been taking a benzodiazepine every night for more than 3 months were recruited through media advertisements or were referred by their family doctors. They were randomly assigned to undergo either cognitive-behavioural therapy plus gradual tapering of the drug (combined treatment) or gradual tapering only. The cognitive-behavioural therapy was provided by a psychologist in 8 weekly small-group sessions. The tapering was supervised by a physician, who met weekly with each participant over an 8-week period. The main outcome measure was benzodiazepine discontinuation, confirmed by blood screening performed at each of 3 measurement points (immediately after completion of treatment and at 3- and 12-month follow-ups).
Results
Of the 344 potential participants, 65 (mean age 67.4 years) met the inclusion criteria and entered the study. The 2 study groups (35 subjects in the combined treatment group and 30 in the tapering group) were similar in terms of demographic characteristics, duration of insomnia and hypnotic dosage. Immediately after completion of treatment, a greater proportion of patients in the combined treatment group had withdrawn from benzodiazepine use completely (77% [26/34] v. 38% [11/29]; odds ratio [OR] 5.3, 95% confidence interval [CI] 1.8–16.2; OR after adjustment for initial benzodiazepine daily dose 7.9, 95% CI 2.4–30.9). At the 12-month follow-up, the favourable outcome persisted (70% [23/33] v. 24% [7/29]; OR 7.2, 95% CI 2.4–23.7; adjusted OR 7.6, 95% CI 2.5–26.6); similar results were obtained at 3 months.
Interpretation
A combination of cognitive-behavioural therapy and benzodiazepine tapering was superior to tapering alone in the management of patients with insomnia and chronic benzodiazepine use. The beneficial effects were sustained for up to 1 year. Applying this multidisciplinary approach in the community could help reduce benzodiazepine use by older people.
Older people frequently take prescribed hypnotics on a long-term basis.1,2,3,4,5 Regular use of benzodiazepine hypnotics is not recommended for protracted periods because their long-term efficacy remains unproven;6 in addition, there is a risk of dependence,7,8 and use of the drugs leads to memory impairment, daytime drowsiness,7,9 falls resulting in fractures10,11 and motor vehicle crashes.12 Guidelines recommend limiting the use of benzodiazepine hypnotics to 4 weeks.9,13 Nevertheless, benzodiazepine use is often prolonged because insomnia is a chronic problem.1,5,14
The usual clinical management of benzodiazepine dependence is gradual tapering, but when used alone this method has not proven highly effective in achieving long-term discontinuation, and 50% to 60% of users resume use of their medication during the month following the end of the withdrawal period.15,16 Behavioural and cognitive therapies are effective treatments for elderly people with insomnia;17,18,19,20,21,22 however, when applied alone for hypnotic users, they do not produce sustained reduction in medication use.18,23 Given that withdrawal of the hypnotic agent and cognitive-behavioural therapy are complementary approaches, we hypothesized that combining them would be more effective than the usual method of progressive withdrawal. In this study we compared the efficacy of combined benzodiazepine tapering and cognitive-behavioural therapy for insomnia with that of benzodiazepine tapering alone in reducing use of hypnotics by older people with insomnia.
Methods
This nonblinded randomized controlled trial had 2 therapeutic arms. The combined treatment group received cognitive-behavioural therapy and gradual benzodiazepine tapering, whereas the other group was managed by gradual tapering alone. The study was approved by the Ethics Committee of the Centre hospitalier universitaire de Québec (Pavillon CHUL), and all participants provided written consent for their participation in the study. Our primary outcome measure was discontinuation of benzodiazepine use, immediately after completion of treatment and up to 1 year later.
We recruited people with chronic insomnia through referral by family physicians and media advertisements. The following inclusion criteria were applied: age 50 years or older; daily benzodiazepine use at bedtime for the past 3 months or more; and diagnosis of chronic insomnia, defined as insomnia for a period of 6 months or more in accordance with the American Sleep Disorders Association.24 In addition, participants had to fulfill 1 of the following 2 criteria: inability to refrain from taking sleeping pills at night because of fear of a bad night's sleep or sleep efficiency of less than 80% over a 2-week period, where sleep efficiency is defined as total sleep time divided by time in bed, multiplied by 100. Participants also had to be experiencing impaired daytime functioning, irritability or mood disturbances. People with cognitive impairment, severe psychiatric problems or physical problems possibly related to insomnia were excluded, as were those taking a benzodiazepine during the daytime or drinking more than 3 alcoholic beverages per day.
A total of 505 people contacted the research nurse, who informed them (by telephone) about the project and verified the inclusion criteria. A total of 161 people were excluded at this stage because they were less than 50 years old or their benzodiazepine use did not meet the inclusion criteria (i.e., they had no consumption, or irregular or daytime consumption). Among the 344 remaining potential subjects, 114 refused to participate further because of lack of interest, 70 were excluded on the basis of physical or psychological problems that might have been related to insomnia, and 41 were excluded for other reasons (e.g., schedule not convenient, transportation problems, alcohol abuse, use of a nonbenzodiazepine hypnotic). The remaining 119 individuals completed validated French versions of the Modified Mini-Mental State,25,26 State Trait Anxiety Inventory27,28 and the Geriatric Depression Scale,29,30 as well as a daily sleep diary31 for a 2-week period. They then underwent a semistructured interview with a psychologist and a physician. At this stage, 54 additional subjects were excluded for various reasons (Fig. 1).
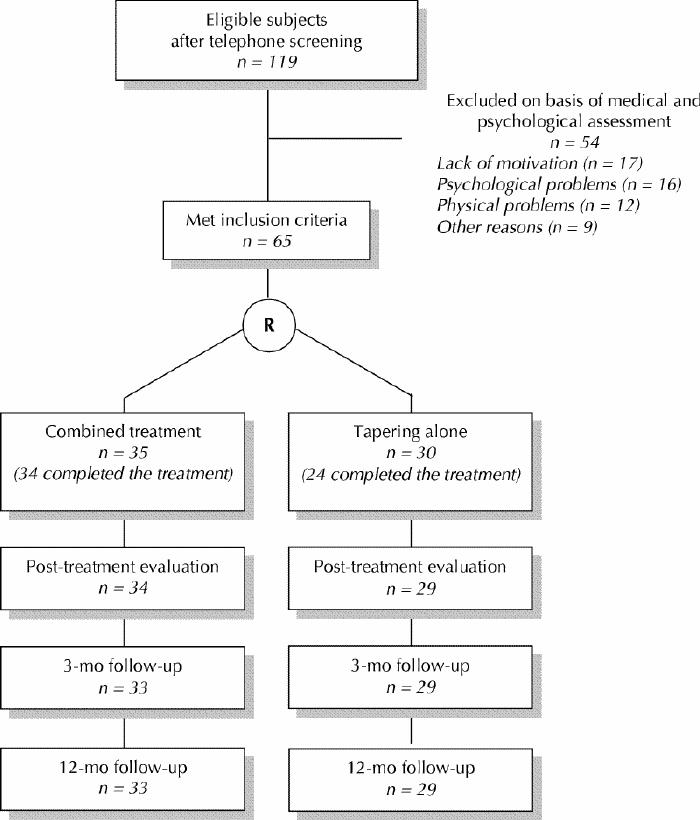
Fig. 1: Flow of patients through a randomized trial of cognitive-behavioural therapy combined with tapering of benzodiazepine use. In the combined treatment group, one patient who completed the treatment was lost to follow-up by the time of the 3-month evaluation. For one additional patient in this group, information about benzodiazepine withdrawal was obtained at 12-month follow-up, but data for the variable “dosage reduction of 50% or more” was not available at that time. Of the 6 subjects who did not complete the tapering-alone treatment, 5 agreed to be contacted at the 3-month and 12-month follow-ups to report on benzodiazepine cessation.
The research nurse randomly assigned the 65 remaining individuals (mean age 67.4 years) to 1 of the 2 therapeutic groups: combined treatment (35 subjects) or gradual tapering alone (30 subjects). First, the nurse randomly assigned a number to each participant. Study randomization was achieved by matching the participant's number with a table of random numbers in which each number corresponded to a predetermined treatment group. We did not perform block randomization. However, because both groups were treated concurrently, we waited until a minimum of 5 participants had been assigned to the combined treatment group before starting the “successive waves” of treatment. The treatment assignment could not be concealed from participants, but aggregate outcome data were not revealed to patients or investigators during the study.
Gradual tapering began concurrently with the initiation of cognitive-behavioural therapy and was supervised by 1 of the 3 study physicians, who met with each participant weekly over an 8-week period. The proposed schedule was a 25% reduction of dosage at 1- or 2-week intervals. At each visit, the physician looked for withdrawal symptoms and prescribed either the same or a lower dosage, depending on the patient's symptoms. The physicians were not permitted to give advice on nonpharmacological treatments of insomnia. All medical visits, of 15 to 20 minutes' duration, were audiotaped to allow one of the authors (L.B.) to give feedback to the physicians during the course of the study.
Cognitive-behavioural treatment as described by Morin31 was provided by an experienced clinical psychologist after special training given by one of the authors (C.M.M.). This intervention involved behavioural, cognitive and educational components. The behavioural component included instructions for stimulus control and procedures for sleep restriction.32,33 The goal of stimulus control was to reinforce the bed as a cue for sleep and to regularize sleep rhythm. Participants were instructed to go to bed only when sleepy, to go into another room when they could not fall asleep, to use the bedroom only for sleeping, to get up at the same time every morning and to avoid daytime naps. Sleep restriction consisted of curtailing the time spent in bed to the amount of time actually spent sleeping. Several studies have shown that restriction of time in bed produces mild sleep deprivation, which consequently reduces the time it takes to fall asleep and the number of awakenings, as well as deepening sleep.34 The prescribed sleep period durations were based on the daily sleep diary completed by participants throughout the study period. The cognitive component addressed irrational thinking that could exacerbate the sleep disorder through emotional arousal. The educational component included sleep hygiene education and information on the adverse effects of benzodiazepines on health. For further details concerning the cognitive-behavioural therapy, see Table 3 at www.cmaj.ca.
The cognitive-behavioural treatment was provided in small groups of 5 to 7 participants, who underwent 8 weekly group sessions of 90 minutes' duration. One month after the last session, the participants who underwent the combined treatment were invited to a “booster” session to reinforce the skills acquired during group therapy. All of the sessions were audiotaped, and one of the authors (C.M.M.) provided feedback to the psychologist during the course of the study.
Benzodiazepine consumption and sleep measures were evaluated by means of the sleep diary completed daily by participants.31 The main outcome measure was benzodiazepine discontinuation, confirmed by blood screening performed at each of 3 measurement points (immediately after completion of treatment and at 3- and 12-month follow-ups). Confirmatory blood specimens were analyzed by electron-capture gas–liquid chromatography.35,36
Differences between proportions of patients in the 2 groups were compared with Fisher's exact test. Continuous variables were compared with the Wilcoxon rank test. All analyses were performed on an intention-to-treat basis.
Unconditional logistic regression was performed to identify factors other than treatment that might be associated with benzodiazepine cessation. Potential confounders were patient characteristics (sex, age, education, occupation, body mass index) and variables related to insomnia at baseline (duration of insomnia, duration of hypnotic use, diazepam-equivalent daily dose). Sex, occupation (retired or homemaker, employed) and daily dose of benzodiazepine (more than, exactly or less than 5 mg/day diazepam equivalent) were introduced as categorical variables. All other potential confounders were introduced as continuous variables.
Calculation of the required sample size was based on the assumption that combined treatment would increase the proportion of patients with a favourable outcome to 75% over the estimated 40% in the tapering-only group. With an α level of 0.10 (1-sided test) and a power of 81%, the analysis required 30 participants per group.
Results
Of the 65 study participants, 7 did not complete the full treatment: 6 in the tapering-only group and 1 in the combined treatment group (Fig. 1). Reasons given included increase in anxiety (n = 2), treatment perceived as ineffective or too demanding (n = 2), lack of time because of new employment (n = 1) and suicide of a family member (n = 1); the participant who dropped out from the combined treatment group gave no specific reason. Five of these subjects agreed to be contacted during follow-up to report on benzodiazepine cessation. One patient receiving the combined treatment moved out of town and was lost to follow-up at 3 months.
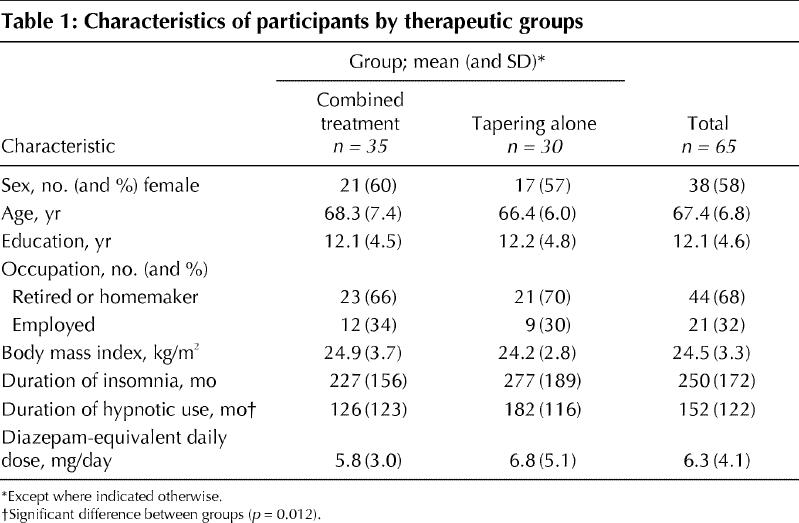
The overall mean duration of insomnia at the start of the study was 250 months, and the mean duration of hypnotic use was 152 months (Table 1). No significant differences were observed between the 2 groups for any of the characteristics recorded, with the exception of a longer baseline duration of hypnotic use in the tapering-only group (p = 0.012).
Table 1
No participant experienced any major adverse reaction during the medication tapering. Only one of the participants who reported complete cessation of hypnotics had a positive test result on plasma screening (at the 12-month follow-up); this person was considered not benzodiazepine-free at that measurement point.
At the evaluation immediately after completion of treatment, a greater proportion of participants in the combined treatment group reported complete hypnotic withdrawal (77% [26/34] v. 38% [11/29]; p = 0.002) (Table 2).
Table 2
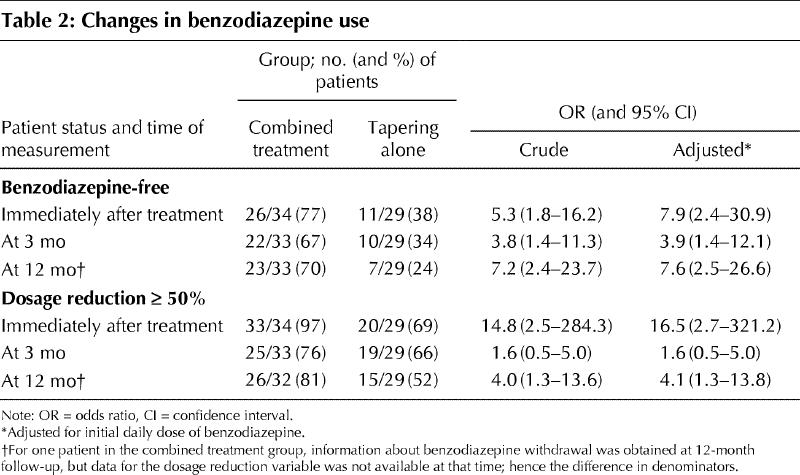
Among all potentially confounding variables included in the logistic regression models, only initial daily dose of benzodiazepine was statistically associated with hypnotic withdrawal immediately after completion of treatment; this was the only confounding variable included in subsequent models. Compared with tapering alone, the combined treatment was strongly associated with benzodiazepine withdrawal, as measured immediately after completion of treatment (odds ratio [OR] 5.3, 95% confidence interval [CI] 1.8–16.2; OR adjusted for initial daily dose of benzodiazepine 7.9, 95% CI 2.4–30.9).
Higher initial daily dose of benzodiazepine (greater than 5 mg/day diazepam equivalent) was associated with lower success in benzodiazepine withdrawal immediately after treatment (OR 0.07, 95% CI 0.01–0.40) relative to a daily dose of less than 5 mg/day. This finding did not persist in later follow-ups.
At the 12-month follow-up, the favourable outcome in terms of benzodiazepine cessation persisted, and 70% (23/33) of the patients who received the combined treatment but only 24% (7/29) of those in the tapering-only group were benzodiazepine-free (OR 7.2, 95% CI 2.4–23.7; OR adjusted for initial daily dose of benzodiazepine 7.6, 95% CI 2.5–26.6). Similar results were obtained at the 3-month follow-up.
Similar differences were observed in the proportion of patients who reduced by at least 50% their daily dose of hypnotic immediately after treatment and at the 12-month follow-up (Table 2). The immediate post-treatment daily dose was lower in the combined treatment group than the tapering-only group (2.84 v. 4.72 mg/day diazepam equivalent), but this difference was not significant (p = 0.196).
Interpretation
Our randomized controlled trial has shown the superiority of a combined intervention, comprising cognitive-behavioural therapy and a gradual tapering of benzodiazepine hypnotics, over the usual clinical approach (tapering alone) for people with chronic insomnia who had been using medication for a protracted period. The benefits in terms of benzodiazepine cessation were still present 1 year after the end of the intervention.
The cognitive-behavioural therapy was provided in small groups rather than individually. In addition to its cost-effectiveness, this format offers many clinical advantages. Group support benefits the participants, helps to keep the therapy focused on sleep problems and increases the likelihood of compliance.31 Personal characteristics (e.g., demographic and insomnia variables) did not seem related to treatment success. Logistic regression analyses showed that the combined treatment and lower initial dosage of benzodiazepine were the only factors related to the success of tapering. Other factors not examined in this study, such as personality traits, psychiatric morbidity and motivation, should be explored in future research.
Few published studies have looked at alternative ways to help people with chronic insomnia to discontinue benzodiazepine use. In a small randomized study, 10 such subjects treated with stimulus control and a withdrawal program were compared with 11 subjects treated with the withdrawal program alone. The 2 interventions were equally effective in reducing the use of hypnotics, as assessed immediately after treatment and at 2-month follow-up.37 However, the variability in the type of hypnotics used (benzodiazepines with or without antidepressants), the short duration of the intervention (2 individual 1-hour sessions) and the low statistical power of the study might explain the discrepancies in results between that study and our own. Morin and colleagues38 reported a clinical replication series involving 21 adults with a diagnosis of drug-dependent insomnia who were treated with cognitive-behavioural therapy combined with a withdrawal plan; 9 of these patients were free of hypnotics immediately after treatment. That study did not include a control group, and a high percentage of follow-up data were missing, which precludes any comparison with the present study. Finally, a study with a multiple-baseline design showed that 4 of 5 older patients with insomnia (mean age 62 years) achieved complete benzodiazepine cessation after cognitive-behavioural treatment combined with benzodiazepine tapering.39 Three of these patients remained benzodiazepine-free at the 3-month follow-up.
In the present study one-third of the participants in the tapering-only group were free of benzodiazepines at the evaluation immediately after completion of treatment. Withdrawal programs have success rates ranging from 26% to 64% among adult populations with mixed disorders.15,40,41,42,43 Comparisons with our study are difficult because of differences in the subject populations, as well as differences between the withdrawal programs. In the only study dealing specifically with insomnia, 60% of the 134 subjects who completed the program were benzodiazepine-free 12 to 18 months after a tapering schedule, which initially involved switching from benzodiazepine to zopiclone for 21 to 30 days.44 However, this nonrandomized study had potentially major flaws: lack of well-defined inclusion criteria for insomnia and benzodiazepine use, high dropout rates and absence of confirmatory testing for benzodiazepine discontinuation.
Because of the nature of this study, blinding of the participants was not feasible. However, contamination bias appears unlikely, as all medical visits were audiotaped and one researcher listened to a sample of these to ensure that physicians had not given specific advice for insomnia. Moreover, any residual bias due to contamination would tend to underestimate the efficacy of the combined intervention.
Without the presence of a placebo group, we cannot be sure that the better outcomes in the combined group were related to the cognitive-behavioural treatment. Other factors, such as the attention and support of the group and the therapist's and patients' expectations, might also explain the results. Further studies are needed to disentangle the effects of the treatment from other components.
The various sleep parameters were assessed by a daily diary; no polysomnographic measures were used. Self-reported evaluation of sleep is considered a valuable and reliable measure of severity of insomnia relative to polysomnography.45,46,47,48 The problem of reactivity (modification of sleep characteristics by a measurement method such as a daily sleep diary) was attenuated by having the patient complete the sleep diary over a 2-week period at baseline and at each measurement point.49
Of the 344 potential participants (those who met the initial criteria for age and benzodiazepine use), only 65 (19%) were included in the study. This raises the issue of the applicability of this approach on a large scale. However, other studies using cognitive-behavioural treatment for insomnia have shown similar proportions of subject inclusion.18,50,51
Because the sample was limited to adults who had isolated chronic insomnia without important psychiatric or medical problems, our findings cannot be generalized to people who do have such problems. Further studies evaluating treatment tailored for insomnia related to conditions such as depression, anxiety disorders and dementia are needed. An important proportion of potential participants (114 of 344) decided not to continue the recruitment procedure because of a lack of interest; therefore, intervention aimed at educating people about the risks associated with dependence on hypnotics is another future direction for research.
Although subject recruitment was difficult, once subjects were enrolled, very few abandoned the study. The percentage of dropouts was low (7/65 or 11%), and the majority of those who did not complete the treatment protocol agreed to be followed for the main outcome measure (benzodiazepine-free status). The higher proportion of dropouts in the tapering-only group might have been related to the perception of a lack of effectiveness or to high anxiety levels (in 4 of the 6 participants who dropped out).
This study has shown that, to achieve discontinuation of chronic benzodiazepine use, a combination of cognitive-behavioural therapy with gradual tapering is more effective than gradual tapering alone. The beneficial effects of this combined strategy on benzodiazepine cessation were maintained over the medium term. Applying this multidisciplinary approach in the community could help reduce benzodiazepine use by older people and prevent health problems related to these medications.
Footnotes
René Verreault's work was supported by the Laval University Chair for Geriatric Research. This study was supported by the National Health Research and Development Program, Health Canada (6605-4573-702).
This article has been peer reviewed.
Contributors: All authors were involved in study conception and design, securing of funding and critical revision of the article for important intellectual content. Lucie Baillargeon coordinated the acquisition of data and was involved in data analyses and interpretation. René Verreault also contributed to data analyses and interpretation.
Acknowledgements: We thank Lucie Misson (research nurse) and Suzanne Gingras (statistics consultant) for their contributions to this project.
Competing interests: None declared.
Correspondence to: Dr. Lucie Baillargeon, Family Medicine Unit, Centre hospitalier universitaire de Québec (CHUL), 2705, boul. Laurier, Sainte-Foy QC G1V 4G2; fax 418 654-2138; lucie.baillargeon@crchul.ulaval.ca
References
- 1.Mellinger GD, Balter MB, Uhlenhuth EH. Insomnia and its treatment. Arch Gen Psychiatry 1985;42:225-32. [DOI] [PubMed]
- 2.Hohagen F, Käppler C, Schramm E, Rink K, Weyerer S, Riemann D, et al. Prevalence of insomnia in elderly general practice attenders and the current treatment modalities. Acta Psychiatr Scand 1994;90:102-8. [DOI] [PubMed]
- 3.Morgan K, Clarke D. Longitudinal trends in late-life insomnia: implications for prescribing. Age Ageing 1997;26:179-84. [DOI] [PubMed]
- 4.Henderson S, Jorm AF, Scott R, Mackinnon AJ, Christensen H, Korten AE. Insomnia in the elderly: its prevalence and correlates in the general population. Med J Aust 1995;162:22-4. [DOI] [PubMed]
- 5.Ohayon MM, Caulet M. Psychotropic medication and insomnia complaints in two epidemiological studies. Can J Psychiatry 1996;41:457-64. [DOI] [PubMed]
- 6.Kupfer DJ, Reynolds CF. Management of insomnia. N Engl J Med 1997; 336: 341-6. [DOI] [PubMed]
- 7.Longo LP, Johnson B. Addiction: Part 1. Benzodiazepines: side effects, abuse risk and alternatives. Am Fam Physician 2000;61:2121-8. [PubMed]
- 8.Matalon A, Yinnon AM, Hurwitz A. Chronic uses of hypnotics in a family practice: patients' reluctance to stop treatment. Fam Pract 1990;7:258-60. [DOI] [PubMed]
- 9.Lader MH. Limitations on the use of benzodiazepines in anxiety and insomnia: Are they justified? Eur Neuropsychopharmacol 1999;9(Suppl 6):S399-S405. [DOI] [PubMed]
- 10.Sorock GS, Shimkin EE. Benzodiazepine sedatives and the risk of falling in a community-dwelling elderly cohort. Arch Intern Med 1988;148:2441-4. [PubMed]
- 11.Ray WA, Griffin MR, Schaffner W, Bauth DK, Melton LJ. Psychotropic drug use and the risk of hip fracture. N Engl J Med 1987;316:363-9. [DOI] [PubMed]
- 12.Hemmelgarn B, Suissa S, Huang A, Boivin JF, Pinard G. Benzodiazepine use and the risk of motor vehicle crash in the elderly. JAMA 1997;278:27-31. [PubMed]
- 13.Insomnia Consensus Conference. Drugs and insomnia. The use of medications to promote sleep. JAMA 1984;251:2410-4. [PubMed]
- 14.Dunbar GC, Perera MH, Jenner FA. Patterns of benzodiazepine use in Great Britain as measured by a general population survey. Br J Psychiatry 1989; 155: 836-41. [DOI] [PubMed]
- 15.Schweizer E, Case WG, Rickels K. Benzodiazepine dependence and withdrawal in elderly patients. Am J Psychiatry 1989;146:529-31. [DOI] [PubMed]
- 16.Rickels K, Schweizer E, Case WG, Greenblatt DJ. Long-term therapeutic use of benzodiazepines. I. Effects of abrupt discontinuation. Arch Gen Psychiatry 1990;47:899-907. [DOI] [PubMed]
- 17.Friedman L, Bliwise DL, Yesavage JA, Salom SR. A preliminary study comparing sleep restriction and relaxation treatments for insomnia in older adults. J Gerontol 1991;46:P1-8. [DOI] [PubMed]
- 18.Morin CM, Azrin NH. Behavioral and cognitive treatments of geriatric insomnia. J Consult Clin Psychol 1988;56:748-53. [DOI] [PubMed]
- 19.Engle-Friedman M, Bootzin R, Hazlewood L, Tsao C. An evaluation of behavioral treatments for insomnia in the older adult. J Clin Psychol 1992;48:77-90. [DOI] [PubMed]
- 20.Anderson MW, Zendell SM, Rosa DP, Rubinstein ML, Herrera CO, Simons O, et al. Comparison of sleep restriction therapy and stimulus control in older insomniacs: an update [abstract]. Sleep Res 1988;17:144.
- 21.Puder R, Lacks P, Bertelson AD, Storandt M. Short-term stimulus control treatment of insomnia in older adults. Behav Ther 1983;14:424-9.
- 22.Morin CM, Colecchi C, Stone J, Sood R, Brink D. Behavioral and pharmacological therapies for late-life insomnia: a randomized controlled trial. JAMA 1999;281:991-9. [DOI] [PubMed]
- 23.Lichstein KL, Johnson RS. Relaxation for insomnia and hypnotic medication use in older women. Psychol Aging 1993;8:103-11. [DOI] [PubMed]
- 24.American Sleep Disorders Association. The international classification of sleep disorders. Lawrence (TX): Allen Press; 1990.
- 25.Teng EL, Chui HC. The modified mini-mental state (3MS) examination. JClin Psychiatry 1987;48:314-7. [PubMed]
- 26.Hébert R, Bravo G, Girouard D. Validation de l'adaptation française du modified mini-mental state (3MS). Rev Gériatr 1992;17:443-50.
- 27.Spielberger CD. Manual for the State-Trait Anxiety Inventory(Form Y). Palo Alto (CA): Consulting Psychologist Press; 1983.
- 28.Gauthier J, Bouchard S. Adaptation canadienne-française de la forme révisée du State-Trait Anxiety Inventory de Spielberger. Can J Behav Sci 1993;25: 559-78.
- 29.Yesavage JA, Brink TL, Rose TL, Lum O, Huang V, Adey M, et al. Development and validation of a geriatric depression screening scale: a preliminary report. J Psychiatr Res 1982-83;17(1):37-49. [DOI] [PubMed]
- 30.Bourque P, Blanchard L, Vézina J. Étude psychométrique de l'échelle de dépression gériatrique. Can J Aging 1990;9:348-55.
- 31.Morin CM. Insomnia: psychological assessment and management. New York: Guilford Press; 1993.
- 32.Spielman AJ, Saskin P, Thorpy MJ. Treatment of chronic insomnia by restriction of time in bed. Sleep 1987;10:45-56. [PubMed]
- 33.Bootzin RR, Epstein D, Wood JM. Stimulus control instructions. In: Hauri P, editor. Case studies in insomnia. New York: Plenum; 1991. p. 19-28.
- 34.Glovinsky PB, Spielman AJ. Sleep restriction therapy. In: Hauri P, editor. Case studies in insomnia. New York: Plenum; 1991. p. 49-63.
- 35.Jennings W. Analytical gas chromatography. San Diego: Academic Press; 1987.
- 36.Greenblatt DJ, Franke K, Shader RI. Analysis of lorazepam and its glucuronide metabolite by electron-capture gas-liquid chromatography. J Chromatogr 1978;146:311-20. [PubMed]
- 37.Riedel BW, Lichstein KL, Peterson BA, Epperson MT, Means MK, Aguillard RN. A comparison of the efficacy of stimulus control for medicated and non medicated insomniacs. Behav Modif 1998;22:3-28. [DOI] [PubMed]
- 38.Morin CM, Stone J, McDonald K, Jones S. Psychological management of insomnia: a clinical replication series with 100 patients. Behav Ther 1994; 25: 291-309.
- 39.Morin CM, Colecchi CA, Ling WD, Sood RK. Cognitive behavior therapy to facilitate benzodiazepine discontinuation among hypnotic-dependent patients with insomnia. Behav Ther 1995;26:733-45.
- 40.Onyett SR, Turpin G. Benzodiazepine withdrawal in primary care: a comparison of behavioral group training and individual sessions. Behav Psychother 1988; 16: 297-312.
- 41.Murphy SM, Tyrer P. A double-blind comparison of the effects of gradual withdrawal of lorazepam, diazepam and bromazepam in benzodiazepine dependence. Br J Psychiatry 1991;158:511-6. [DOI] [PubMed]
- 42.Romach MK, Kaplan HL, Busto UE, Somer G, Sellers EM. A controlled trial of ondansetron, a 5-HT3 antagonist, in benzodiazepine discontinuation. J Clin Psychopharmacol 1998;18:121-31. [DOI] [PubMed]
- 43.Rickels K, Case WG, Schweizer E, Garcia-Espana F, Fridman R. Long-term benzodiazepine users 3 years after participation in a discontinuation program. Am J Psychiatry 1991;148:757-61. [DOI] [PubMed]
- 44.Shapiro CM, Sherman D, Peck DF. Withdrawal from benzodiazepines by initially switching to zopiclone. Eur Psychiatry 1995;10(Suppl 3):145s-151s. [DOI] [PubMed]
- 45.Coates TJ, Killen JD, George J, Marchini E, Silverman S, Toresen C. Estimating sleep parameters: a multitrait-multimethod analysis. J Consult Clin Psychol 1982;50:345-52. [PubMed]
- 46.Hoch CC, Reynolds CF, Kupfer DJ, Berman SR, Houck PR, Stack JA. Empirical note: self-report versus recorded sleep in healthy seniors. Psychophysiology 1987;24:293-9. [DOI] [PubMed]
- 47.Spiegel R. Subjective sleep assessment and polygraphic criteria. In: Sleep and sleeplessness in advanced age. Vol. 5 of Advances in sleep research series (Weitzman ED, editor). New York: Spectrum; 1981. p. 169-88.
- 48.Nüesch E, Spiegel R. Objective correlates of subjective sleep assessments in young and elderly subjects. In: Koela WP, Levin P, editors. Sleep 1976: 3rd European Congress on Sleep Research. New York and Basel: Karger; 1977. p. 281-5.
- 49.Lacks P, Morin CM. Recent advances in the assessment and treatment of insomnia. J Consult Clin Psychol 1992;60:586-94. [DOI] [PubMed]
- 50.Morin CM, Kowatch RA, Barry T, Walton E. Cognitive-behavior therapy for late-life insomnia. J Consult Clin Psychol 1993;61:137-46. [DOI] [PubMed]
- 51.Campbell AJ, Robertson MC, Gardner MM, Norton RN, Buchner DM. Psychotropic medication withdrawal and a home-based exercise program to prevent falls: a randomized controlled trial. J Am Geriatr Soc 1999;47:850-3. [DOI] [PubMed]


