Abstract
We performed a comprehensive cognitive, neuroimaging, and genetic study of 31 patients with primary progressive aphasia (PPA), a decline in language functions that remains isolated for at least 2 years. Detailed speech and language evaluation was used to identify three different clinical variants: nonfluent progressive aphasia (NFPA; n = 11), semantic dementia (SD; n = 10), and a third variant termed logopenic progressive aphasia (LPA; n = 10). Voxel-based morphometry (VBM) on MRIs showed that, when all 31 PPA patients were analyzed together, the left perisylvian region and the anterior temporal lobes were atrophied. However, when each clinical variant was considered separately, distinctive patterns emerged: (1) NFPA, characterized by apraxia of speech and deficits in processing complex syntax, was associated with left inferior frontal and insular atrophy; (2) SD, characterized by fluent speech and semantic memory deficits, was associated with anterior temporal damage; and (3) LPA, characterized by slow speech and impaired syntactic comprehension and naming, showed atrophy in the left posterior temporal cortex and inferior parietal lobule. Apolipoprotein E ε4 haplotype frequency was 20% in NFPA, 0% in SD, and 67% in LPA. Cognitive, genetic, and anatomical features indicate that different PPA clinical variants may correspond to different underlying pathological processes.
Isolated speech and language difficulties are often the first symptoms of focal forms of neurodegenerative diseases, particularly frontotemporal lobar degeneration (FTLD) and corticobasal degeneration (CBD).1,2 Alzheimer’s disease (AD) patients also have been shown to present with atypical focal cognitive manifestations, including fluent and nonfluent progressive aphasia.3–7 When speech and language deficits remain the only complaint for at least 2 years, the term primary progressive aphasia (PPA) has been applied.8
Pathologically, the most frequent finding in PPA is an FTLD-type of damage such as dementia lacking distinctive pathology (DLDH)9–11 or Pick’s disease.12,13 Cases with AD,3 Creutzfeldt–Jakob disease,1,4 and FTLD with motor neuron disease (FTLD-MND) pathology also have been reported15 (for review, see Mesulam,16 Grossman,17 and Black18). Kertesz first included CBD in the FTLD/Pick’s spectrum of diseases and recently reported four PPA cases with pathologically proven CBD.12,19
Different clinical presentations of PPA have been reported, but large studies that investigate both cognitive and neuroimaging findings in the same group of patients are still lacking. Here, we consider the clinical variants that have been more consistently described. The nonfluent progressive aphasia variant (NFPA) or “PPA with agrammatism” is characterized by “labored” speech, agrammatism in production, and/or comprehension, variable degrees of anomia, and phonemic paraphasias, in the presence of relatively preserved word comprehension.1,20,21 Semantic dementia (SD), also sometimes called “fluent progressive aphasia,” is characterized by fluent, grammatically correct speech, loss of word and object meaning, surface dyslexia, and relatively preserved syntactic comprehension skills.22 The most recent clinical criteria for FTLD include NFPA and SD as two possible clinical presentations of FTLD.1 SD also has been included in the PPA spectrum, as a “PPA-plus” syndrome.16 In the early 1990s an additional syndrome was described called “progressive aphemia” or “anarthria,” in which patients showed mainly a severe speech disorder.23–25 A “logopenic” variant also has been described, with word-finding difficulties and decreased output but relatively preserved syntax and phonology.26,27 Recently, Mesulam16 has introduced another type of PPA called “PPA with comprehension (verbal semantics) deficits” characterized by fluent speech and anomia but not the multimodal semantic deficits typical of SD. Kertesz and colleagues27 raised the possibility that some of these clinical variants could represent different stages of the same disease.
Neuroimaging and neuropathology findings have shown that isolated speech and language deficits in PPA correspond to a mainly left hemisphere anatomical involvement.1,3,7,28 However, the association of specific PPA clinical variants with discrete regions in the speech and language network remains vague. The anatomical basis of the cognitive impairment in SD has been investigated previously,29–32 but no study has directly compared SD with other variants of PPA. Functional imaging studies have shown left frontal hypomethabolism in the nonfluent clinical variant.23,33,34 Recent work from the Mesulam’s group35 compared a group of PPA patients and normal controls using voxel-based morphometry (VBM), although cases were not classified as having a particular clinical variant of PPA. Atrophy was found in the left superior temporal gyrus and inferior parietal lobule.
In this study, we performed a VBM analysis in a neurologically, cognitively, and genetically well-characterized cohort of 31 PPA patients to identify the overall network of brain regions involved in PPA, and the specific patterns of atrophy associated with each PPA clinical variant. Localization of gray matter loss and differences in APOE ε4 haplotype frequency in each clinical variant provide useful information regarding the possible underlying causative diagnosis in PPA.
Patients and Methods
Patients and Control Group
Cognitive, neuroimaging, and genetic data were collected for 31 patients with PPA diagnosed at the University of California at San Francisco (UCSF) Memory and Aging Center (Table 1). Clinical history, neurological examination, and a general neuropsychological screening (including a short naming test and two word-generation tasks) were used to obtain the PPA diagnosis. Neuroimaging findings were used only to exclude other causes of focal brain damage including extensive white matter disease. Apolipoprotein E (APOE) genotyping was obtained using standard procedures.36
Table 1.
Demographics and Neuropsychological Screen Data for All PPA Patients
| PPA,
Mean (sd) (N = 31) |
Controls,
Mean (sd) (N = 10) |
|
|---|---|---|
| Demographic/functional/genetic (maximum) | ||
| Age | 67.62 (8.2) | 69.5 (5.4) |
| Education | 16.03 (3.1) | 16.3 (2.7) |
| Male/female patients | 13/18 | 5/5 |
| MMSE (30) | 23.8 (5.1) | 29.5 (0.7) |
| CDR total | 0.6 (0.4) | 0.0 |
| ApoE4 frequency: | 30% | NA |
| Years from first symptom | 4.5 (1.8) | NA |
| Language | ||
| Phonemic fluency | 6.8 (3.7) | 16.6 (6.8) |
| Semantic fluency | 7.6 (4.5) | 21.2 (3.6) |
| Abbreviated BNT (15) | 8.8 (5.1) | 14.4 (0.7) |
| Visuospatial functions | ||
| Modified Rey–Osterrieth copy (17) | 14.9 (2.1) | 15.1 (1.7) |
| Cube copy (2) | 1.6 (0.6) | 1.6 (0.7) |
| VOSP number location (10) | 9.0 (1.2) | NA |
| Visual memory | ||
| WMS-III Faces: | ||
| Faces I (sealed score) | 8.3 (2.8) | 13.0 (3.3) |
| Faces II (sealed score) | 9.0 (4.0) | 14.6 (3.2) |
| Modified Rey–Osterrieth delay (17) | 8.13 (4.4) | 10.9 (3.9) |
| Verbal memory | ||
| CVLT-MS (9): 30″ Free Recall | 3.6 (2.7) | 7.9 (1.6) |
| 10-min. free recall | 3.2 (3.1) | 7.3 (1.6) |
| 10-min recognition | 6.5 (2.9) | 8.7 (0.9) |
| Verbal executive functions | ||
| Digit span backward | 3.5 (1.5) | 4.9 (1.1) |
| Modified Trails no. of lines/min | 12.9 (14.8) | 37.2 (9.8) |
PPA = primary progressive aphasia; sd = standard deviation; NA = not applicable; BNT = Boston Naming Test; WMS = Wechsler Memory Scale; CVLT-MS = California Verbal Learning Test–Mental Status; VOSP = visual object and space perception battery.
Clinical history demonstrated that speech and language deficits were the presenting symptoms and remained isolated for at least 2 years. At the time of study, all patients were at least 3 years into the disease, and language/speech deficits were still the main cause of functional impairment. Exclusion criteria for PPA included history of everyday episodic memory impairment as first and/or primary symptom and significant deficits in tests of visual memory and/or visuospatial functions on neuropsychological screening. Patients also were excluded if they showed motor and ocular signs typical of progressive supranuclear palsy (PSP), CBD, or MND.
Patients were included in the study prospectively within a 2-year period. During the same time period, 15 additional patients were referred to UCSF for prominent language or speech disorder. They were excluded from the study because the screening visit showed generalized cognitive impairment (five AD probable and four nontestable patients); significant vascular changes on magnetic resonance imaging (MRI; two cases); significant asymmetric extrapyramidal syndrome, dystonia, or alien limb (three CBD cases); or upper and lower motor neuron signs (one FTLD-MND case).
Sixty-four age- and sex-matched healthy subjects were used as controls for the VBM study. None of the control subjects had any history of neurological or psychiatric disorders, and all showed normal MRI. The mean age was 68.2 years (range, 56 – 81 years; 32 men, 32 women). Cognitive data from 10 of the 64 subjects were used as a control for the cognitive analysis.
The study was approved by the UCSF committee on human research. All subjects provided written informed consent before participating.
Cognitive Testing
Each patient participated in a neuropsychological screening battery and a detailed speech and language evaluation. The neuropsychological screening was used to determine if patients met inclusion criteria for PPA. Once PPA was diagnosed, the speech and language battery was used to identify different PPA clinical variants. Only language testing, not neuroimaging findings, was used to classify patients into clinical variants.
Neuropsychological Screening Battery
General intellectual function was assessed using the Mini-Mental State Examination.37 Initial language screening included the abbreviated Boston Naming Test (15 items)38 (BNT) and semantic (number of animals/1 min) and phonemic (number of D words/1 min) word generation. The California Verbal Learning Test–Mental Status Version39 (CVLT-MS), a modified version of the Rey–Osterrieth complex figure with a 10-minute free recall delay trial and the Faces subtest from the Wechsler Memory Scales–Third Edition (WMS-III)40 were used to evaluate verbal and nonverbal episodic memory. Visuospatial abilities were assessed by the copying of a modified Rey–Osterrieth figure and a transparentcube, and by the Number Location Test from the Visual Object Space Perception Battery.41 A sequencing task (modified Trails B test42) and backward digit span assessed executive functioning. Ability to perform five arithmetic calculations also was assessed. Praxis was evaluated by asking patients to perform seven buccofacial, transitive limb, and intransitive limb praxis tasks, each rated on a two-point scale. All tests were administered both to patients and the 10 age-matched normal controls to determine the normative range of scores for nonstandardized tests.
Speech and Language Evaluation
The tasks used to assess speech and language functioning addressed the symptoms and signs specified in the current clinical criteria for PPA.16
SPEECH AND LANGUAGE PRODUCTION
Speech was tested using the Motor Speech Evaluation.43 The Motor Speech Evaluation elicits speech samples with such tasks as vowel prolongation, oral reading, picture description, and the repetition of syllables, monosyllabic, and multisyllabic words and phrases. The examiner determines the presence or absence of dysarthria and apraxia of speech as well as a severity rating (1–7) for each task. Apraxia of speech is a “motor speech disorder resulting from impairment of the capacity to program sensorimotor commands for the positioning and movements of muscles for the volitional production of speech.”44 As described by Wertz and colleagues,43 apraxic errors include initiation difficulty, sound substitutions, omissions or transpositions of syllables, slow rate, equal or even stress, and inappropriate stops and starts. Typically, difficulty articulating multisyllabic words is diagnostic of apraxia of speech.
Spontaneous speech and syntactic production were evaluated using the “Spontaneous Speech” section from the Western Aphasia Battery45 (WAB). The Repetition subtest of the WAB also was used.
LEXICAL RETRIEVAL AND SEMANTIC MEMORY
Confrontation naming was evaluated using the 60-item version of the BNT. An experimental three-alternative, verbally presented, forced-choice word recognition task for the missed items also was added. The “Pyramid and Palm Trees” test evaluated verbal and visual semantic abilities.46 The “Auditory Word Recognition” subtest of the WAB tested comprehension of spoken single words.
SYNTACTIC COMPREHENSION
Sentence and syntactic comprehension was tested using the WAB “Sequential Commands” and, more extensively, by selected subtests of the Curtiss–Yamada Comprehensive Language Evaluation–Receptive47 (CYCLE-R). Eleven subtests of the CYCLE-R were administered, each containing five sentences. Patients were asked to match the meaning of an auditorially presented sentence with the corresponding line drawing in a three- or four-picture array. All subtests involved complete declarative sentences referring to a full state of affairs (referent/s and predication). They spanned a range of sentence types, comprising different levels of morphosyntactic complexity, ranging from simple constructions (simple declaratives and possession: levels 2 and 3) to more elaborated structures (active voice, passive voice, double embedding: levels 4, 5, 6) and complex structures (object clefting, subject relative clauses, negative passives, object relative clauses, and object relative clauses with relativized object: levels 7, 8, 9). The numbers denominating the levels correspond to the age at which children normally learn to comprehend the considered type of sentence.
READING SKILLS
Single-word reading was tested by the “Regularity and Reading” subtest of the Psycholinguistic Assessments of Language Processing in Aphasia48 (PALPA). Reading aloud of the “Grandfather passage” was assessed as part of the Motor Speech Evaluation.
Primary Progressive Aphasia Subgroup Classification
Patients initially were classified as having PPA using clinical history and general neuropsychological examination. Data from a detailed speech and language evaluation then were used to further classify patients into different clinical subgroups.
NFPA was defined using criteria for the “PPA with agrammatism”16 variant of PPA and for the “Progressive Aphasia nonfluent” variant of FTLD.1 NFPA is characterized by nonfluent speech output, “labored articulation,” agrammatism, difficulty with comprehension of complex syntactic structures, word-finding deficits, and preserved single-word comprehension (see also Hodges and Patterson,20 Rhee and colleagues,49 and Hillis and colleagues50). Specifically, patients needed to show at least two of the following: (1) motor speech deficits; (2) agrammatism in language production; (3) spared single-word comprehension and impaired comprehension of only the most complex syntactic structures (CYCLE, levels 8/9).
SD was defined using the description by Hodges and colleagues22, 51 as well as the Neary criteria for FTLD. SD patients present with fluent, grammatical speech; confrontation naming deficits; semantic deficits for words and, initially less severely, for objects; surface dyslexia; and relatively spared syntactic comprehension. Specifically, patients needed to show at least three of the following: (1) no grammatical errors and normal language output (circumlocutions and usage of nonspecific words were accepted); (2) scores below two standard deviations from the control mean in the 60-item BNT; (3) scores below two standard deviations from the control mean in the Pyramid and Palm Trees test; and (4) normal syntactic comprehension as measured by the CYCLE.
Ten patients who met general PPA clinical criteria based on history and neuropsychological screening did not show a pattern of speech and language deficit compatible with NFPA or SD. To be consistent with previous reports, we used the term logopenic for this group because their language output was slow in rate, grammatically simple but correct, and halted by frequent word-finding pauses. Nonfluent, logopenic speech, and relative sparing of semantics and single-word comprehension in the presence of severe syntactic deficits excluded an SD diagnosis. Preserved articulation, absence of agrammatism, and generalized syntactic comprehension excluded NFPA. The pattern of language impairment specific to this group are given in the Results section.
Statistical Analysis of Cognitive Data
A post hoc statistical analysis of the speech and language results was conducted to better characterize the specific deficits and illustrate the patterns of impairment in each group. Analyses of variance were used to show overall group differences, and the Scheffe’s method was used for post hoc comparisons. Normative data reported in test booklets were used for the BNT and the PALPA reading test. The Kruskal–Wallis and the Mann–Whitney U tests were used when non-parametric testing was necessary. To characterize nonlanguage cognitive performance in each PPA variant, we also conducted a post hoc statistical analysis of the neuropsychological data. Statistical analyses were performed with SPSS (version 10.0.5 for Windows; SPSS, Chicago, IL).
Formal statistical analysis of the genetic data was not performed because of the low number of subjects in the groups. Frequencies of APOE ε4 haplotype are reported.
Imaging Data
MAGNETIC RESONANCE IMAGING SCANNING
MRI scans were obtained on a 1.5T Magnetom VISION system (Siemens, Iselin, NJ). A volumetric magnetization prepared rapid gradient-echo MRI (MPRAGE, TR/TE/TI = 10/4/300 milliseconds) was used to obtain T1-weighted images of the entire brain, 15-degree flip angle, coronal orientation perpendicular to the double spin-echo sequence, 1.0 × 1.0 mm2 in-plane resolution and 1.5mm slab thickness.
VOXEL-BASED MORPHOMETRY
VBM is a technique for the detection of regional brain atrophy by voxel-wise comparison of gray matter volumes between groups of subjects.52,53 The technique comprises an image preprocessing step (spatial normalization, segmentation, modulation, and smoothing) followed by statistical analysis. Both stages are implemented in the SPM99 software package (www.fil.ion.ucl.ac.uk/spm). To optimize the spatial normalization, we created an ad hoc age-matched template image. Affine and nonlinear transformations were applied to spatially normalize patients’ and controls’ images to the template. Each normalized image then was segmented into gray, white, and cerebrospinal fluid compartments. Gray matter voxel values were multiplied by the Jacobian determinants derived from the spatial normalization step to preserve the initial volumes. Images then were spatially smoothed with a 12mm full-width at half-maximum isotropic Gaussian kernel. Total intracranial volume was used as a confounding covariate in an analysis of covariance. Age and sex for each subject were entered into the design matrix as nuisance variables. Regionally specific differences in gray matter volumes were assessed using the general linear model, and the significance of each effect was determined using the theory of Gaussian fields. Specific statistical analyses were performed to investigate the overall network of regions involved in PPA and regions specific to each PPA subgroup. We accepted a statistical threshold of p value less than 0.05 corrected for multiple comparisons.
VBM recently has been shown to be sensitive in detecting changes in gray matter volumes in neurodegenerative disorders.53 The spatial resolution of the technique is 12mm, and the risk of false-negatives when considering single subjects limits its routine clinical use.
Results
Cognitive Data: Post Hoc Analysis of Speech and Language Performance in Each Primary Progressive Aphasia Variant
NONFLUENT PROGRESSIVE APHASIA
As expected, the NFPA group scored lowest in the WAB fluency task (Table 2). The major reason for dysfluency was significant apraxia of speech that was found in 9 of 11 patients. Four patients also showed mild dysarthria. Varying degrees of morphological and syntactic errors were present in speech production, repetition, and passage reading tasks. Single-word comprehension was unimpaired, confrontation naming on the BNT was at the 50th percentile (with good recognition of the unnamed items), and semantic memory was within normal limits. Syntactic comprehension was impaired only for the most complex structures (CYCLE level 9), whereas performance on the WAB “Sequential Commands” was within normal limits. Similar to the other patient groups, the NFPA group performed below the first percentile in the single-word reading task. Errors were equally distributed between regular and irregular words.
Table 2.
Post Hoc Analysis of Speech and Language Performance
| NFPA
Mean (sd) (N = 11) |
SD
Mean (sd) (N = 10) |
LPA
Mean (sd) (N = 10) |
Controls
Mean (sd) (N = 10) |
|
|---|---|---|---|---|
| Speech and language production (maximum) | ||||
| Speech fluency on WAB (10) | 5.4 (3.1)a | 8.9 (0.6)b | 8.0 (1.5)a | 10 (0) |
| Apraxia of speech rating | 3.4 (2.6)c | 0 (0) | 1.7 (1.8) | NA |
| Dysarthria rating (7 = max deficit) | 2.7 (2.8) | 0 (0) | 0 (0) | NA |
| WAB repetition (100) | 64.5 (36)a | 85.3 (11) | 71.6 (18)a | 99.5 (0.9) |
| Phonemic fluency (from screening) | 6.6 (4.3)a | 6.4 (3.0)a | 7.5 (3.8)a | 16.6 (6.8) |
| Semantic fluency (from screening) | 10.1 (4.3)a | 4.3 (3.4)a,b | 8.0 (4.1)a | 21.2 (3.6) |
| Lexical retrieval and semantic memory: | ||||
| WAB word recognition Total (60): | 60 (0) | 48.8 (12)a,b,c | 58.5 (1.6) | 6.0 (0) |
| Single categories (6) | ||||
| Real objects | 6.0 (0) | 4.6 (1,6)a,b,c | 6.0 (0) | 6.0 (0) |
| Drawn objects | 6.0 (0) | 4.9 (1,8) | 6.0 (0) | 6.0 (0) |
| Shapes | 6.0 (0) | 3.3 (2.1)a,b,c | 5.9 (0.3) | 6.0 (0) |
| Letters | 6.0 (0) | 6.0 (0) | 6.0 (0) | 6.0 (0) |
| Numbers | 6.0 (0) | 6.0 (0) | 6.0 (0) | 6.0 (0) |
| Colors | 6.0 (0) | 5.6 (0.7) | 6.0 (0) | 6.0 (0) |
| Furniture | 6.0 (0) | 5.2 (1.4) | 6.0 (0) | 6.0 (0) |
| Body parts | 6.0 (0) | 4.9 (1.3)a,b,c | 6.0 (0) | 6.0 (0) |
| Fingers | 6.0 (0) | 4.0 (2.1)a,b | 5.2 (0.7) | 6.0 (0) |
| Left and right | 6.0 (0) | 4.2 (2)a,b | 5.4 (1.4) | 6.0 (0) |
| Boston Naming Test (60) | 50 (5.7)c | 10 (4.5)a,b | 30 (13)b | NA |
| Recognized items (% of unnamed) | 94% (17) | 23% (21)a,b | 73% (41) | NA |
| Total Pyramids/Palm Trees (52) | 49.5 (2.1) | 35.3 (8.1)a,b,c | 46.8 (2.8) | 51.8 (0.4) |
| Syntactic comprehension | ||||
| WAB sequential commands (80) | 69 (10) | 73 (8.6) | 59.4 (21)a | 80 (0) |
| CYCLE (2 = simplest, 9 = most complex) | ||||
| Total (55) | 49.5 (4) | 51 (5.1)c | 38 (13)a | 53.8 (0.9) |
| Cycle 2, 3 (10) | 10 (0) | 9.7 (0.7) | 9.4 (0.8) | 10 (0) |
| Cycle 4 (15) | 14.4 (0.7)c | 14.1 (1.2)c | 9.9 (4)a | 15 (0) |
| Cycle 5, 7 (10) | 9.2 (0.8)c | 9.6 (1)c | 5.3 (3.5)a | 10 (0) |
| Cycle 8 (10) | 9.0 (1,2)c | 9.7 (0.5)c | 6 (2.7)a | 9.7 (0.4) |
| Cycle 9 (10) | 7 (2.2)a,c | 8.7 (1.2)b,c | 4.7 (2.2)a | 9 (1) |
p < 0.05 vs controls.
p < 0.05 vs NFPAs.
p < 0.05 vs LPAs.
NFPA = nonfluent progressive aphasia; SD = sematic dementia; LPA = logopenic progressive aphasia; sd = standard deviation; WAB = Western Aphasia Battery; NA = not applicable; CYCLE = Curtiss–Yamada Comprehensive Language Evaluation–Receptive.
SEMANTIC DEMENTIA
The SD group did not differ significantly from controls on the WAB picture description task (see Table 2). Speech was normal in rate, well articulated, and grammatically correct. Nonspecific words, such as “thing,” or supraordinate category names (ie, “animal” for “dog”) often were used to circumvent word-finding difficulties. Only three patients had reached a stage in the disease in which they had difficulty comprehending conversational speech and showed generalized object and/or face recognition deficits. Word and sentence repetition were spared. SD patients showed a significant overall deficit in single-word comprehension, particularly for shapes, whereas comprehension of color names was relatively spared. The SD group showed the worst performance on the 60-item BNT, along with severely impaired scores on BNT recognition and on the semantic association test. SDs performed within normal limits in the comprehension of even the most complex syntactic structures (CYCLE level 9). In the context of an overall deficit in single-word reading, SD patients had greater difficulty reading irregular than regular words (ratio regular/irregular of 1.5).
LOGOPENIC PROGRESSIVE APHASIA
Patients obtained a score intermediate between NFPAs and SDs in the WAB picture description task. They showed a pattern of speech output that has been defined as “logopenic,” that is, slow, made of syntactically simple but correct sentences, with frequent word-finding pauses. Typically, the logopenic group had generalized difficulty on syntactic comprehension tasks, and, unlike the SD and NFPAs groups, LPAs were significantly impaired in all but the simplest (levels 2 and 3) CYCLE subtests. Repetition also was significantly impaired compared with controls (p < 0.05). Logopenic patients performed below the first percentile on confrontation naming but were able to recognize 73% of the unnamed items.
Single-word comprehension and semantic association abilities were within normal limits. Considering that patients were at least 3 years into the disease and showed logopenic speech, relative spared semantics with marked syntactic comprehension deficits, a diagnosis of initial SD is unlikely. Reading of single words was below the first percentile with errors almost equally distributed between regular and irregular words.
Cognitive Data: Post Hoc Analysis of General Neuropsychological Performance
There were no significant differences among groups in sex, age, education, or disease duration (Table 3). Consistent with our inclusion and exclusion criteria, none of the three patient groups differed from the control group on the visuospatial and visual memory tests. All groups were significantly impaired compared with controls on word-generation tasks, verbal immediate recall (CVLT-MS: trials 1– 4 total), and verbally mediated executive tasks (digits-backward and modified Trails). CDR scores were significantly lower in each patient group compared with controls.
Table 3.
Post Hoc Analysis of Neuropsychological Performance by PPA Subgroup
| NFPA
Mean (sd) (N = 11) |
SD
Mean (sd) (N = 10) |
LPA
Mean (sd) (N = 10) |
Controls
Mean (sd) (N = 10) |
|
|---|---|---|---|---|
| Demographic/functional/genetic (maximum) | ||||
| Age | 67.9 (8.1) | 63.0 (5.8) | 72.0 (8.5) | 69.1 (7.6) |
| Education | 14.9 (2.3) | 15.7 (3.0) | 17.6 (3.6) | 16.3 (2.7) |
| Males/Females | 3/8 | 5/5 | 5/5 | 5/5 |
| MMSE | 26.0 (3.4) | 23.1 (6.5)a | 22.2 (4.6)a | 29.5 (0.7) |
| CDR Total | 0.5 (0.4)a | 0.5 (2.7)a | 0.7 (0.4)a | 0.0 (0.0) |
| ApoE4 frequency | 20% | 0% | 67% | NA |
| Years from first symptom | 4.4 (2.5) | 4.0 (1.2) | 4.5 (0.8) | NA |
| Visuospatial functions | ||||
| Modified Rey–Osterrieth Copy (17) | 14.2 (2.2) | 16.2 (1.0) | 14.4 (2.3) | 15.1 (1.7) |
| Cube copy (2) | 1.5 (0.5) | 1.9 (0.3) | 1.3 (0.8) | 1.6 (0.7) |
| Visual memory | ||||
| WMS-III Faces: | ||||
| Faces I (scaled score) | 8.8 (3.8) | 6.6 (1.7)a | 9.8 (1.3) | 13.0 (3.3) |
| Faces II (scaled score) | 9.5 (4.2) | 6.4 (2.1)a | 11.5 (4.2) | 14.6 (3.2) |
| Modified Rey–Osterrieth Delay (17) | 9.3 (4.4) | 8.3 (5.0) | 6.7 (3.7) | 10.9 (3.9) |
| Verbal memory | ||||
| CVLT-MS (9) | ||||
| Trials 1–4 total | 20.8 (7.2)a,b | 13.0 (6.1)a,c | 14.0 (2.2)a,c | 28.7 (3.1) |
| 30-sec free recall | 5.6 (2.7) | 1.9 (2.4)a,c | 3.0 (1.3)a,c | 7.9 (1.6) |
| 10-min free recall | 5.4 (3.3) | 1.3 (2.4)a,c | 2.5 (1.7)a | 7.3 (1.6) |
| 10-min recognition | 7.6 (1.6) | 3.6 (3.5)a,b,c | 7.8 (1.6) | 8.7 (0.9) |
| Verbal executive functions | ||||
| Digit span backward | 2.9 (1.6)a | 4.8 (1.0)a,b | 2.9 (0.7)a | 4.9 (1.1) |
| Modified Trails no. of lines/min | 16.5 (17.4)a | 17.9 (15.4)a | 3.6 (2.8)a | 37.2 (9.8) |
| Praxis (14) | 10.7 (2.9)a | 10.9 (3.4) | 12.7 (1.8) | 14.0 (0.0) |
| Calculation (5) | 4.1 (1.0)b | 3.9 (1.7)b | 2.2 (1.1)a,c | 4.5 (0.5) |
p < 0.05 vs controls.
p < 0.05 vs LPAs.
p < 0.05 vs NFPAs.
PPA = primary progressive aphasia; NFPA = nonfluent progressive aphasia; sd. = standard deviation; SD = semantic dementia; LPA = logopenic progressive aphasia; MMSE = Mini-Mental State Examination; WMS = Wechsler Memory Scales; CVLT-MS = California Verbal Learning Test–Mental Status.
NONFLUENT PROGRESSIVE APHASIA
The NFPA group performed significantly better on verbal memory tasks than the other two patient groups (p < 0.05), although their scores were still lower than controls (p < 0.05; see Table 3). They did not differ from the other groups on nonverbal memory or executive testing. In contrast with the other groups, they performed within normal limits on MMSE and short BNT. They also showed the worst performance on the praxis test.
SEMANTIC DEMENTIA
The SD group had significantly lower scores than the other two groups on tests of verbal recognition (p < 0.01). However, SDs performed significantly better than either the LPAs or the NFPAs on a test of verbal working memory (backward digit span; p < 0.01). Their semantic word-generation scores were significantly lower than the NFPA group, although phonemic word-generation scores among the groups were equivalent.
LOGOPENIC PROGRESSIVE APHASIA
The LPA group scored worst on calculations. Their immediate and 30-second recall of verbal information was significantly worse than the NFPA patients (p < 0.05), but their 10-minute recognition memory and their nonverbal memory did not differ significantly from controls.
Neurological and Genetic Findings
NONFLUENT PROGRESSIVE APHASIA
In six patients, neurological examination showed mild motor symptoms, such as diffuse motor slowing, reduced dexterity, and mild rigidity in the right arm and hand. No patient showed dystonia, myoclonus, alien hand syndrome, or reported frequent falls. Conjugated eye movement examination showed slowness of lateral gaze, more marked toward the right in seven patients. No supranuclear gaze palsy was observed. No signs of MND and no swallowing troubles were detected. The frequency of APOE ε haplotype in this group was 20% (Table 3).
SEMANTIC DEMENTIA
Neurological examination was completely normal in all patients. The frequency of the APOE ε4 haplotype in this group was 0%.
LOGOPENIC PROGRESSIVE APHASIA
Neurological examination showed minimal extrapyramidal signs in the right arm and hand of one patient. The frequency of APOE ε4 haplotype in this group was 67%.
Neuroimaging Data
ALL PPAS VERSUS CONTROLS
When all 31 PPA patients were compared with controls, a wide network of brain regions (see Fig, A) including the whole left perisylvian region, the anterior temporal lobes bilaterally (more extensively on the left), and the basal ganglia bilaterally (p < 0.05 corrected for multiple comparisons) was found to be significantly atrophied.
Fig.
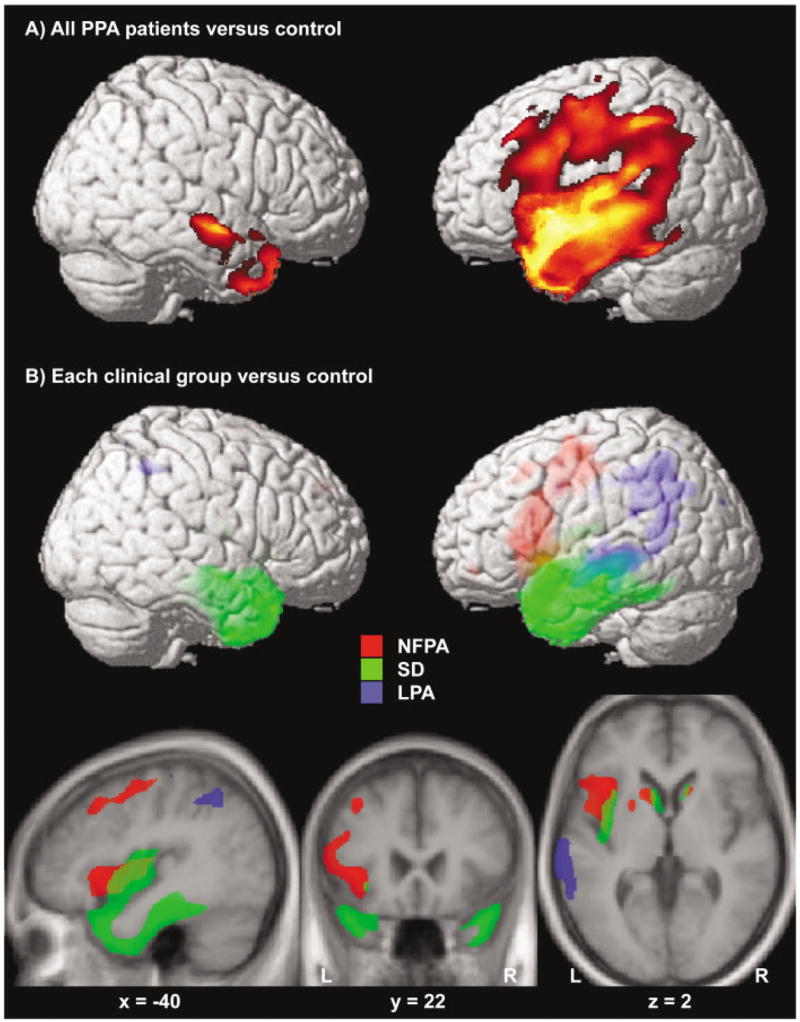
(A) Areas significantly atrophied in all primary progressive aphasia (PPA) patients versus controls. (B) Areas of significant atrophy in each clinical subgroup versus controls are indicated in three different colors (red for nonfluent progressive aphasia [NFPA], green for semantic dementia [SD], and blue for logopenic progressive aphasia [LPA]). All figures were obtained within SPM using a statistical threshold of p value less than 0.05 corrected for multiple comparisons for each contrast. Regions of significant gray matter loss were superimposed on a three-dimensional rendering of the Montreal Neurological Institute standard brain (A, B) and on axial, coronal, and sagittal sections of the mean image of the scans used to obtain the template image (B). The coordinates of the sections correspond to the peak of the left insular cluster in the contrast NFPA versus control. Color saturation in B indicates depth from the cortical surface (less saturated = deeper).
By separately comparing each clinical variant to the control group, we identified which regions were differentially involved in each clinical variant.
NONFLUENT PROGRESSIVE APHASIA
When the NFPA group was compared with controls, significant atrophy was found in the pars opercularis, triangularis (BA 44/45, ie, Broca’s area), and a small region of the pars orbitalis (BA 47) of the left inferior frontal gyrus (p < 0.001 corrected for multiple comparisons; Table 4; in red in Fig, B). Highly significant gray matter loss was also found in the left precentral gyrus of the insula (p < 0.001 corrected). Atrophy extended to the inferior precentral sulcus and gyrus (BA 4/6; p < 0.001 corrected) and anteriorly to the middle frontal gyrus (BA 8; p < 0.001 corrected). Significant atrophy also was found in the caudate nucleus bilaterally extending to the putamen on the left (p < 0.01 corrected).
Table 4.
VBM Results: Each Group versus Controls
| Brain region (Brodman area) | x | y | z | T value | Z-score |
|---|---|---|---|---|---|
| NFPA | |||||
| Left inferior frontal gyrus (44/45/47) | −48 | 15 | 25 | 8.2 | 7.1 |
| −53 | 13 | 17 | 7.9 | 6.9 | |
| −50 | 50 | −9 | 5.5 | 5.1 | |
| Left precentral gyrus of the insula | −40 | 22 | 2 | 7.7 | 6.7 |
| Left precentral sulcus/gyrus (4/6) | −43 | 4 | 49 | 7.9 | 6.8 |
| −54 | −10 | 45 | 6.6 | 5.9 | |
| −36 | −4 | 60 | 6.3 | 5.7 | |
| Left middle frontal gyrus (8) | −38 | 20 | 48 | 7.5 | 6.6 |
| Bilateral caudate nucleus | −11 | 12 | 5 | 6.7 | 6.0 |
| 16 | −4 | 20 | 5.9 | 5.4 | |
| Left putamen | −22 | 10 | −3 | 5.8 | 5.3 |
| SD | |||||
| Bilateral amygdala/anterior hippocampus | −24 | −6 | −21 | 13.5 | Inf |
| 25 | −1 | −24 | 9.3 | 7.7 | |
| Bilateral temporal pole (38/20/21) | −31 | −5 | −39 | 12.2 | Inf |
| −36 | 17 | −42 | 9.7 | Inf | |
| −48 | 14 | −22 | 9.9 | Inf | |
| 29 | −1 | −40 | 11.0 | Inf | |
| 29 | 16 | −37 | 9.7 | Inf | |
| 47 | 22 | −28 | 9.4 | 7.8 | |
| Bilateral ant fusiform (37) | −40 | −20 | −26 | 10.2 | Inf |
| 44 | −20 | −28 | 6.1 | 5.5 | |
| Bilateral middle temporal gyrus (21) | −64 | −10 | −18 | 9.5 | 7.8 |
| −64 | −19 | −7 | 6.9 | 6.2 | |
| 68 | −12 | −16 | 7.9 | 6.9 | |
| Bilateral posterior insula (long gyri) | −39 | −5 | 12 | 6.3 | 6.4 |
| 37 | −7 | 15 | 5.9 | 5.4 | |
| Ventromedial frontal (25) | −1 | 11 | −10 | 6.1 | 5.5 |
| Bilateral caudate nucleus | −9 | 3 | 15 | 7.4 | 6.5 |
| 12 | 0 | 15 | 6.2 | 5.6 | |
| Right posterior thalamus | 6 | −15 | 13 | 6.1 | 5.6 |
| LPA | |||||
| Left inferior parietal lobule (39/40) | −45 | −54 | 49 | 7.8 | 6.8 |
| −56 | −53 | 34 | 6.8 | 6.1 | |
| −53 | −36 | 43 | 6.7 | 6.0 | |
| −60 | −42 | 30 | 5.8 | 5.3 | |
| Left middle temporal gyrus/superior temporal sulcus (21/22) | −68 | −24 | −3 | 7.3 | 6.4 |
| −63 | −47 | 1 | 5.6 | 5.1 | |
| Precuneus (31) | −8 | −54 | 47 | 7.2 | 6.4 |
| Right angular gyrus (39) | 45 | −48 | 48 | 6.3 | 5.7 |
| Left hippocampus | −27 | −10 | −16 | 6.1 | 5.6 |
VBM = voxel-based morphometry; NFPA = nonfluent progressive aphasia; SD = semantic dementia; LPA = logopenic progressive aphasia.
SEMANTIC DEMENTIA
The SD group showed a large cluster of significant atrophy versus controls including the medial and lateral portions of the anterior temporal lobes bilaterally (see Table 4; green area in Fig, B). The clusters included bilateral amygdala/anterior hippocampus (p < 0.001 corrected), bilateral anterior inferior, middle and superior temporal gyri (BA 38/20/21; p < 0.001 corrected), and the anterior fusiform gyri bilaterally (BA 37; p < 0.001 corrected). The posterior portion of the insula, bilateral caudate nuclei, and the ventromedial frontal region also were involved (p < 0.001 corrected; see Fig, B).
LOGOPENIC PROGRESSIVE APHASIA
In the logopenic group, a large cluster of gray matter loss included the angular gyrus (BA 39/40; p < 0.001 corrected), the posterior third of the middle temporal gyrus and of the superior temporal sulcus (BA 21; p < 0.001 corrected). In LPA atrophy thus was located more posteriorly than in the SD group (see Table 4; in blue in Fig, B), but the two variants overlapped in the middle and posterior thirds of the middle temporal gyrus (at a Talairach coordinate of around − 20). Smaller clusters of significant atrophy also were found in the left anterior hippocampus, in the right angular gyrus (BA 39) and in the precuneus (BA 31).
Discussion
We performed a comprehensive study of 31 PPA patients and found that, when appropriate speech and language measures are used to identify three divergent clinical presentations, patterns of specific focal atrophy emerge: left inferior frontal and anterior insular regions in NFPA, bilateral anterior temporal lobes in SD, and left temporoparietal area in LPA. Duration of disease was similar across groups, and neuropsychological, neurological, and genetic findings confirmed the differences between the three clinical variants. We discuss the correspondence between cognitive and anatomical focality in PPA and suggest that FTLD pathology might not always be the underlying process in patients who meet clinical criteria for PPA.
When all 31 PPA patients were compared with controls, the whole network of brain regions involved in speech and language processing showed atrophy (left perisylvian and anterior temporal areas), confirming previous findings of asymmetrical involvement of the left hemisphere in PPA. When linguistic features were used to identify three different clinical variants, specific patterns of focal anatomical damage emerged.
Nonfluent Progressive Aphasia
The main features of the NFPA group were significant apraxia of speech, syntactic deficits for complex morphosyntactic structures, and atrophy in the left inferior frontal gyrus, premotor cortex, and anterior insula regions. These results are consistent with previous findings on the role of these regions in motor speech and syntax processing. The left precentral gyrus of the insula has been associated with apraxia of speech in vascular patients,54 and one case of nonfluent progressive aphasia due to AD showed neuronal loss that included the left insula.55 The finding of significant left anterior insula atrophy in a group of progressive neurodegenerative patients who show significant apraxia of speech confirms this anatomical-cognitive association and provides an important localization tool for the clinician. The role of Broca’s area and of more anterior left pre-frontal regions in syntactic comprehension and/or motor speech56,57 is still debated. Lesions confined to Broca’s area result in mild and transient language disturbances,58 whereas the full clinical picture of Broca’s aphasia is caused by larger lesions in the left frontal lobe.58 On the other hand, recent functional neuroimaging studies have shown Broca’s area activation in learning grammatical rules.59,60 Metabolic imaging studies previously have shown frontal lobe hypometabolism in a few cases of “progressive aphemia”23,25 who show different levels of language comprehension deficits. It is likely that NFPA and “progressive aphemia” represent parts of a clinical spectrum, in which damage in the insula/premotor inferior frontal area and/or in more anterior prefrontal regions causes various degrees of speech and/or syntactic impairment.
The NFPA group was also the only group to show significant buccofacial and limb apraxia and moderate extrapyramidal signs in the right hand and arm. No signs of MND were found. Consistently, this group showed atrophy in the inferior frontal premotor areas involved with the control of facial movements61 and the caudate nuclei, more extensively on the left.
Pathological confirmation will be necessary to definitely determine the cause in each of these clinical syndromes. However, significant left frontal atrophy argues that NFPA could be the left frontal variant of FTLD. Previous pathological studies have demonstrated that Pick’s or DLDH type pathology can show a similar anatomical distribution.9 Alternatively, asymmetric motor findings, praxis deficits, and atrophy in brain regions involved in motor functions raise the possibility of a CBD cause.2 Longer clinical follow-up will determine whether the motor involvement in NFPA could predict an evolution to the FTLD-MND syndrome.62
Semantic Dementia
The cognitive and anatomical features of SD have been extensively studied. Our findings emphasize the need for more specific cognitive measures to correctly differentiate mild, early presentations of SD from the more classic language deficits found in the logopenic variant. In particular, we confirmed the striking dissociation in SD between impaired performance in confrontation naming, semantic association tasks, and single-word comprehension versus intact syntactic comprehension. Preserved speech fluency (rate, grammatical content, and articulatory abilities) was also important in differentiating SD from NFPA and LPA.
We found bilateral anterior temporal atrophy in SD, confirming previous studies that associated these regions with semantic memory deficits.29–32 SD has been associated mainly with FTLD-like pathology,12 but large pathological studies are still needed. The finding that APOE ε4 frequency in the SD group was 0% further supports this view.
Logopenic Progressive Aphasia
We identified a third group of patients who met general clinical criteria for PPA, but who, after detailed speech and language evaluation, did not meet criteria for NFPA or SD. Because patients showed a slow rate of speech output and word-finding pauses, and consistently with previous reports, we called this group “logopenic.” Agrammatism in production and articulation deficits were not typical of LPA patients. Single-word comprehension, recognition of nonnamed items, and performance on the semantic association test were relatively spared, whereas syntactic comprehension was markedly impaired. The deficit was not limited to the most complex structures, as in the NFPA, but also included other constructions, such as simple passives. This finding, together with a significant deficit in sentence repetition, suggests that a short-term phonological memory deficit could be the core mechanism underlying the clinical presentation of LPA. Our VBM results are compatible with this idea, showing that the left posterior temporal and parietal lobules were the areas most atrophied in LPA. Both patient studies and functional imaging activation studies have, in fact, pointed to the inferior parietal lobule as the site for the phonological store portion of the phonological loop.63–65
A recent study from the Mesulam’s group reported the findings of a VBM analysis on 11 PPA patients, who were not classified as belonging to a specific clinical variant.35 Results showed a left temporoparietal pattern of atrophy very similar to the one we found in LPA. This is consistent with the fact that, based on the available data, most of their patients demonstrated logopenic speech output without articulation difficulties, and thus might correspond to our LPA variant. Our group of LPA patients also showed small clusters of atrophy in the right parietal lobe and in the left anterior hippocampus. Although anterior hippocampal atrophy also has been found in SD,29,32 neuroimaging and pathological evidence has shown that damage to the left temporoparietal junction is typical of probable AD presenting mainly with language symptoms.5,66–69 Furthermore, parietal involvement has been associated with probable AD both in neuroimaging analysis in vivo34,70 and in pathological studies postmortem.71 Although pathological confirmation is needed for final diagnosis, the temporoparietal pattern of atrophy and genetic evidence of a higher than expected frequency of the APOE ε4 haplotype72–74 in LPA (67%) suggest that many of these patients might have an atypical form of AD. Alternatively, the possibility that this genetic profile could influence the location or type of FTLD-like pathology also should be considered.
In conclusion, a comprehensive speech and language evaluation was necessary to identify three clinical variants of PPA. A specific and focal pattern of brain atrophy within the speech and language network and distinctive neuropsychological and genetic profiles were associated with each clinical syndrome. The different cognitive, anatomical, and genetic profiles in NFPA, SD, and LPA suggest that PPA is highly heterogeneous and that different variants may correspond to different pathological profiles. Further studies combining detailed cognitive evaluation, image analysis in vivo, and pathological findings are necessary to confirm this hypothesis.
Acknowledgments
The study was supported by grants from the John Douglas French Alzheimer’s Foundation (M.L.G.T., B.L.M.), the McBean Foundation (B.L.M.), the Larry Hillblom Foundation (2002/2F, B.L.M.), the Koret Foundation (99-0102, B.L.M.), the NIH (National Institute on Aging, AG 19724, B.L.M.), and the State of California (03-75271 DHS/ADP/ARCC and 01-15945 DHS/ADP, B.L.M.).
We thank patients and their families for the time and effort they dedicate to our research. We also thank G. Yu, and J. Goldman, H. Feiler, D. Phayre, and K. Wilhelmsen for providing genetic data.
References
- 1.Neary D, Snowden JS, Gustafson L, et al. Frontotemporal lobar degeneration: a consensus on clinical diagnostic criteria. Neurology. 1998;51:1546–1554. doi: 10.1212/wnl.51.6.1546. [DOI] [PubMed] [Google Scholar]
- 2.Kertesz A, Martinez-Lage P, Davidson W, Munoz DG. The corticobasal degeneration syndrome overlaps progressive aphasia and frontotemporal dementia. Neurology. 2000;55:1368–1375. doi: 10.1212/wnl.55.9.1368. [DOI] [PubMed] [Google Scholar]
- 3.Galton CJ, Patterson K, Xuereb JH, Hodges JR. Atypical and typical presentations of Alzheimer’s disease: a clinical, neuropsychological, neuroimaging and pathological study of 13 cases. Brain. 2000;123:484–498. doi: 10.1093/brain/123.3.484. [DOI] [PubMed] [Google Scholar]
- 4.Greene JDW, Patterson K, Xuereb J, Hodges JR. Alzheimer’s disease and nonfluent progressive aphasia. Arch Neurol. 1996;53:1072–1078. doi: 10.1001/archneur.1996.00550100158027. [DOI] [PubMed] [Google Scholar]
- 5.Pogacar S, Williams RS. Alzheimer’s disease presenting as slowly progressive aphasia. R I Med J. 1984;67:181–185. [PubMed] [Google Scholar]
- 6.Petersen RC. Clinical subtypes of Alzheimer’s disease. Dement Geriatr Cogn Disord. 1998;9:16–24. doi: 10.1159/000051199. [DOI] [PubMed] [Google Scholar]
- 7.Kempler D, Metter EJ, Riege WH, et al. Slowly progressive aphasia: three cases with language, memory, CT and PET data. J Neurol Neurosurg Psychiatry. 1990;53:987–993. doi: 10.1136/jnnp.53.11.987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mesulam MM. Slowly progressive aphasia without generalized dementia. Ann Neurol. 1982;11:592–598. doi: 10.1002/ana.410110607. [DOI] [PubMed] [Google Scholar]
- 9.Turner RS, Kenyon LC, Trojanowski JQ, et al. Clinical, neuroimaging, and pathologic features of progressive nonfluent aphasia. Ann Neurol. 1996;39:166–173. doi: 10.1002/ana.410390205. [DOI] [PubMed] [Google Scholar]
- 10.Snowden JS, Neary D, Mann DM, et al. Progressive language disorder due to lobar atrophy. Ann Neurol. 1992;31:174–183. doi: 10.1002/ana.410310208. [DOI] [PubMed] [Google Scholar]
- 11.Rossor MN, Revesz T, Lantos PL, Warrington EK. Semantic dementia with ubiquitin-positive tau-negative inclusion bodies. Brain. 2000;123:267–276. doi: 10.1093/brain/123.2.267. [DOI] [PubMed] [Google Scholar]
- 12.Kertesz A, Hudson L, Mackenzie IR, Munoz DG. The pathology and nosology of primary progressive aphasia. Neurology. 1994;44:2065–2072. doi: 10.1212/wnl.44.11.2065. [DOI] [PubMed] [Google Scholar]
- 13.Graff-Radford NR, Damasio AR, Hyman BT, et al. Progressive aphasia in a patient with Pick’s disease: a neuropsychological, radiologic, and anatomic study. Neurology. 1990;40:620–626. doi: 10.1212/wnl.40.4.620. [DOI] [PubMed] [Google Scholar]
- 14.Mandell AM, Alexander MP, Carpenter S. Creutzfeld-Jakob disease presenting as isolated aphasia. Neurology. 1989;39:55–58. doi: 10.1212/wnl.39.1.55. [DOI] [PubMed] [Google Scholar]
- 15.Caselli RJ, Windebank AJ, Petersen RC, et al. Rapidly progressive aphasic dementia and motor neuron disease. Ann Neurol. 1993;33:200–207. doi: 10.1002/ana.410330210. [DOI] [PubMed] [Google Scholar]
- 16.Mesulam MM. Primary progressive aphasia. Ann Neurol. 2001;49:425–432. [PubMed] [Google Scholar]
- 17.Grossman M. Frontotemporal dementia: a review. J Int Neuropsychol Soc. 2002;8:566–583. doi: 10.1017/s1355617702814357. [DOI] [PubMed] [Google Scholar]
- 18.Black SE. Focal cortical atrophy syndromes. Brain Cogn. 1996;31:188–229. doi: 10.1006/brcg.1996.0042. [DOI] [PubMed] [Google Scholar]
- 19.Kertesz A, Munoz DG. Primary progressive aphasia and Pick complex. J Neurol Sci. 2003;206:97–107. doi: 10.1016/s0022-510x(02)00345-3. [DOI] [PubMed] [Google Scholar]
- 20.Hodges JR, Patterson K. Nonfluent progressive aphasia and semantic dementia: a comparative neuropsychological study. J Int Neuropsychol Soc. 1996;2:511–524. doi: 10.1017/s1355617700001685. [DOI] [PubMed] [Google Scholar]
- 21.Grossman M, Mickanin J, Onishi K, et al. Progressive non-fluent aphasia: language, cognitive and PET measures contrasted with probable Alzheimer’s disease. J Cogn Neurosci. 1996;8:135–154. doi: 10.1162/jocn.1996.8.2.135. [DOI] [PubMed] [Google Scholar]
- 22.Hodges JR, Patterson K, Oxbury S, Funnell F. Semantic dementia. Progressive fluent aphasia with temporal lobe atrophy. Brain. 1992;115:1783–1806. doi: 10.1093/brain/115.6.1783. [DOI] [PubMed] [Google Scholar]
- 23.Tyrrell PJ, Kartsounis LD, Frackowiak RS, et al. Progressive loss of speech output and orofacial dyspraxia associated with frontal lobe hypometabolism. J Neurol Neurosurg Psychiatry. 1991;54:351–357. doi: 10.1136/jnnp.54.4.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cohen L, Benoit N, Van Eeckhout P, et al. Pure progressive aphemia. J Neurol Neurosurg Psychiatry. 1993;56:923–924. doi: 10.1136/jnnp.56.8.923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Broussolle E, Bakchine S, Tommasi M, et al. Slowly progressive anarthria with late anterior opercular syndrome: a variant form of frontal cortical atrophy syndromes. J Neurol Sci. 1996;144:44–58. doi: 10.1016/s0022-510x(96)00096-2. [DOI] [PubMed] [Google Scholar]
- 26.Weintraub S, Rubin NP, Mesulam M-M. Primary progressive aphasia: longitudinal course, neuropsychological profile, and language features. Arch Neurol. 1990;47:1329–1335. doi: 10.1001/archneur.1990.00530120075013. [DOI] [PubMed] [Google Scholar]
- 27.Kertesz A, Davidson W, McCabe P, et al. Primary progressive aphasia: diagnosis, varieties, evolution. J Int Neuropsychol Soc. 2003;9:710–719. doi: 10.1017/S1355617703950041. [DOI] [PubMed] [Google Scholar]
- 28.Fukui T, Kertesz A. Volumetric study of lobar atrophy in Pick complex and Alzheimer’s disease. J Neurol Sci. 2000;174:111–121. doi: 10.1016/s0022-510x(00)00261-6. [DOI] [PubMed] [Google Scholar]
- 29.Rosen HJ, Gorno-Tempini ML, Goldman WP, et al. Patterns of brain atrophy in frontotemporal dementia and semantic dementia. Neurology. 2002;58:198–208. doi: 10.1212/wnl.58.2.198. [DOI] [PubMed] [Google Scholar]
- 30.Mummery C, Patterson K, Price C, et al. A voxel-based morphometry study of semantic demntia: relationship between temporal lobe atrophy and semantic memory. Ann Neurol. 2000;47:36–45. [PubMed] [Google Scholar]
- 31.Galton CJ, Patterson K, Graham K, et al. Differing patterns of temporal atrophy in Alzheimer’s disease and semantic dementia. Neurology. 2001;56:216–225. doi: 10.1212/wnl.57.2.216. [DOI] [PubMed] [Google Scholar]
- 32.Chan D, Fox NC, Scahill RI, et al. Patterns of temporal lobe atrophy in semantic dementia and Alzheimer’s disease. Ann Neurol. 2001;49:433–442. [PubMed] [Google Scholar]
- 33.Tyrrell PJ, Warrington EK, Frackowiak RSJ, Rossor MN. Heterogeneity in progressive aphasia due to focal cortical atrophy. A clinical and PET study. Brain. 1990;113:1321–1336. doi: 10.1093/brain/113.5.1321. [DOI] [PubMed] [Google Scholar]
- 34.Grossman M, Payer F, Onishi K, et al. Language comprehension and regional cerebral defects in frontotemporal degeneration and Alzheimer’s disease. Neurology. 1998;50:157–163. doi: 10.1212/wnl.50.1.157. [DOI] [PubMed] [Google Scholar]
- 35.Sonty SP, Mesulam MM, Thompson CK, et al. Primary progressive aphasia: PPA and the language network. Ann Neurol. 2003;53:35–49. doi: 10.1002/ana.10390. [DOI] [PubMed] [Google Scholar]
- 36.Addya K, Wang YL, Leonard DG. Optimization of apolipoprotein E genotyping. Mol Diagn. 1997;2:271–276. doi: 10.1054/MODI00200271. [DOI] [PubMed] [Google Scholar]
- 37.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state” A practical method for grading the mental state of patients for the clinician. J Psychiat Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 38.Kaplan EF, Goodglass H, Weintraub S. The Boston Naming Test. Philadelphia: Lea & Febiger; 1983. [Google Scholar]
- 39.Delis DC, Kramer JH, Kaplan E, Ober BA. California Verbal Learning Test. 2. San Antonio, TX: The Psychological Corporation; 2000. [Google Scholar]
- 40.Wechsler D. Wechsler Memory Scale (WMS-III) 3. San Antonio, TX: The Psychological Corporation; 1997. [Google Scholar]
- 41.Warrington EK, James M. Visual Object and Space Perception Battery. Bury St. Edmunds, Suffolk, UK: Thames Valley Test Co; 1991. [Google Scholar]
- 42.Reitan RM. Validity of the Trailmaking Test as an indication of organic brain damage. Percept Mot Skills. 1958;8:271–276. [Google Scholar]
- 43.Wertz RT, LaPointe LL, Rosenbek JC. Apraxia of speech: the disorders and its management. New York: Grune and Stratton; 1984. p. 318. [Google Scholar]
- 44.Duffy JR. Motor speech disorders. San Louis: Mosby; 1995. [Google Scholar]
- 45.Kertesz A. Western Aphasia Battery. London, Ontario, Canada: University of Western Ontario Press; 1980. [Google Scholar]
- 46.Howard D, Patterson K. Pyramids and palm trees: a test of semantic access from pictures and words. Suffolk: 1992. [Google Scholar]
- 47.Curtiss S, Yamada J. Curtiss-Yamada Comprehensive Language Evaluation. 1988. Unpublished test. [Google Scholar]
- 48.Kay J, Lesser R, Coltheart M. Psycholinguistic assessment of language processing in aphasia. Hove, UK: Lawrence Erlbaum Associates; 1992. [Google Scholar]
- 49.Rhee J, Antiquena P, Grossman M. Verb comprehension in frontotemporal degeneration: the role of grammatical, semantic and executive components. Neurocase. 2001;7:173–184. doi: 10.1093/neucas/7.2.173. [DOI] [PubMed] [Google Scholar]
- 50.Hillis AE, Tuffiash E, Caramazza A. Modality-specific deterioration in naming verbs in nonfluent primary progressive aphasia. J Cogn Neurosci. 2002;14:1099–1108. doi: 10.1162/089892902320474544. [DOI] [PubMed] [Google Scholar]
- 51.Hodges JR, Patterson K, Ward R, et al. The differentiation of semantic dementia and frontal lobe dementia (temporal and frontal variants of frontotemporal dementia) from early Alzheimer’s disease: a comparative neuropsychological study. Neuropsychology. 1999;13:31–40. doi: 10.1037//0894-4105.13.1.31. [DOI] [PubMed] [Google Scholar]
- 52.Ashburner J, Friston KJ. Voxel-based morphometry—the methods. Neuroimage. 2000;11:805–821. doi: 10.1006/nimg.2000.0582. [DOI] [PubMed] [Google Scholar]
- 53.Good CD, Scahill RI, Fox NC, et al. Automatic differentiation of anatomical patterns in the human brain: validation with studies of degenerative dementias. Neuroimage. 2002;17:29–46. doi: 10.1006/nimg.2002.1202. [DOI] [PubMed] [Google Scholar]
- 54.Dronkers NF. A new brain region for coordinating speech articulation. Nature. 1996;384:159–161. doi: 10.1038/384159a0. [DOI] [PubMed] [Google Scholar]
- 55.Harasty JA, Halliday GM, Xuereb J, et al. Cortical degeneration associated with phonologic and semantic language impairments in AD. Neurology. 2001;56:944–950. doi: 10.1212/wnl.56.7.944. [DOI] [PubMed] [Google Scholar]
- 56.Alexander MP, Naeser MA, Palumbo C. Broca’s area aphasias: aphasia after lesions including the frontal operculum. Neurology. 1990;40:353–362. doi: 10.1212/wnl.40.2.353. [DOI] [PubMed] [Google Scholar]
- 57.Schiff HB, Alexander MP, Naeser MA, Galaburda AM. Aphemia Clinical-anatomic correlations. Arch Neurol. 1983;40:720–727. doi: 10.1001/archneur.1983.04050110038005. [DOI] [PubMed] [Google Scholar]
- 58.Mohr JP, Pessin MS, Finkelstein S, et al. Broca aphasia: pathologic and clinical. Neurology. 1978;28:311–324. doi: 10.1212/wnl.28.4.311. [DOI] [PubMed] [Google Scholar]
- 59.Tettamanti M, Alkadhi H, Moro A, et al. Neural correlates for the acquisition of natural language syntax. Neuroimage. 2002;17:700–709. [PubMed] [Google Scholar]
- 60.Fiebach CJ, Schlesewsky M, Friederici AD. Syntactic working memory and the establishment of filler-gap dependencies: insights from ERPs and fMRI. J Psycholinguist Res. 2001;30:321–338. doi: 10.1023/a:1010447102554. [DOI] [PubMed] [Google Scholar]
- 61.Geschwind N. The apraxias: neural mechanisms of disorders of learned movement. Am Sci. 1975;63:188–195. [PubMed] [Google Scholar]
- 62.Lomen-Hoerth C, Anderson T, Miller B. The overlap of amyotrophic lateral sclerosis and frontotemporal dementia. Neurology. 2002;59:1077–1079. doi: 10.1212/wnl.59.7.1077. [DOI] [PubMed] [Google Scholar]
- 63.Paulesu E, Frith CD, Frackowiak RS. The neural correlates of the verbal component of working memory. Nature. 1993;362:342–345. doi: 10.1038/362342a0. [DOI] [PubMed] [Google Scholar]
- 64.Vallar G, Di Betta AM, Silveri MC. The phonological short-term store-rehearsal system: patterns of impairment and neural correlates. Neuropsychologia. 1997;35:795–812. doi: 10.1016/s0028-3932(96)00127-3. [DOI] [PubMed] [Google Scholar]
- 65.Damasio H, Damasio AR. The anatomical basis of conduction aphasia. Brain. 1980;103:337–350. doi: 10.1093/brain/103.2.337. [DOI] [PubMed] [Google Scholar]
- 66.Martin A, Brouwers P, Lalonde F, et al. Towards a behavioral typology of Alzheimer’s patients. J Clin Exp Neuropsychol. 1986;8:594–610. doi: 10.1080/01688638608405178. [DOI] [PubMed] [Google Scholar]
- 67.Green J, Morris JC, Sandson J, et al. Progressive aphasia: a precursor of global dementia? Neurology. 1990;40:423–429. doi: 10.1212/wnl.40.3_part_1.423. [DOI] [PubMed] [Google Scholar]
- 68.Benson DF, Zaias BW. Progressive aphasia: a case with postmortem correlation. Neuropsychiatry Neuropsychol Behav Neurol. 1991;4:215–223. [Google Scholar]
- 69.Harasty JA, Halliday GM, Kril JJ, Code C. Specific temporoparietal gyral atrophy reflects the pattern of language dissolution in Alzheimer’s disease. Brain. 1999;122:675–686. doi: 10.1093/brain/122.4.675. [DOI] [PubMed] [Google Scholar]
- 70.Grossman M, Payer F, Onishi K, et al. Constraints on the cerebral basis for semantic processing from neuroimaging studies of Alzheimer’s disease. J Neurol Neurosurg Psychiatry. 1997;63:152–158. doi: 10.1136/jnnp.63.2.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Brun A, Englund E. Regional pattern of degeneration in Alzheimer’s disease: neuronal loss and histopathological grading. Histopathology. 1981;5:549–564. doi: 10.1111/j.1365-2559.1981.tb01818.x. [DOI] [PubMed] [Google Scholar]
- 72.Tsai MS, Tangalos EG, Petersen RC, et al. Apolipoprotein E: risk factor for Alzheimer disease. Am J Hum Genet. 1994;54:643–649. [PMC free article] [PubMed] [Google Scholar]
- 73.Mayeux R, Saunders AM, Shea S, et al. Utility of the apolipoprotein E genotype in the diagnosis of Alzheimer’s disease. N Engl J Med. 1998;338:506–511. doi: 10.1056/NEJM199802193380804. [DOI] [PubMed] [Google Scholar]
- 74.Tsuboi Y, Josephs KA, Cookson N, Dickson DW. APOE E4 is a determinant for Alzheimer type pathology in progressive supranuclear palsy. Neurology. 2003;60:240–245. doi: 10.1212/01.wnl.0000044340.37138.a9. [DOI] [PubMed] [Google Scholar]


