Abstract
Background
While a great deal is known regarding the performance of muscle with intact tendon, little is known about muscle performance when tendon is surgically lengthened or shortened. This knowledge may allow surgeons to more accurately predict functional outcome following tendon repair when correcting a simple tendon laceration or performing a more complex vascularized neuromuscular transfer.
Materials and Methods
We studied muscle performance 12 weeks following extensor tendon repairs producing altered tendon lengths. Forty male Fischer 344 rats underwent division of the proximal and distal tendons of the extensor digitorum longus muscle. Tendons were immediately repaired producing tendons with increased length, decreased length, or pre-surgical length (control). Observation confirmed that altered tendon length produced inverse changes in initial resting muscle tension.
Results
Muscle in the Decreased Tendon Length group demonstrated a 15.2% greater muscle mass, 4.9% greater muscle length, 9.6% greater physiologic cross-sectional area, 12.6% greater maximum isometric force, and 31.9% greater maximum power relative to the Control Tendon Length group (p < 0.05). The Increased Tendon Length group did not differ significantly from the Control Tendon Length group for any measurement. Histologically, muscles set with a decreased tendon length demonstrated normal appearing hypertrophied fibers, without evidence of detrimental histological effects such as fibrosis, denervation, necrosis, inflammation, fiber type changes, or fiber splitting.
Conclusion
These data support the clinical practice of setting muscles with increased passive tension when performing tendon transfer surgeries. Conversely, setting muscles with decreased tension does not necessarily result in a force or power deficit.
Keywords: Tendon Injuries, Muscle Contraction, Muscle Tension, Muscle Fibers, Fast-Twitch
INTRODUCTION
Tendon division and repair is a component of many common surgical procedures including vascularized neuromuscular transfers, tendon transfer surgeries, tenodeses, tenotoplasties, tenorrhaphies, and tenotomyotomies. The purpose of these procedures is to restore or supplement functional movement following muscular or neurological injury or disease. The optimum tension at which a muscle is set following tenotomy and repair has not been systematically defined, with the surgical dictum being to set muscles with increased passive tension when repairing tendons. Surgeons typically rely on subjective observations such as tendon tension and extremity position to guide repair. Studies show that relying on surgical experience to set muscle tension results in muscle sarcomere lengths that result in suboptimal force output on a classic Blix curve [1]. The purpose of this study is to determine whether long term muscle performance differences result from altering the tendon length during surgical tendon repair in otherwise healthy muscle.
For neurovascular muscle transfer, Guelinckx found that tendon repair alone is responsible for most of the muscle function deficit seen in a neurovascularized muscle transfer model [2]. Additional studies also demonstrate force and power deficits following simple tenotomy and repair [2–4]. Surgically lengthening tendon, thus decreasing resting tension, is shown to decrease muscle mass and total force output [5]. However, following a 3 week recovery, normal force output is recovered when pre-surgical muscle tension is restored 7 days after tenotomy [6]. Thus we question whether failure to optimize tendon length led to the earlier study results [2–4]. Also, the power producing capacity of muscle after undergoing tenotomy and repair at altered resting tendon length has not been studied.
Altered tendon length that increases resting muscle tension triggers several adaptive mechanisms in skeletal muscle. When muscle length is permanently changed, sarcomere number alters to accommodate an absolute optimal sarcomere length [6–11]. Loading or stretching a muscle to increase passive tension up-regulates slow myosin mRNA production [12, 13]. Increased expression of slow and intermediate MHC isoforms is also observed [14]. Levels of autocrine/paracrine musculotrophic IGF-1 isoforms increase when muscle is chronically stretched [15]. On the contrary, muscle disuse and/or unloading reduces slow-oxidative and fast-oxidative MHC content [16].
We hypothesize that tendon repair that alters tendon length inversely alters the optimal muscle length for contractile performance after recovery. Additionally we hypothesize that increased muscle optimal resting length at recovery results in hypertrophied muscle with increased muscle force and power production capacity when compared to muscle with tendon at control length.
MATERIALS AND METHODS
General
Forty retired, breeder, male, Fischer-344 (Charles River Laboratory, Kingston, NY) rats were studied. Rat body mass ranged from 320 to 450 g at the initial surgery date. All animal care and surgical procedures were approved by the University of Michigan’s University Committee on Use and Care of Animals (UCUCA) and were in strict accordance with the National Research Council’s Guide for the Care and Use of Laboratory Animals [17]. Rats were individually housed in a restricted-access, specific pathogen-free university facility, given food and water ad libitum, and exposed to a 12-hour light-dark cycle. For all surgical procedures, rats were anesthetized with an initial intraperitoneal injection of sodium pentobarbital (65 mg/kg); supplementary doses were administered to maintain a deep plane of general anesthesia. All surgical procedures were conducted under aseptic conditions.
Surgery Groups
The main effect independent variable in this study was resting extensor digitorum longus (EDL) tendon length following the surgical intervention. Rats were randomly assigned to one of five surgical groups which were later collapsed into three groups based on change in tendon lengths, as summarized in Table 1. In the Sham group, the proximal and distal tendons were exposed and freed from surrounding structures. In the Unchanged group, the proximal and distal tendons were transected and immediately repaired creating a tendon with pre-surgery length. In the Tenotomy group, proximal and distal tendons were transected and not repaired. In the Tendon-Added group, 2 mm and 6 mm segments of contralateral plantaris tendon were autografted into the proximal and distal EDL tendons, respectively, to create a muscle with increased tendon length. In both the Tenotomy and Tendon-Added groups, the ends of the muscle were seen to slack following repair. In the Tendon-Removed group, 2 mm and 6 mm segments of tendon were removed from the proximal and distal tendons, respectively, to create a muscle with decreased tendon length. In the Tendon-Removed group, the tendons were noted to be under tension, and the toes were prominently dorsiflexed at the time of repair. The total tendon length change of 8 mm is approximately 50% of previously reported EDL fiber lengths [18]. Changes in muscle tension by altering tendon length were confirmed for each rat by methods used in human tendon operations, i.e., tension on the tendon at the time of repair, visible changes in postoperative joint position, and observing slacked muscle boundaries.
TABLE 1.
Summary of the five original surgical treatments, results on EDL tendon length, and final statistical groups.
| Surgical Group | Surgical Treatment Steps | Tendon Length Effect | Statistical Group |
|---|---|---|---|
| Sham (n = 8) |
|
Control Length | Control Tendon Length (n = 16) |
|
| |||
| Unchanged (n = 8) |
|
Control Length | |
|
| |||
| Tenotomy (n = 8) |
|
Increased Length | Increased Tendon Length (n = 16) |
|
| |||
| Tendon-Added (n = 8) |
|
Increased Length | |
|
| |||
| Tendon-Removed (n = 8) |
|
Decreased Length | Decreased Tendon Length (n = 8) |
Surgical Groups were combined to form Statistical Groups based on similar effects on tendon length. The Sham and Unchanged surgical groups were combined to form the Control Tendon Length group. The Tenotomy and Tendon-Added groups were combined to form the Increased Tendon Length group. The Tendon-Removed group became the Decreased Tendon Length group.
The five surgical groups were then collapsed to form three groups according to similar surgical effect on tendon length. The Sham (n = 8) and Unchanged (n = 8) groups, each with no change in resting tendon length, were combined to form the Control Tendon Length group (n = 16). The Tenotomy (n = 8) and Tendon-Added (n = 8) groups, each producing an increased tendon length, were combined to form the Increased Tendon Length group (n = 16). The original Tendon-Removed (n = 8) group, producing a decreased tendon length, formed the Decreased Tendon Length group (n = 8). Surgical groups were combined when observations of postoperative myotendinous position at the initial operation, animal behavior during recovery, and appearance at the subsequent testing operation were similar between groups with the same effect on initial resting muscle length. A preliminary statistical analysis revealed no differences between groups with similar resting muscle lengths.
For all surgeries, short longitudinal incisions were created to explore and free the proximal and distal EDL tendons from surrounding structures. Tenotomies were performed on the proximal tendon midway between the femoral origin and proximal myotendinous junction; the distal tendons were divided midway between the extensor retinaculum and insertion on the distal-most phalanxes. A modified Kessler-Mason-Allen suture technique with 6-0 polyester sutures (Ethicon, Somerville, NJ) was used for tendon repair. All tendons were repaired end-to-end, with the distal tendons repaired individually. For all rats, a 9 mm tendon graft was removed from the contralateral plantaris muscle distal tendon. In the original Tendon-Added surgical group, the contralateral plantaris tendon was autografted in order to increase EDL tendon length. Observations of toe position, tendon tension, and visible movement of the muscle ends were used to confirm changes in muscle tension. The experimental limb was passively moved to confirm tendon sliding prior to skin closure. The wounds were closed with 4-0 absorbable chromic gut suture (Ethicon, Somerville, NJ). Limbs were not immobilized following surgery, and rats were allowed to freely move around in their cages during recovery.
Twelve weeks postoperatively, maximum force and power capacities of the operated EDL muscle were measured in situ. After force and power measurements were completed, the EDL muscles on the left and right sides were harvested, blotted dry, cleaned of adherent fat or connective tissue, weighed, frozen in isopentane at −160° C, and stored at −70° C for subsequent histological treatment.
In Situ Measurements
Measurements of the maximal force and power capacity were conducted using methods well-established in our laboratory [18–21]. The EDL muscle was surgically isolated from the surrounding muscle taking care to preserve the motor nerve and vascular pedicle. The distal tendon was clamped to the motor arm of an Aurora Dual Model Lever System, Model 305B controller (Aurora Scientific; Aurora, Ontario, Canada). The rat hind limb was secured to a platform, the peroneal nerve was exposed, the tibial nerve and all other tendons crossing the ankle were severed. Throughout the evaluation, body temperature and muscle temperature were maintained at 37°C. Stimulation and data acquisition were controlled with customized programming using Labview software (National Instruments, Austin, TX) and PC based data acquisition hardware (Dell Computer Corp.; Austin, TX). Supramaximal stimulation between 4 and 20 volts was delivered through a shielded bipolar silver wire electrode (Harvard Apparatus, Inc; Holliston, MA) placed under the peroneal nerve. Isometric tensions between 100 and 10,000 mN could be reliably recorded while the Aurora motor arm maintained a stable position. During measurements of power output, the lever arm was programmed to move from a length representing 105% of optimal fiber length to a position of 95%, achieving an isovelocity contraction over 10% of resting muscle fiber length. Muscles were allowed to rest for 2 minutes between individual contractions [18, 20–22].
Maximum Isometric Force (P0) Measurement
Optimal muscle length was determined by measuring the length of the EDL muscle at which the strongest twitch contractions were generated following supramaximal stimuli. Optimal fiber length was derived by multiplying the optimal muscle length by an established EDL muscle fiber length to EDL muscle length ratio of 0.4 [19]. All subsequent isometric force measurements were made at optimal length. The EDL muscle was then stimulated for 300 msec at increasing frequencies (from 30 to 350 Hz) with 2 minutes rest between isometric contractions. The highest force generated was defined as the maximal isometric force.
Maximum Power Measurement
Power was measured during isovelocity shortening contractions with displacement through 10% (105% to 95%) of the optimum fiber length [18, 20, 22–24]. Muscle power was sampled during repeated individual shortening contraction trials at increasing velocities (1.5 to 6.3 fiber lengths/second). The power measurement was obtained using the customized computer software’s (Labview, National Instruments, Austin, TX) function which allows for integration of a force curve. Maximum power was identified as the highest power achieved during these trials. Optimum velocity was defined as the shortening velocity at which maximum power generation occurred. Power is defined as the product of force and velocity; therefore, the average force during power movement was derived by dividing the maximum power by the optimum velocity.
Specific Force and Normalized Power
Force, when normalized to muscle “physiologic” cross sectional area (PCSA), is known as specific force [18, 25, 26]. Muscle PCSA was calculated using Equation 1. An average ratio of fiber length to optimal muscle length of 0.40 was used from established data [19]. The EDL has a parallel muscle fiber architecture, therefore a θ = 0° was used [18]. We used an established muscle density constant of 1.06 mg/mm3 [18, 25, 26]. Power, when customarily normalized to muscle mass, is known as normalized power. Thus, normalized power was calculated by dividing maximal muscle power by muscle mass [18, 27, 28].
Histology
Histological techniques were used to identify fiber type morphology, fiber cross sectional area, morphologic abnormalities, denervation status, and changes in collagen deposition. Hematoxylin and eosin stained muscle sections were observed for the presence of internal nuclei, fiber necrosis, and inflammatory responses [29, 30]. Additional muscle sections were incubated for the presence of calcium activated myosin-type adenosine triphosphatase activity [31–34] to determine fiber type distribution and average fiber areas by fiber type. Cross-sectional areas of at least 50 EDL muscle fibers of each fiber type were quantified for each muscle section using computerized planimetry (Bioquant-R&M Biometrics, Nashville, TN). Using an immunohistologic labeling technique for neural cell adhesion molecule (NCAM) antigen, individual EDL muscle fibers were identified as being either denervated or innervated [26, 35, 36]. A modified Gomori trichrome stain was used on additional sections to visualize collagen content [37]. Sections were viewed through a Leitz Laborlux S bright field microscope fitted for fluorescence microscopy (Leica, Wetzlar, Germany).
Data Analysis
The data were analyzed using SAS for Windows (SAS Institute Inc., Cary, NC). The mean and standard deviation were determined for each dependent variable for each experimental group. The statistical model included two main effect variables: tenorrhaphy (tenorrhaphy or no tenorrhaphy) and tendon length change (no-change, increased, or decreased length); the interaction between main-effects was included in the model. The GLM (general linear models) procedure in SAS was used to test for differences between main effects. Tukey’s pairwise comparison tests were applied for multiple comparisons of individual group means when the GLM was significant for each main effect but not significant for the interaction. The body mass at surgery and body mass at evaluation were compared using a repeated measures one-way analysis of variance (ANOVA) with tendon length change as the grouping independent variable. The percentage change in body mass during the study was compared using a Kruskal-Wallis nonparametric test. An alpha-level equal to 0.05 was selected a priori as being an appropriately stringent test of significance for all tests.
RESULTS
Descriptive Data
All rats survived the initial surgery and the 12 week recovery period. Descriptive and functional data are summarized in Table 2. Mean body mass for the Control Tendon Length, Increased Tendon Length, and Decreased Tendon Length groups differed neither at the time of initial surgery nor at final evaluation. Following the 12 week recovery period, EDL muscle mass for the muscle contralateral to the experimental leg was not significantly different among the groups. However for the experimental leg, the Decreased Tendon Length group demonstrated hypertrophy compared to the Control Tendon Length and Increased Tendon Length groups. EDL muscle mass was 15.2% and 19.8% greater in the Decreased Tendon Length group relative to the Control and Increased Tendon Length groups, while optimal muscle length was 4.9% and 4.6% longer, and PCSA larger by 9.6% and 14.7%, respectively. The Increased Tendon Length group did not differ from the Control Tendon Length group on any of the descriptive or contractile dependent variables.
TABLE 2.
Summary of EDL muscle descriptive, force capacity, and power output data by surgical group.
| Groups Based on Change in Tendon Length
|
|||
|---|---|---|---|
| Control Tendon Length | Increased Tendon Length | Decreased Tendon Length | |
| Operated Leg EDL Muscle Mass (mg) | 178 ± 9 (16) | 172 ±13 (15) | 206 ±14*, † (8) |
| Unoperated Leg, EDL Muscle Mass (mg) | 172 ± 7 (8) | 165 ±13 (9) | 174 ± 6 (5) |
| Optimal Resting Muscle Length (mm) | 37.1 ± 1.4 (16) | 37.2 ± 1.5 (15) | 38.9 ± 1.3*, † (8) |
| Physiologic Cross-Sectional Area (mm2) | 11.4 ± 0.7 (16) | 10.9 ± 0.9 (15) | 12.5 ± 0.8*, † (8) |
| Maximum Isometric Force (mN) | 3985 ± 427 (13) | 3854 ± 279 (12) | 4488 ± 432*, † (8) |
| Specific Force (mN/mm2) | 350 ± 38 (13) | 350 ± 24 (12) | 361 ± 39 (8) |
| Maximum Power (mW) | 40.3 ± 3.8 (12) | 41.0 ± 9.0 (8) | 53.2 ± 6.3*, † (6) |
| Normalized Power (mW/g) | 225 ±16 (12) | 231 ± 38 (8) | 265 ± 38* (6) |
| Absolute Velocity (mm/s) | 38.4 ± 5.5 (12) | 43.7 ± 8.7 (8) | 43.4 ± 10.2 (6) |
| Relative Velocity (Lf/s) | 2.59 ± 0.4 (12) | 2.98 ± 0.6 (8) | 2.78 ± 0.6 (6) |
| Average Force during Isovelocity Maximal Power Movement (N) | 1.08 ± 0.2 (12) | 0.95 ± 0.2 (8) | 1.27 ± 0.3 † (6) |
Abbreviations used are: grams (g), milligrams (mg), millimeters (mm), square millimeters (mm2), Newtons (N), milli-Newtons (mN), milliwatts (mW), fiber length (Lf), seconds (s). Values listed are the mean ± standard deviation. The numbers of rats that completed each testing phase are listed in parentheses.
Indicates value significantly different from the Control Tendon Length group.
Indicates value significantly different from the Increased Tendon Length group. (p < .05).
Muscle Isometric Force
The Decreased Tendon Length group produced 12.6% more maximal isometric force than the Control Tendon Length group (4488 vs. 3985 mN, respectively), and also significantly more force (16.5%) than the Increased Tendon Length group (3854 ± 279 mN). When maximal isometric forces were normalized to individual muscle PCSA, the resulting specific forces were not significantly different among the experimental groups (361 ± 39, 350 ± 38, and 350 ± 24 mN/mm2 for the Decreased, Control, and Increased Tendon Length groups, respectively).
Muscle Power
The Decreased Tendon Length group produced 32% more maximum power than the Control Tendon Length group and 30% more power than the Increased Tendon Length group (53.2 ± 6.3, 40.3 ± 3.8, 41.0 ± 9.0 mW, respectively). When the maximal power value was adjusted for muscle mass, the mean normalized power produced by the Decreased Tendon Length group remained statistically higher compared with the Control Tendon Length group (265± 38 vs. 225 ± 16 mW/g, respectively), but did not differ significantly from the Increased Tendon Length group (231 ± 38 mW/g). Individual analysis of the two components used to derive power, i.e. force and velocity, indicated that the absolute velocity at which maximal power was achieved was not significantly affected. The absolute velocities of 38.4 mm/s, 43.7 mm/s, and 43.4 mm/s for the Control, Increased, and Decreased Tendon Length groups, respectively, were not significantly different. Similarly, the relative velocities in fiber lengths/second [18, 20, 22–24] did not differ significantly among the groups: 2.59 for the Control, 2.98 for the Increased, and 2.78 for the Decreased Tendon Length group. However, the average force during the maximal power movement was 36% higher for the Decreased Tendon Length group than the Increased Tendon Length group (1.27 vs. 0.95 N, respectively), but was not significantly different from the Control Tendon Length group (1.08 N).
Histology
Hematoxylin and eosin staining of the EDL muscle sections did not reveal any obvious microscopic abnormalities such as the presence of central nuclei, fiber necrosis, or any inflammatory response in the muscles. There was no evidence for gross pathologic fibrosis in any of the cross sections upon examination of trichrome stained cross sections. Regardless of group assignment, NCAM labeling identified between zero and four denervated muscle fibers for each cross section which is entirely normal for EDL muscle [26]. The histological data from myofibular ATPase reacted sections are summarized in Table 3. There were no changes in the percentages of fibers classified as Type I, Type IIa, or Type IIb among the three groups of the main independent effect. Analysis of the average muscle fiber cross sectional area (CSA) by each specific fiber type revealed significant hypertrophy in the Type IIa and Type IIb fibers of the Decreased Tendon Length group compared to both the Control and Increased Tendon Length groups (Figure 1). The CSA of the Decreased Tendon Length group Type IIa fibers was increased by 41.7% while average Type IIb fiber was increased by 31.5% when compared to the Control Tendon Length group. The Type I fiber average cross sectional area did not differ significantly among the groups.
TABLE 3.
Summary of ATPase fiber typing data by groups based on change in tendon length.
| Tenorrhapy Groups Based on Change in Tendon Length
|
||||
|---|---|---|---|---|
| Muscle Fiber Types | Control Length (6) | Increased Length (6) | Decreased Length (5) | |
| Distribution of fibers (%) | Type I | 4.9 ± 1.6 | 4.7 ± 1.8 | 7.0 ± 3.9 |
| Type IIa | 54.6 ± 8.5 | 55.7 ± 5.2 | 50.0 ± 5.7 | |
| Type IIb | 40.4 ± 7.0 | 39.6 ± 3.7 | 43.0 ± 2.9 | |
| Fiber cross sectional area (um2) | Type I | 947 ± 171 | 925 ± 200 | 1137 ± 123 |
| Type IIa | 3810 ± 372 | 3674 ± 482 | 5400 ± 758*, † | |
| Type IIb | 1525 ± 243 | 1386 ± 171 | 2006 ± 376*, † | |
Abbreviations used are: percentage (%) and micrometers2 (um2). Values listed are the mean ± standard deviation. N values are listed in parentheses.
Indicates value significantly different from the Control Tendon Length group.
Indicates value significantly different from the Increased Tendon Length group. (p < .05).
Figure 1.
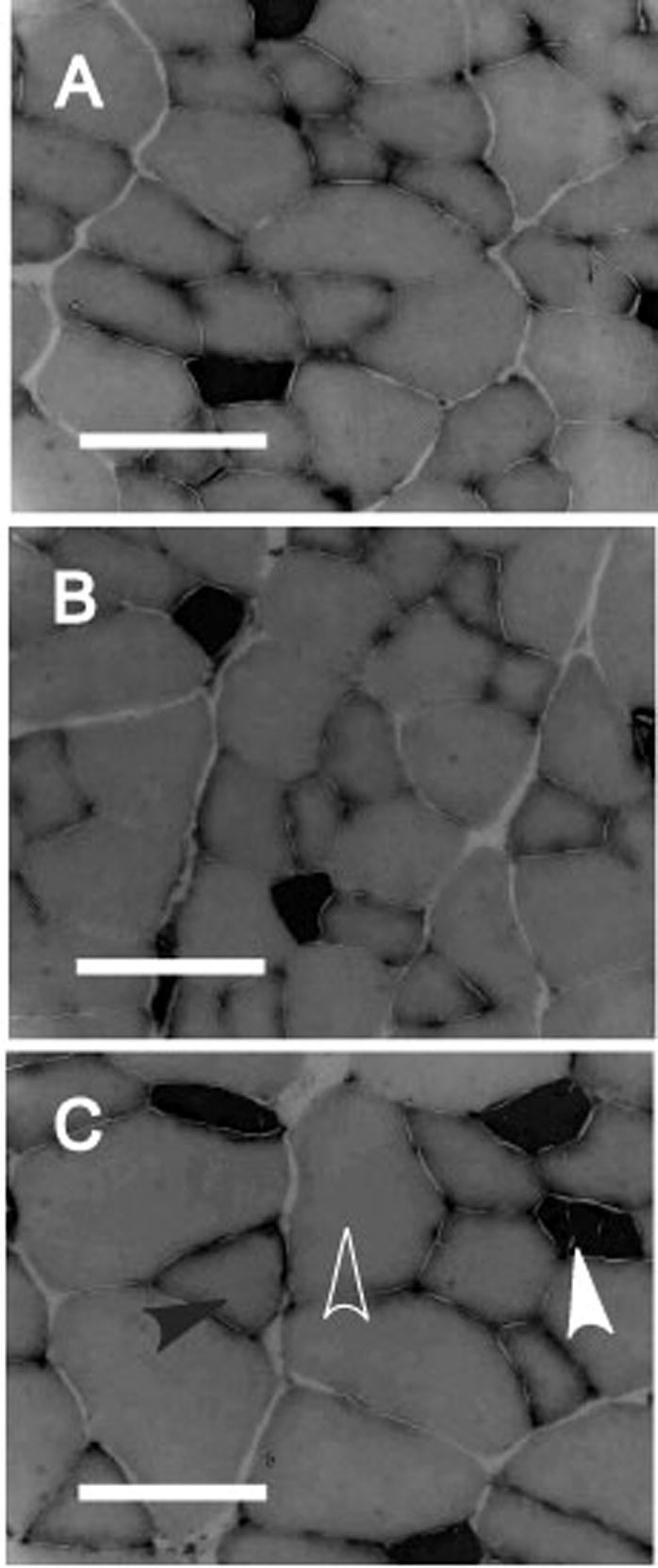
Representative histomicrographs of EDL muscle cross sections for each group based on tendon length. Myosin ATPase stain at pH 4.5. A) Control Tendon Length muscle, B) Increased Tendon Length muscle, C) Decreased Tendon Length muscle. Solid white arrowhead denotes Type I fiber, white outline arrowhead denotes Type IIa fiber, and solid black arrowhead denotes Type IIb fiber. Data on fiber type percentage and cross-sectional area are presented in Table 3. Scale bar is 100 micrometers.
DISCUSSION
The purpose of this study was to determine if surgical alteration of tendon length produces measurable differences in muscle size, fiber type, and force and power output. It is well established that surgical and non-surgical increases in dynamically produced muscle tension result in larger muscles [14, 38–41], with increased force output [39–45], and our findings are consistent with these prior studies.
We hypothesize that tendon repair that alters tendon length inversely alters optimal muscle length after recovery. This hypothesis is supported in our Decreased Length Group as the optimal resting muscle length was 38.9 mm after 12 weeks of recovery representing a 4.9% increase in muscle length. There were no significant differences in our Increased Length Group and therefore our hypothesis was not supported in this regard. More importantly, we hypothesize that increased muscle optimal resting length at recovery results in hypertrophied muscle with increased muscle force and power production capacity when compared to muscle with tendon at pre-surgical length. Our results support this hypothesis.
We imply that the EDL muscle in our Decreased Tendon Length group experienced increased tension during some of the recovery period, and, in fact, we observed both exaggerated toe and ankle dorsiflexion and knee extension for at least three post operative days. Our results support the long-held clinical axiom that muscles should be set at “increased tension” when performing tendon or muscle transfers. Because many tendon and free muscle transfer procedures substitute a smaller muscle for a larger one or result in a relative biomechanical disadvantage for the transferred muscle (palmaris longus with an ulna pulley for opponensplasty), the strength and power of motions restored with tendon and muscle transfers is often relatively weak. So, in this setting, the increased muscle force resulting from setting at increased resting tension is beneficial for reconstruction. In other settings, this muscular hypertrophy may unbalance the muscle force moments across the joint leading to poor coordination, synergistic muscle atrophy, antagonist muscle hypertrophy, and tendon re-injury. Note that our data indicate no decrement in muscle function if resting tension is restored to baseline, or even if a slight decrease in tension exists after repair. In the setting of simple tendon repair, these observations support repairing traumatically lacerated tendons at their original tensions, because, at least theoretically, it is possible that compensatory hypertrophy from increased resting muscle tension could unbalance the muscle force moments across the joint. We foresee the need to study whether joint angle effectiveness is changed when optimal muscle length is increased in a procedure-specific manner.
An important clinical consideration is the possibility of setting a muscle with extreme tension, thus causing fiber damage. Although our experiment was not designed to answer this question, our 8 mm shortening of EDL tendon (50% of optimal muscle fiber length) produced a profound stretch far greater than what would be created clinically. This “supraphysiolgic” stretch did not result in permanent muscle damage, but rather a robust hypertrophic response, as evidenced by normal appearing muscle fibers on histological examination and the proportional increase in muscle force output with respect to PCSA. This pattern of muscle performance suggests that pathologic loss of function either did not take place, or was reversed during the recovery period in our Decreased Tendon Length Group. Repeating this study, with data collection at different lengths of recovery, would elucidate this process. While the adaptive process appears robust in rat muscle, it remains to be determined if human muscle would respond in a similar manner. Future experiments will be required to determine this with more certainty.
We selected 8 mm, representing 50% of our rat population’s EDL optimal fiber length, based on preliminary indications that this length change would produce a muscle with extreme tension or slack while maintaining technical feasibility. It would be interesting to repeat the study with a less profound fiber length change to evaluate for a threshold length that produces alterations in muscle performance. Determining this threshold would allow surgeons to optimize muscle performance while protecting the tendon repair.
Our Increased Tendon Length group did not demonstrate a force deficit relative to the Control Tendon Length Group. To avoid injuring the muscle belly and neurovascular bundle during our initial surgery, we limited our exposure to only the ends of the muscle and its associated tendons. We therefore did not isolate the muscle from the neighboring tibialis anterior and extensor hallucis longus muscles. As a result, the adjacent muscles may have “splinted” the EDL at or near its optimum resting length. Prior study shows that, at 1 day post tenotomy, the EDL sarcomere length is only slightly shorter than control muscle, while both the soleus and gastrocnemius sarcomere lengths are significantly decreased following tenotomy [8, 46]. Additionally, simply acutely dissecting extra-muscular connective tissue results in reduced muscle force output [47]. Subsequent to our experiment, it was shown that rat supraspinatus muscle, when simply tenotomized at its humeral insertion, returns to its pre-surgical mass and muscle size when allowed a 16 week recovery [48]. These findings support our “splinting” assertion. This has important clinical implications in that minor changes in tendon length may not produce functionally significant alterations in muscle performance if the muscle belly is not extensively dissected from its surroundings.
We find no shifts in the relative number of Type I, Type IIa, or Type IIb fibers when comparisons are made among the Control, Decreased, and Increased Tendon Length groups. This finding argues against formation of new fibers with increased tension and removal of fibers with decreased tension, but supports re-establishing the baseline composition, and is consistent with prior studies [5, 48]. Our resultant hypertrophied Type IIa and Type IIb fibers appear to run counter to the studies previously mentioned, where muscle demonstrates hypertrophy of slow fibers and a decrease in fast glycolytic fiber content when subjected to increased load [12, 14, 16, 49]. We speculate that slow fiber myosin production may have initially increased relative to fast heavy chain myosin production, however with time the slow fiber content may have receded to baseline once the muscle was being used while the fast fiber content increased with over use (resistance training) due to increased tension. Studies with sampling times during recovery are needed to evaluate this possibility.
In the Decreased Tendon Length group, the toes appeared spread maximally, the ankle joint was profoundly dorsiflexed, and the knee joint remained in a flexed position in the immediate postoperative period. Usage of the surgically altered limb immediately postoperatively varied among rats in this group, however, within one week all limb position changes normalized and usage among the surgical groups was indistinguishable. After one week, subjective behavioral and functional changes, including walking patterns and ability to stand for feeding, were not observed. Objectively, rat body mass did not differ among groups, indicating no functionally relevant changes in limb usage. The mechanisms responsible for the normalization of limb position and function are speculative, and may result from muscle stretch or tear, tendon stretch, scar gap, or suture failure at the tendon repair sites.
We did not attempt to simulate the immobilization and physical therapy protocol that most patients who undergo muscle or tendon transfers experience. In the clinical setting, patients generally have the affected joints immobilized in a splint for the majority of the first few weeks after surgery. During this time, they are commonly placed on an intermittent passive range of motion protocol to maintain joint mobility without putting stress on their tendon repairs. After tendon healing has occurred, they are generally prescribed active range of motion exercises to maximize excursion and strength of the affected muscles. Although it would have been optimal to mimic this clinical scenario in our model, the rat model made splinting and frequent therapy impractical. Because the passive range of motion protocols employed in clinical tendon transfer patients are all designed to range joints while not subjecting the muscle-tendon unit to increased tension, we would expect that this intervention would not have appreciable effect on muscle function. However, we would expect that, as typically employed clinically, splinting would have resulted in a decrease in muscle functional capacity (physical detraining). Although introducing this factor may have resulted in a change in the absolute muscle forces observed, it is unlikely that the relative changes observed would be altered.
The decision to combine the original Unchanged and Sham groups into the Control Tendon Length group, and the Tenotomy and Tendon-Added groups in the Increased Tendon Length group, were based on observations at the time of surgical intervention. In the Unchanged group, there was no tension on the repair at the initial surgery, and rat limb position and behavior during the recovery phase were indistinguishable from the Sham group. Similarly, for both the Tenotomy and Tendon-Added groups, muscle ends were seen to slack maximally at the time of initial operative intervention, and postoperative behavior was not readily distinguishable. Significant scar tissue was present in both groups at the testing phase. Based on our operative, post-recovery, and behavioral observations, we restructured the groups based on alteration in tendon length. A preliminary statistical analysis demonstrated no differences post-recovery between groups with similar tendon length changes.
There are limitations to our study and, as a consequence, point the way to future studies. The main limitation is that the experiments were performed on a single muscle with a parallel fiber architecture. Because the fiber architecture of skeletal muscles have significantly more influence on contractile properties than fiber type composition or fiber size, it is not clear that our current observations would translate to other muscles with pinnate fiber arrangements. This is obviously important to determine, because many of the tendon transfers employed in reconstructive hand surgery involve the use of pinnate muscles. In addition, the muscle studied is primarily a fast-fibered gait accessory muscle in the rat, so changes observed may differ in a mixture fiber type muscle used for posture control.
CONCLUSION
Following a long-term recovery period, a muscle set with increased tension due to a reduction of tendon length hypertrophies, contracts with increased isometric maximal force, and produces greater maximal power during optimized isovelocity shortening contractions.
| Equation 1. Calculation of muscle physiologic cross sectional area (PCSA) |
Where mmass = muscle mass (mg), θ = muscle fiber pinnation angle, Lo = muscle fiber length (mm), ρ =mammalian skeletal muscle density constant (mg/mm3).
Acknowledgments
Portions of this paper were presented in preliminary form at the annual meeting of the Plastic Surgery Research Council 49th Annual Meeting, Ann Arbor, MI, June, 2004
We thank Joshua D. Hack, B.S., for completing the myosin ATPase histology.
This work was support in part by the National Institute of Neurological Disorders and Stroke Grant NS 34380 and the NIH Short Term Training Health Professional Schools Grant T35 HL07690-23
Abbreviations
- CSA
Cross sectional area
- EDL
Extensor Digitorum Longus
- IGF
Insulin-like Growth Factor
- MHC
Myosin Heavy Chain
- PCSA
Physiologic cross sectional area
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Friden J, Lieber RL. Evidence for muscle attachment at relatively long lengths in tendon transfer surgery. J Hand Surg [Am] 1998;23:105. doi: 10.1016/S0363-5023(98)80097-X. [DOI] [PubMed] [Google Scholar]
- 2.Guelinckx PJ, Faulkner JA, Essig DA. Neurovascular-Anastomosed Muscle Grafts in Rabbits - Functional Deficits Result from Tendon Repair. Muscle & Nerve. 1988;11:745. doi: 10.1002/mus.880110710. [DOI] [PubMed] [Google Scholar]
- 3.Kadhiresan VA, Guelinckx PJ, Faulkner JA. Tenotomy and Repair of Latissimus-Dorsi Muscles in Rats - Implications for Transposed Muscle Grafts. Journal of Applied Physiology. 1993;75:1294. doi: 10.1152/jappl.1993.75.3.1294. [DOI] [PubMed] [Google Scholar]
- 4.Burton HW, Stevenson TR, White TP, Hartman J, Faulkner JA. Force Deficit of Vascularized Skeletal-Muscle Grafts in Rabbits. Journal of Applied Physiology. 1989;66:675. doi: 10.1152/jappl.1989.66.2.675. [DOI] [PubMed] [Google Scholar]
- 5.Frey M, Gruber H, Freilinger G. The importance of the correct resting tension in muscle transplantation: experimental and clinical aspects. Plast Reconstr Surg. 1983;71:510. doi: 10.1097/00006534-198304000-00011. [DOI] [PubMed] [Google Scholar]
- 6.Abrams RA, Tsai AM, Watson B, Jamali A, Lieber RL. Skeletal muscle recovery after tenotomy and 7-day delayed muscle length restoration. Muscle Nerve. 2000;23:707. doi: 10.1002/(sici)1097-4598(200005)23:5<707::aid-mus7>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 7.Simpson AH, Williams PE, Kyberd P, Goldspink G, Kenwright J. The response of muscle to leg lengthening. J Bone Joint Surg Br. 1995;77:630. [PubMed] [Google Scholar]
- 8.Baker JH, Hall-Craggs EC. Changes in sarcomere length following tenotomy in the rat. Muscle Nerve. 1980;3:413. doi: 10.1002/mus.880030505. [DOI] [PubMed] [Google Scholar]
- 9.Tabary JC, Tabary C, Tardieu C, Tardieu G, Goldspink G. Physiological and structural changes in the cat’s soleus muscle due to immobilization at different lengths by plaster casts. J Physiol. 1972;224:231. doi: 10.1113/jphysiol.1972.sp009891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Williams PE, Goldspink G. The effect of immobilization on the longitudinal growth of striated muscle fibres. J Anat. 1973;116:45. [PMC free article] [PubMed] [Google Scholar]
- 11.Argaw A, Desaulniers P, Gardiner PF. Enhanced neuromuscular transmission efficacy in overloaded rat plantaris muscle. Muscle Nerve. 2004;29:97. doi: 10.1002/mus.10535. [DOI] [PubMed] [Google Scholar]
- 12.Goldspink G, Scutt A, Loughna PT, Wells DJ, Jaenicke T, Gerlach GF. Gene expression in skeletal muscle in response to stretch and force generation. Am J Physiol. 1992;262:R356–R363. doi: 10.1152/ajpregu.1992.262.3.R356. [DOI] [PubMed] [Google Scholar]
- 13.Dix DJ, Eisenberg BR. Redistribution of myosin heavy chain mRNA in the midregion of stretched muscle fibers. Cell Tissue Res. 1991;263:61. doi: 10.1007/BF00318400. [DOI] [PubMed] [Google Scholar]
- 14.Gregory P, Low RB, Stirewalt WS. Changes in skeletal-muscle myosin isoenzymes with hypertrophy and exercise. Biochem J. 1986;238:55. doi: 10.1042/bj2380055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McKoy G, Ashley W, Mander J, Yang SY, Williams N, Russell B, Goldspink G. Expression of insulin growth factor-1 splice variants and structural genes in rabbit skeletal muscle induced by stretch and stimulation. J Physiol. 1999;516(Pt 2):583. doi: 10.1111/j.1469-7793.1999.0583v.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Loughna PT, Izumo S, Goldspink G, Nadal-Ginard B. Disuse and passive stretch cause rapid alterations in expression of developmental and adult contractile protein genes in skeletal muscle. Development. 1990;109:217. doi: 10.1242/dev.109.1.217. [DOI] [PubMed] [Google Scholar]
- 17.Institute of Laboratory Animals Resources. National Research Council’s Guide for the Care and Use of Laboratory Animals. 7. Washington, DC: National Academy Press; 1996. Ref Type: Computer Program. [Google Scholar]
- 18.Yoshimura K, Asato H, Cederna PS, Urbanchek MG, Kuzon WM. The effect of reinnervation on force production and power output in skeletal muscle. J Surg Res. 1999;81:201. doi: 10.1006/jsre.1998.5498. [DOI] [PubMed] [Google Scholar]
- 19.Urbanchek MS, Chung KC, Asato H, Washington LN, Kuzon WM., Jr Rat walking tracks do not reflect maximal muscle force capacity. J Reconstr Microsurg. 1999;15:143. doi: 10.1055/s-2007-1000085. [DOI] [PubMed] [Google Scholar]
- 20.Brooks SV, Faulkner JA, McCubbrey DA. Power outputs of slow and fast skeletal muscles of mice. J Appl Physiol. 1990;68:1282. doi: 10.1152/jappl.1990.68.3.1282. [DOI] [PubMed] [Google Scholar]
- 21.Yoshimura K, Asato H, Jejurikar SS, Cederna PS, Urbanchek MG, Kuzon WM., Jr The effect of two episodes of denervation and reinnervation on skeletal muscle contractile function. Plast Reconstr Surg. 2002;109:212. doi: 10.1097/00006534-200201000-00032. [DOI] [PubMed] [Google Scholar]
- 22.Brooks SV, Faulkner JA. Forces and powers of slow and fast skeletal muscles in mice during repeated contractions. J Physiol. 1991;436:701. doi: 10.1113/jphysiol.1991.sp018574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brooks SV, Faulkner JA. Contractile Properties of Skeletal-Muscles from Young, Adult and Aged Mice. Journal of Physiology-London. 1988;404:71. doi: 10.1113/jphysiol.1988.sp017279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yoshimura K, Asato H, Jejurikar SS, Cederna PS, Urbanchek MG, Kuzon WM., Jr The effect of two episodes of denervation and reinnervation on skeletal muscle contractile function. Plast Reconstr Surg. 2002;109:212. doi: 10.1097/00006534-200201000-00032. [DOI] [PubMed] [Google Scholar]
- 25.Mendez J, Keys A. Density and composition of mammalian muscle. Metabolism. 1960;9:184. [Google Scholar]
- 26.Urbanchek MG, Picken EB, Kalliainen LK, Kuzon WM., Jr Specific force deficit in skeletal muscles of old rats is partially explained by the existence of denervated muscle fibers. J Gerontol A Biol Sci Med Sci. 2001;56:B191–B197. doi: 10.1093/gerona/56.5.b191. [DOI] [PubMed] [Google Scholar]
- 27.Lynch GS, Hinkle RT, Faulkner JA. Force and power output of diaphragm muscle strips from mdx and control mice after clenbuterol treatment. Neuromuscular Disorders. 2001;11:192. doi: 10.1016/s0960-8966(00)00170-x. [DOI] [PubMed] [Google Scholar]
- 28.Lynch GS, Hinkle RT, Chamberlain JS, Brooks SV, Faulkner JA. Force and power output of fast and slow skeletal muscles from mdx mice 6–28 months old. Journal of Physiology-London. 2001;535:591. doi: 10.1111/j.1469-7793.2001.00591.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Luna LG. Routine Staining Procedures. In: Luna LG, editor. Manual of Histologic Staining Methods of the Armed Forces Institute of Pathology. New York: McGraw-Hill Book Company; 1960. pp. 32–46. [Google Scholar]
- 30.Dubowitz V, Sewry C, Fitzsimons R. Muscle Biopsy A Practical Approach. London: Bailliere Tindall; 1985. [Google Scholar]
- 31.Brooke MH, Kaiser KK. Some comments on the histochemical characterization of muscle adenosine triphosphatase. J Histochem Cytochem. 1969;17:431. doi: 10.1177/17.6.431. [DOI] [PubMed] [Google Scholar]
- 32.Brooke MH, Kaiser KK. Three human myosin ATPase systems and their importance in muscle pathology. Neurology. 1970;20:404. [PubMed] [Google Scholar]
- 33.Chayen J, Bitensky L, Butcher RG. Practical Histochemistry. London: John Wiley & Sons Ltd; 1973. Adenosine Triphosphatases; pp. 122–127. [Google Scholar]
- 34.Padykula HA, Herman E. Factors affecting the activity of adenosine triphosphatase and other phosphatases as measured by histochemical techniques. J HistoChem & Cytochem. 1955;3:161. doi: 10.1177/3.3.161. [DOI] [PubMed] [Google Scholar]
- 35.Sanes JR, Hall ZW. Antibodies that bind specifically to synaptic sites on muscle fiber basal lamina. J Cell Biol. 1979;83:357. doi: 10.1083/jcb.83.2.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kalliainen LK, Jejurikar SS, Liang LW, Urbanchek MG, Kuzon WM., Jr A specific force deficit exists in skeletal muscle after partial denervation. Muscle Nerve. 2002;25:31. doi: 10.1002/mus.1216. [DOI] [PubMed] [Google Scholar]
- 37.MacNiven I Stable trichrome for the demonstration of the intermyofibrillary network in muscle biopsies. Journal of Histotechnology. 1994;17:59. [Google Scholar]
- 38.Goldberg AL, Etlinger JD, Goldspink DF, Jablecki C. Mechanism of work-induced hypertrophy of skeletal muscle. Med Sci Sports. 1975;7:185. [PubMed] [Google Scholar]
- 39.Walsh JV, Jr, Burke RE, Rymer WZ, Tsairis P. Effect of compensatory hypertrophy studied in individual motor units in medial gastrocnemius muscle of the cat. J Neurophysiol. 1978;41:496. doi: 10.1152/jn.1978.41.2.496. [DOI] [PubMed] [Google Scholar]
- 40.Freeman PL, Luff AR. Contractile properties of hindlimb muscles in rat during surgical overload. Am J Physiol. 1982;242:C259–C264. doi: 10.1152/ajpcell.1982.242.5.C259. [DOI] [PubMed] [Google Scholar]
- 41.Roy RR, Meadows ID, Baldwin KM, Edgerton VR. Functional significance of compensatory overloaded rat fast muscle. J Appl Physiol. 1982;52:473. doi: 10.1152/jappl.1982.52.2.473. [DOI] [PubMed] [Google Scholar]
- 42.Booth FW. Time course of muscular atrophy during immobilization of hindlimbs in rats. J Appl Physiol. 1977;43:656. doi: 10.1152/jappl.1977.43.4.656. [DOI] [PubMed] [Google Scholar]
- 43.Booth FW, Shanely RA. The biochemical basis of the health effects of exercise: an integrative view. Proc Nutr Soc. 2004;63:199. doi: 10.1079/pns2004337. [DOI] [PubMed] [Google Scholar]
- 44.Booth FW, Thomason DB. Molecular and cellular adaptation of muscle in response to exercise: perspectives of various models. Physiol Rev. 1991;71:541. doi: 10.1152/physrev.1991.71.2.541. [DOI] [PubMed] [Google Scholar]
- 45.Lieber RL. Skeletal muscle architecture: implications for muscle function and surgical tendon transfer. J Hand Ther. 1993;6:105. [PubMed] [Google Scholar]
- 46.Baker JH, Hall-Craggs EC. Changes in length of sarcomeres following tenotomy of the rat soleus muscle. Anat Rec. 1978;192:55. doi: 10.1002/ar.1091920105. [DOI] [PubMed] [Google Scholar]
- 47.Smeulders MJC, Kreulen M, Hage JJ, Baan GC, Huijing PA. Progressive surgical dissection for tendon transposition affects length-force characteristics of rat flexor carpi ulnaris muscle. Journal of Orthopaedic Research. 2002;20:863. doi: 10.1016/S0736-0266(01)00181-4. [DOI] [PubMed] [Google Scholar]
- 48.Barton ER, Gimbel JA, Williams GR, Soslowsky LJ. Rat supraspinatus muscle atrophy after tendon detachment. Journal of Orthopaedic Research. 2005;23:259. doi: 10.1016/j.orthres.2004.08.018. [DOI] [PubMed] [Google Scholar]
- 49.Dix DJ, Eisenberg BR. Myosin mRNA accumulation and myofibrillogenesis at the myotendinous junction of stretched muscle fibers. J Cell Biol. 1990;111:1885. doi: 10.1083/jcb.111.5.1885. [DOI] [PMC free article] [PubMed] [Google Scholar]


