Abstract
Tyk2 belongs to the Janus kinase (JAK) family of receptor associated tyrosine kinases, characterized by a large N-terminal region, a kinase-like domain and a tyrosine kinase domain. It was previously shown that Tyk2 contributes to interferon-α (IFN-α) signaling not only catalytically, but also as an essential intracellular component of the receptor complex, being required for high affinity binding of IFN-α. For this function the tyrosine kinase domain was found to be dispensable. Here, it is shown that mutant cells lacking Tyk2 have significantly reduced IFN-α receptor 1 (IFNAR1) protein level, whereas the mRNA level is unaltered. Expression of the N-terminal region of Tyk2 in these cells reconstituted wild-type IFNAR1 level, but did not restore the binding activity of the receptor. Studies of mutant Tyk2 forms deleted at the N terminus indicated that the integrity of the N-terminal region is required to sustain IFNAR1. These studies also showed that the N-terminal region does not directly modulate the basal autophosphorylation activity of Tyk2, but it is required for efficient in vitro IFNAR1 phosphorylation and for rendering the enzyme activatable by IFN-α. Overall, these results indicate that distinct Tyk2 domains provide different functions to the receptor complex: the N-terminal region sustains IFNAR1 level, whereas the kinase-like domain provides a function toward high affinity ligand binding.
The intracellular protein-tyrosine kinases of the Janus kinase (JAK) family play an essential role in cytokine signaling: they interact with receptor components and undergo tyrosine phosphorylation and enzymatic activation upon ligand binding. Their activation is the first step of a cascade of intracellular phosphorylation events ultimately leading to the transcriptional activation of target genes (1, 2). Signaling through the receptor for type I interferons (IFN-α and -β) requires two members of the JAK family, Tyk2 and JAK1 (3, 4). Both enzymes are associated with the receptor, which is composed of IFN-α receptor (IFNAR) 1 (5) and IFNAR2–2, the longer splice variant of the IFNAR2 gene (6, 7). Tyk2 was shown to interact with the membrane-proximal region of IFNAR1 (8, 9), and JAK1 with IFNAR2–2 (10, 11). The stoichiometry of the activated receptor/JAK complex is not known and might differ for the different type I IFNs. Ligand-induced dimerization or oligomerization of the receptor components leads to asymmetric trans-phosphorylation and consequent catalytic activation of Tyk2 and JAK1 (4, 12, 13).
Tyk2 shares with the other members of the JAK family a unique structural framework (see Fig. 1A) comprising seven conserved JAK homology (JH) regions (14). The most C-terminal one (JH1) is a tyrosine kinase (TK) domain, which is flanked by the JH2 or kinase-like (KL) domain of unknown function. The remaining five blocks of homology (JH3 to JH7) extend toward the N terminus of the protein and exhibit variable degrees of conservation among the family members, the most conserved being JH4 with a central core of 18 identical residues. No defined function has been attributed to date to any of these five JH regions. There is evidence that multiple JH regions of JAK2 are necessary to mediate association with the growth hormone and the granulocyte-macrophage colony-stimulating factor receptor (15–17). Studies of chimeric JAK1/JAK2 constructs have shown that the JH7 and/or JH6 of JAK2 are involved in binding to the IFN-γR2 subunit of the IFN-γ receptor. On the other hand, reciprocal experiments also showed that the entire JH7 to JH3 portion of JAK1 was required for association to the IFN-γR1 subunit. Thus, it is likely that the structural requirements for cytokine receptor association of the JAKs vary in different JAK/receptor complexes (18).
Figure 1.
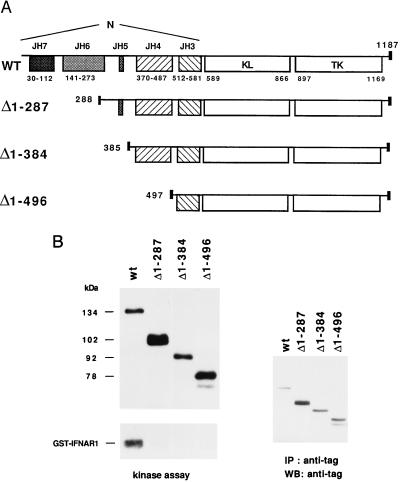
(A) Schematic representation of wt and deleted Tyk2 proteins. The position and size of the JH domains and the first amino acid of each mutant protein are indicated. The small black box corresponds to the VSV-G epitope. (B) Basal in vitro kinase activity of wt and deleted Tyk2 forms. Whole cell extracts of the indicated transfectant were immunoprecipitated with anti-VSV-G antibodies. One-third of the immunoprecipitate was subjected to an in vitro kinase assay (Left) and the remaining material was directly resuspended in SDS/sample buffer. Samples were then fractionated on SDS/7% polyacrylamide gel, transferred to Hybond C-super membrane and subjected to autoradiography (Left) or probed with a monoclonal anti-VSV-G antibody, followed by goat anti-mouse 125I-conjugated, and subjected to autoradiography (Right). Phosphorylated and iodinated bands were quantified with a PhosphorImager.
We have used the Tyk2-deficient cell line 11,1 to gain insight into the structure-function relation of Tyk2. Through the analysis of deleted forms of Tyk2, it was found that Tyk2 contributes to IFN-α signaling as an active tyrosine kinase amplifying the autocatalytic activation of JAK1 and downstream phosphorylation events (13), and as an intracellular component of the receptor complex, essential for high affinity binding of IFN-α (19). Although the TK domain was found to some extent dispensable for this function, the KL domain appeared to be required (20). However, the mechanism by which Tyk2 contributes to the IFN-α binding capacity of the receptor complex is not known. In this work, we have investigated the contribution of the N-terminal region (here referred to as the N region) to the activities of Tyk2. Through the analysis of deleted Tyk2 mutant forms expressed in the Tyk2-deficient cell line 11,1, we show that the N region (i) does not modulate the basal catalytic activity of Tyk2; (ii) is required for IFN-α-induced activation of the enzyme and for signaling; and (iii) sustains by itself normal expression of the receptor chain IFNAR1 in 11,1 cells, though it is not sufficient to rescue the binding activity of the receptor.
MATERIALS AND METHODS
Cell Culture.
Parental 2fTGH and mutants 11,1 (UIA), U1B, and U1C were described previously (19, 21). 11,1-derived clones expressing the mutated Tyk2 forms ΔKL, ΔTK, N, Y1054F/Y1055F, and K930R were described in refs. 13 and 20. Cells were grown in DMEM containing 10% heat-inactivated fetal calf serum and 250 μg/ml of hygromycin. Transfections and selection in G418 (450 μg/ml) were performed as reported (20). Cell survival in hypoxantine/aminopterin/thymidine medium was assayed in the presence of different concentrations of IFN-α2 (recombinant IFN-α2b, kindly provided by D. Gewert, Wellcome).
Plasmids.
All Tyk2 deletion mutants were cloned in the pRc/cytomegalovirus vector (Invitrogen). A Tyk2 cDNA fragment corresponding to amino acids 288-1187 was subcloned into pBluescript KS+ (bs), to make the intermediate bsΔ1–287 plasmid. Δ1–384 and Δ1–497 were obtained from bsΔ1–287 by removal of the EcoRI/PmlI (Δ1–384) or the EcoRI/SphI (Δ1–496) fragments (the EcoRI site is in the vector). Ends were made blunt with Klenow and the plasmid recircularized. The three cDNAs were subcloned into the NotI/XbaI sites of pRc/CMV. To obtain ΔJH4 (or Δ385–496), an intermediate plasmid was constructed from bsΔ1–287: the PmlI/SphI fragment (encoding amino acids 385–496) was removed, protruding ends blunted and relegated. SacII, which cuts 96-bp upstream of PmlI, and XbaI, which cuts in the vector polylinker, were used to swap the fragment containing the deletion into pRc/Tyk2 (13) digested with XbaI and partially digested with SacII (to avoid digestion in the 3′ untranslated region of Tyk2). Δ385–1187 is a pRc/Tyk2 with a stop codon after amino acid 385 that was generated by incorrect ligation at PmlI during construction of an unrelated plasmid. Δ1–51 lacks amino acids 1–51 of Tyk2: a SalI fragment of bsTyk2 was substituted with a SalI PCR fragment which spanned the sequence from amino acids 52–170 (SalI site). The resulting cDNA was cloned into the HindIII/XbaI sites of pRc/CMV. All plasmids, with the exception of Δ385–1187, contain the vesicular stomatitis virus glycoprotein (VSV-G) epitope tag (22).
Antibodies, Immunoprecipitation, Western Blot Analysis, and In Vitro Kinase Assay.
The anti-Tyk2 mAb, T10–2, was described elsewhere (12, 13). The EA12 monoclonal anti-IFNAR1 antibody used in immunoprecipitations and the GB8 monoclonal anti-IFNAR1 antibody used in immunoblot were described (23). Anti-VSV-G antibodies were kindly provided by M. Arpin (Institut Curie, Paris). Preparation of cell extracts, immunoprecipitations, and in vitro kinase assay were performed essentially as described (13). Immunoblottings were routinely revealed with an enhanced chemiluminescence detection system (Amersham). In the experiment shown in Fig. 1B, Western blot analysis was performed using a 125I-conjugated secondary antibody (Amersham) and quantified by a PhosphorImager (Molecular Dynamics).
Interferon Binding Assays.
Recombinant IFN-α2c, from Adolf Ernst (Boehringer Institute, Vienna) was labeled with 125I to an incorporation of 80 Bq/fmol (24). Cells close to confluency in 12-wells plates were incubated at 37°C with different IFN concentrations for 30 min, a time where the steady-state binding is attained in Tyk2 minus cells and where the peak binding is reached in wild-type (wt) cells (20). Plates were placed on ice, washed, detached with trypsin/EDTA, and counted. Nonspecific backgrounds were estimated by measuring the 125I-IFN uptakes on U5A, a 2fTGH derivative cell line lacking any displaceable binding (6).
RESULTS
Effect of N-Terminal Truncations on Tyk2 Activities.
The extra-catalytic or N region of Tyk2 spans ≈590 amino acids, i.e., half of the protein. As an initial step toward narrowing down its function(s), we generated the panel of staggered N-terminal deletion mutants depicted in Fig. 1A. Δ1–287, deleted of the first 287 amino acids, lacks the JH7 and JH6 regions; Δ1–384, deleted of the first 384 amino acids, lacks also JH5; Δ1–496 starts at position 497 and thus retains only the JH3 domain. The mutant cDNAs, cloned in pRc/CMV, were stably transfected in the Tyk2-deficient cell line 11,1 and the biological and enzymatic activities of the proteins were analyzed in representative neomycin-resistant clones. We chose clones expressing the exogenous protein at levels approaching that of a previously characterized wt Tyk2-expressing clone (WT in ref. 13).
As a test of the biological activity of the mutant proteins, we analyzed the ability of neomycin-resistant clones to survive in hypoxanthine/aminopterin/thymidine-containing medium, in the presence of increasing concentrations of IFN-α (from 10 to 10,000 international units/ml). As reported (19), this medium allows survival of 11,1 derivatives that have reverted to IFN-α sensitivity. Whereas wt cells survived at 10 international units/ml of IFN-α, cells expressing the N-terminal truncated mutants could not survive at any dose of IFN-α. Thus, all three mutants were biologically inactive.
Next, we analyzed the effect of the deletions on the basal and IFN-α induced kinase activity of the protein. For this, immunoprecipitated wt and mutated Tyk2 proteins were assayed for autophosphorylation in an in vitro kinase assay. To measure phosphorylation of a relevant substrate, the fusion protein glutathione S-transferase (GST)-IFNAR1cyt, containing the intracellular region of the receptor subunit IFNAR1, was added to the reaction (13). The amount of Tyk2 protein immunoprecipitated in each sample was accurately quantified by Western blot using an iodinated secondary antibody. Fig. 1B shows that all mutants retained a basal autophosphorylation activity that, when normalized for protein amount, was comparable to that of the wt protein. On the other hand, the ability of the three mutants to phosphorylate GST-IFNAR1cyt was severely impaired. None of the truncated proteins displayed higher activities (autophosphorylation or GST-IFNAR1cyt phosphorylation) in response to IFN-α (data not shown). Thus, the N region does not appear to modulate the basal autophosphorylation ability of Tyk2. However, the JH7 and/or JH6 region(s) are required for efficient phosphorylation of GST-IFNAR1cyt and for rendering the kinase activatable by IFN-α.
We have previously shown that the absence of inducible kinase activity is per se not sufficient to abolish the biological activity of Tyk2, because mutant Y1054F/Y1055F, an active Tyk2 mutated in two adjacent tyrosines in the TK domain activation loop, can sustain residual signaling, though unable to be catalytically induced (13). On the other hand, we know that Tyk2 contributes to IFN-α signaling by allowing high affinity IFN-α binding to the receptor and that the KL, but not the TK domain, is required for this function (20). Thus, the binding of IFN-α was measured on the cells expressing the truncated mutants described above. Although these mutants retained an intact KL domain, they were found unable to reconstitute wt IFN-α binding (see below), indicating that the N region of Tyk2 has a role in the formation of a functional receptor complex.
Reduced IFNAR1 Protein Level in Tyk2-Deficient Cells.
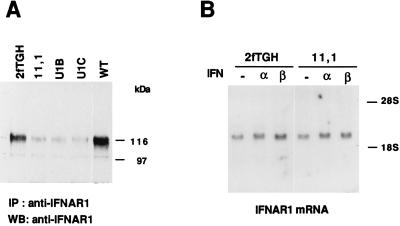
Because Tyk2 is known to interact with the receptor component IFNAR1 and this interaction could underline Tyk2 involvement in receptor function, we compared the level of IFNAR1 in Tyk2-expressing cells vs. Tyk2-negative cells. IFNAR1 is a highly glycosylated protein (23) that migrates as a broad band with an apparent molecular mass ranging from 110 to 150 kDa, depending on the cell type. Fig. 2A shows anti-IFNAR1 immunoblots from the parental 2fTGH cell line (19), three independent Tyk2-deficient mutants, (11,1, U1B, and U1C) (21) and complemented WT cells. The steady-state level of IFNAR1 was found severely reduced in the three mutants when compared with the parental 2fTGH, and was restored to normality in WT cells. To rule out the possibility of loss of reactivity of the IFNAR1 antibody due to an altered conformation of IFNAR1 in the absence of Tyk2, analogous experiments were performed with two other antibodies, directed toward distinct ectodomain epitopes, and identical results were obtained (data not shown). To investigate further this finding, the steady-state IFNAR1 mRNA level was analyzed by Northern blot in parental 2fTGH and mutant 11,1 cells and was found identical in the two cell lines (Fig. 2B). Treatment with IFN-α or IFN-β for 4 hr had no effect on the mRNA levels. Thus, the dependence of IFNAR1 from Tyk2 is a translational or posttranslational event.
Figure 2.
IFNAR1 expression in 2fTGH cells and derivatives. (A) Whole cell extracts (1 mg) were immunoprecipitated with the anti-IFNAR1 monoclonal EA12 antibody, fractionated on SDS/7% polyacrylamide gel and blotted with the anti-IFNAR1 monoclonal GB8 antibody. (B) Northern transfers of 10 μg of total RNA extracted from subconfluent untreated (−), IFN-α, or IFN-β treated cells (4 hr) were hybridized to an IFNAR1 cDNA specific probe. Comparable signals in each lane were obtained with a β-actin probe (not shown).
Multiple JH Regions Required To Restore IFNAR1 Protein Level.
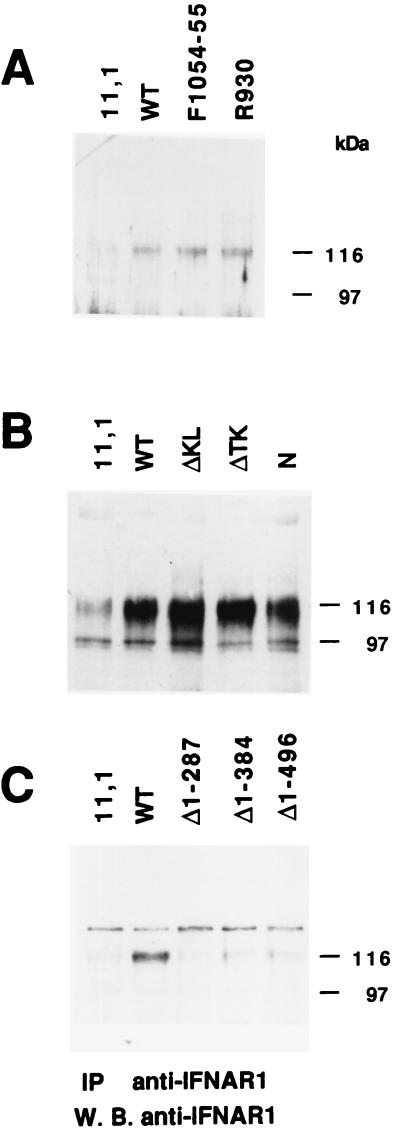
We next analyzed the steady-state level of IFNAR1 in 11,1-derived clones expressing various mutated forms of Tyk2. The kinase-inactive Tyk2 mutant (K930R) and the uninducible Tyk2 mutant (Y1054F/Y1055F) have been previously described (13). Fig. 3A shows that both proteins were able to sustain IFNAR1 expression, thus establishing that the enzymatic activity of Tyk2 is not required. To globally delimite the portion(s) of Tyk2 required for this function, we analyzed IFNAR1 level in three previously characterized 11,1 transfectants expressing deleted forms of Tyk2: ΔTK lacks the TK domain, ΔKL lacks the KL domain and N lacks both kinase domains (see figure 1 in ref. 20 and Fig. 4A). Deletion of one or the other kinase domain did not affect the ability of Tyk2 to sustain IFNAR1 expression (Fig. 3B, lanes 3 and 4). Most importantly, the N protein was by itself sufficient to restore IFNAR1 (Fig. 3B, lane 5; see also Fig. 4B, lane 5). In the experiment shown in Fig. 3B a larger number of cells was processed, which allowed detection of residual IFNAR1 in 11,1 cells (≈one-fifth the level in wt cells). To attempt to further delimit a region within the N-terminal portion of Tyk2 required to sustain IFNAR1 expression, we analyzed the level of IFNAR1 in the clones expressing the N-terminal truncation mutants (see Fig. 1). None of the three mutants was able to restore IFNAR1 to the level seen in WT cells (Fig. 3C), suggesting that a minimal region comprising JH7 and/or JH6 is required.
Figure 3.
IFNAR1 level in 11,1 expressing mutated forms of Tyk2. Anti-IFNAR1 immunoprecipitation and Western blot analysis were performed as in Fig. 2A. (A) IFNAR1 level in cells expressing the K930R point mutant, and the Y1054F/Y1055F mutant. (B) IFNAR1 level in cells expressing Tyk2 mutants deleted of either or both kinase domains. Five milligrams of lysates were processed. (C) IFNAR1 levels in cells expressing the N-terminal mutants, as indicated. The slower migrating band was seen only in this experiment.
Figure 4.
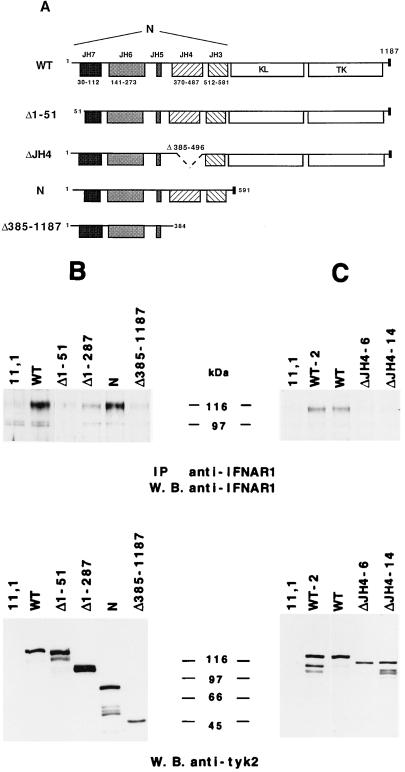
(A) Schematic representation of four N-terminal deleted Tyk2 mutants: Δ1–51, ΔJH4, N, and Δ385–1187. (B and C) IFNAR1 level in 11,1 cells expressing mutated forms of Tyk2. (Upper) IFNAR1 protein level was analyzed in each cell line as described in Fig. 2. (Lower) The level of expression of each mutant protein was analyzed by direct anti-Tyk2 Western blot from 20 μg of crude extract.
Three additional Tyk2 mutants were constructed and stably expressed in 11,1 cells: Δ1–51, which lacks the first 51 amino acids; ΔJH4, which has an internal deletion of 111 amino acids spanning the highly conserved JH4 region; and, finally, Δ385–1187, which retains only JH7, JH6, and JH5 (Fig. 4A). The level of mutant protein expressed in each clone was monitored by anti-Tyk2 Western blot (Lower, Fig. 4 B and C). Removal of 51 amino acids at the N terminus abolished Tyk2 ability to restore IFNAR1 (Fig. 4B, lanes 2 and 3). When compared with N, Δ385–1187 was also inactive, suggesting that the JH4 and/or JH3 regions are required (Fig. 5B, lanes 5 and 6). The ΔJH4 protein was inactive as well, as can be seen in Fig. 4C where two wt clones were compared with two ΔJH4-expressing clones.
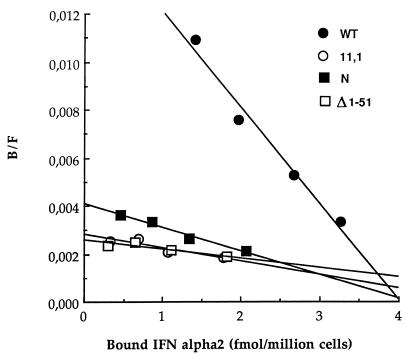
Figure 5.
Binding of IFN-α2 to 11,1 cells expressing deleted Tyk2 forms. Scatchard plot of direct binding of 125I-labeled IFN-α2c at 37°C.
Overall, these data showed that an intact N region was required and sufficient to restore wt level of IFNAR1 in 11,1 cells. Interestingly, however, this did not correlate with greater IFN-α binding activity. As can be seen in the Scatchard plot shown in Fig. 5, 11,1 cells expressing the N region of Tyk2 and thus complemented for the IFNAR1 level, showed the same low apparent affinity IFN-α2 binding as cells expressing a reduced IFNAR1 level, such as 11,1 or Δ1–51 expressing cells.
DISCUSSION
In this study, N-terminal truncated versions of Tyk2 were stably expressed in a Tyk2-deficient mutant cell line to gain some insight into the function(s) of the N region. One aim was to test whether this large portion of the protein contained domains positively or negatively regulating the catalytic activity of the protein. From in vitro kinase assays it was shown that the basal autophosphorylation activity of Tyk2 was not affected by deletions in the N region. Similar observations have been made for JAK2 (17, 25). A JAK2 mutant lacking the N region and 40% of the KL domain was described as hyperphosphorylated in insect cells and hyperactive in an in vitro kinase assay, suggesting the presence of an inhibitory domain within the deleted portion of the enzyme (25). This finding is not supported by our data. Note that in our study particular care was taken to normalize the in vitro autophosphorylated Tyk2 to the amount of immunoprecipitated protein. Thus, we are confident that the intrinsic activity of each truncation mutant is comparable to that of the wt protein, and we found no evidence for the presence of inhibitory domains within the N region. The discrepancy could be due either to the different cellular systems used or to the absence of part of the KL domain in the JAK2 mutant studied by (25).
Although the first 496 amino acids (JH7 to JH4) of Tyk2 are dispensable for its autophosphorylation activity, they are required for efficient phosphorylation of GST-IFNAR1cyt, suggesting the presence within N of a domain of interaction with IFNAR1. The N region is also required to sustain in vivo a normal steady-state level of expression of this receptor chain: the loss of IFN-α activation and of signaling capacity of the N-terminal deleted proteins is thus likely to be the direct consequence of their impaired ability to rescue IFNAR1 expression. However, we cannot use our cellular system to establish a correlation between the ability of the various Tyk2 mutants to interact with IFNAR1 and their ability to rescue IFNAR1 expression: in fact, being the steady-state level of IFNAR1 protein influenced by Tyk2 itself (Fig. 2A), coimmunoprecipitation studies of the two proteins are not feasible. On the other hand, we know from in vitro studies of recombinant Tyk2 and IFNAR1 proteins, that the N region interacts with the cytoplasmic portion of IFNAR1, and that an intact JH7 region is required, because deletion of the first 51 amino acids of the recombinant N protein abolishes its interaction with recombinant IFNAR1cyt (M.F.R. and S.P., unpublished work).
So far, we have been unable to identify a discrete JH domain responsible for rescuing IFNAR1. Deletion of the N-terminal 51 residues may impinge into and disrupt the JH7 region, which was shown to be required for interaction of JAK1 and JAK2 with the γR1 and the γR2 subunits, respectively, of the IFN-γ receptor (18). An internal deletion of the highly conserved JH4 region also inactivates the function of Tyk2. Similarly, restoration of IFNAR1 seen in N-expressing cells is abolished upon removal of JH3 and JH4. Thus, these data suggest that the structural determinant responsible for rescuing IFNAR1 in vivo consists of several noncontiguous segments of N required to maintain a correct three-dimensional configuration.
A question that arises is whether the reduced amount of IFNAR1 in cells lacking Tyk2 can account for the impairment of high affinity IFN-α binding in these cells (19, 20). The role of IFNAR1 in binding of type I IFNs has been demonstrated by a number of studies (26). IFNAR1 was shown to interact with the helix C of the IFN-α molecule (27); IFNAR1 expression is required to reconstitute high affinity binding of human IFN-α/β in murine cells (28); antibodies directed toward the ectodomain of IFNAR1 were shown to neutralize both the binding and antiviral activities of all IFN-α/β species (29, 30). In the murine system, deletion or inactivation of the IFNAR1 gene render cells or mice resistant to the activities of all type I IFNs (31, 32). Recent studies by Cutrone and Langer (33) suggest that IFNAR1 modulates the affinity and the differential regulation of the IFNAR2/IFNAR1 complex toward the different type I IFNs. In particular, these authors showed that the contribution of IFNAR1 to the binding of IFN-β is small when compared with other type I IFNs. Interestingly, the contribution of Tyk2 to IFN-β binding and signaling is less manifest, because 11,1 cells retain high affinity binding sites and partial responsiveness to IFN-β (20). More work is needed to establish if the residual IFNAR1 present in 11,1 cells mediates these activities. Most important, however, is the finding that restoration of normal IFNAR1 level as seen in cells expressing N or ΔKL (Fig. 3B) is not sufficient to rescue high affinity IFN-α binding (Fig. 5) (20). This suggests that distinct domains of Tyk2 provide different functions: the N region sustains IFNAR1 level, most likely through a direct interaction, and the KL domain provides a function toward high affinity ligand binding, possibly through its interaction with another partner of the complex.
It is not known whether the “dependence” of IFNAR1 from Tyk2 is the consequence of the direct interaction between the two proteins or whether other proteins of the receptor complex are involved. In this respect, the study of mutant cells lacking other components of the complex can be particularly instructive. The level of IFNAR1 in U5 cells, defective for the expression of IFNAR2–2 (6), and in U4C cells, lacking JAK1 (4) is comparable to the level seen in parental 2fTGH cells (M.C.G. and S.P., unpublished observations). Thus, IFNAR2–2 and JAK1 do not seem to affect the expression level of IFNAR1. It remains to be studied whether IFNAR2–2 protein level is similarly dependent from JAK1. Interestingly, impaired IFN-α binding has been reported in JAK1-deficient U4A cells (34) (G.U., unpublished observations), but it is not yet known whether this correlates with reduced IFNAR2–2 protein levels. Apart from this possibility, the phenomenon that is described here appears unique to the Tyk2/IFNAR1 complex because, to our knowledge, reduced levels of cytokine receptors (or impaired ligand binding) in the absence of interaction with a JAK family member have never been reported.
The mechanism by which Tyk2 influences IFNAR1 protein level is currently under study. The simplest interpretation is that of an increased turnover rate of IFNAR1 in the absence of Tyk2: the cytoplasmic region of IFNAR1 might be improperly folded and thus targeted to degradation or, conversely, the interaction with Tyk2 could mask degradation targeting sites on IFNAR1. Another possibility is that posttranslational modifications, such as glycosylation, could be altered in the absence of Tyk2 and affect the stability of IFNAR1. In this regard, it was reported that a transiently overexpressed CD4-IFNAR1cyt chimera unable to bind Tyk2 had an altered mobility in SDS/PAGE, when compared with the Tyk2-binding chimera. This mobility shift was attributed to altered glycosylation or some other modifications consequent to the failure to bind Tyk2 (35). Tyk2 may be required for correct assembly of the receptor complex and its delivery to the cell surface. In its absence IFNAR1 could be retained in the endoplasmic reticulum (36). As noted above, however, other receptor components (IFNAR2–2 and JAK1) are not required for IFNAR1 expression. Finally, the hypothesis that the requirement for Tyk2 is cotranslational cannot be ruled out. So far, our attempts to compare the stability of IFNAR1 in the presence and in the absence of Tyk2 have been unsuccessful. The low level of IFNAR1 protein, its high glycosylation and the quality of the available antibodies may account for our inability to pulse label IFNAR1. Most important is our observation that overexpression of IFNAR1 in 11,1 cells abolishes the dependence toward Tyk2. In fact, comparable levels of IFNAR1 are present in 11,1 and 2fTGH cells upon transient transfection of IFNAR1 cDNA (M.C.G. and S.P., unpublished observations). This finding suggests that overexpression of the receptor subunit in 11,1 may titrate an intracellular component targeting IFNAR1 to degradation.
One question of considerable interest is whether the dependence of IFNAR1 level from Tyk2 is of any biological significance in the physiology of the IFN response and/or in the regulation of the cytokine network. Tyk2 was reported to be ubiquitously expressed in different human cell lines (37). It will be interesting to investigate whether Tyk2 expression is modulated under specific stimuli and whether this can influence the IFN-α response through receptor down-modulation. Furthermore, Tyk2 is known to bind to components of the interleukin 10 and of the interleukin 12 receptors (38, 39) and the question arises whether induced expression of these receptors in certain cell types can modulate IFN-α binding by sequestering Tyk2 and thus limiting its availability to IFNAR1.
Acknowledgments
We dedicate this work to the memory of Tommaso Meo. We wish to thank O. Acuto, P. Benaroch, and E. Coccia for critical reviewing of the manuscript. M.C.G. was supported by Associazione Italiana per la Ricerca sul Cancro and by Fondation pour la Recherche Médicale; G.B. was the recipient of a European Economic Community fellowship; M.F.R. was supported by a World Health Organization fellowship. This work was supported in part by the Association pour la Recherche sur le Cancer (Grant ARC 1152 to S.P.), the Institut National Santé et de la Recherche Médicale, and the Ministère de la Recherche et de l’Enseignement Supérieur. The work at the Institut Génétique Moléculaire was supported by grants from the Direction des Recherches, Etudes et Téchniques (94/2513A), Association pour la Recherche sur le Cancer, and the Ligue Nationale contre le Cancer.
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
Abbreviations: JAK, Janus kinase; KL, kinase-like; TK, tyrosine kinase; IFN, interferon; IFNAR, interferon α receptor; GST, glutathione S transferase; VSV-G, vesicular stomatitis virus glycoprotein; wt, wild type; N region, N-terminal region.
References
- 1.Ihle J N. Adv Immunol. 1995;60:1–35. doi: 10.1016/s0065-2776(08)60582-9. [DOI] [PubMed] [Google Scholar]
- 2.Leaman D W, Leung S, Li X, Stark G R. FASEB J. 1996;10:1578–1588. [PubMed] [Google Scholar]
- 3.Velazquez L, Fellous M, Stark G R, Pellegrini S. Cell. 1992;70:313–322. [PubMed] [Google Scholar]
- 4.Müller M, Briscoe J, Laxton C, Guschin D, Ziemiecki A, Silvennoinen O, Harpur A G, Barbieri G, Witthuhn B A, Schindler C, Pellegrini S, Wilks A F, Ihle J N, Stark G R, Kerr I M. Nature (London) 1993;366:129–166. doi: 10.1038/366129a0. [DOI] [PubMed] [Google Scholar]
- 5.Uzé G, Luftalla G, Gresser I. Cell. 1990;60:225–234. doi: 10.1016/0092-8674(90)90738-z. [DOI] [PubMed] [Google Scholar]
- 6.Lutfalla G, Holland S J, Cinato E, Monneron D, Reboul J, Rogers N C, Smith J M, Stark G R, Gardiner K, Mogensen K E, Kerr I M, Uzé G. EMBO J. 1995;14:5100–5108. doi: 10.1002/j.1460-2075.1995.tb00192.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Domanski P, Witte M, Kellum M, Rubinstein M, Hackett R, Pitha P, Colamonici O R. J Biol Chem. 1995;270:21606–21611. doi: 10.1074/jbc.270.37.21606. [DOI] [PubMed] [Google Scholar]
- 8.Colamonici O, Yan H, Domanski P, Handa R, Smalley D, Mullersman J, Witte M, Krishnan K, Krolewski J. Mol Cell Biol. 1994;14:8133–8142. doi: 10.1128/mcb.14.12.8133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yan H, Krishnan K, Greenlund A C, Gupta S, Lim J T, Schreiber R D, Schindler C W, Krolewski J J. EMBO J. 1996;15:1064–1074. [PMC free article] [PubMed] [Google Scholar]
- 10.Novick D, Cohen B, Rubinstein M. Cell. 1994;77:391–400. doi: 10.1016/0092-8674(94)90154-6. [DOI] [PubMed] [Google Scholar]
- 11.Domanski P, Colamonici O R. Cytokine Growth Factor Rev. 1996;7:143–151. doi: 10.1016/1359-6101(96)00017-2. [DOI] [PubMed] [Google Scholar]
- 12.Barbieri G, Velazquez L, Scrobogna M, Fellous M, Pellegrini S. Eur J Biochem. 1994;223:427–435. doi: 10.1111/j.1432-1033.1994.tb19010.x. [DOI] [PubMed] [Google Scholar]
- 13.Gauzzi M C, Velazquez L, McKendry R, Mogensen K E, Fellous M, Pellegrini S. J Biol Chem. 1996;271:20494–20500. doi: 10.1074/jbc.271.34.20494. [DOI] [PubMed] [Google Scholar]
- 14.Ziemiecki A, Harpur A G, Wilks A F. Trends Cell Biol. 1994;4:207–212. doi: 10.1016/0962-8924(94)90143-0. [DOI] [PubMed] [Google Scholar]
- 15.Tanner J W, Chen W, Young R L, Longmore G D, Shaw A S. J Biol Chem. 1995;270:6523–6530. doi: 10.1074/jbc.270.12.6523. [DOI] [PubMed] [Google Scholar]
- 16.Frank S J, Yi W, Zhao Y, Goldsmith J F, Gilliland G, Jiang J, Sakai I, Kraft A. J Biol Chem. 1995;270:14776–14785. doi: 10.1074/jbc.270.24.14776. [DOI] [PubMed] [Google Scholar]
- 17.Zhao Y, Wagner F, Frank S J, Kraft A S. J Biol Chem. 1995;270:13814–13818. doi: 10.1074/jbc.270.23.13814. [DOI] [PubMed] [Google Scholar]
- 18.Kohlhuber F, Rogers N C, Watling D, Feng J, Guschin D, Briscoe J, Witthuhn B A, Kotenko S V, Pestka S, Stark G R, Ihle J N, Kerr I M. Mol Cell Biol. 1997;17:695–706. doi: 10.1128/mcb.17.2.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pellegrini S, John J, Shearer M, Kerr I M, Stark G R. Mol Cell Biol. 1989;9:4605–4612. doi: 10.1128/mcb.9.11.4605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Velazquez L, Mogensen K E, Barbieri G, Fellous M, Uze G, Pellegrini S. J Biol Chem. 1995;270:3327–3334. doi: 10.1074/jbc.270.7.3327. [DOI] [PubMed] [Google Scholar]
- 21.McKendry R, John J, Flavell D, Muller M, Kerr I M, Stark G R. Proc Natl Acad Sci USA. 1991;88:11455–11459. doi: 10.1073/pnas.88.24.11455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kreis T E. EMBO J. 1986;5:931–941. doi: 10.1002/j.1460-2075.1986.tb04306.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ling L E, Zafari M, Reardon D, Brickelmeier M, Goelz S E, Benjamin C D. J Interferon Cytokine Res. 1995;15:55–61. doi: 10.1089/jir.1995.15.55. [DOI] [PubMed] [Google Scholar]
- 24.Mogensen K E, Uzé G. Methods Enzymol. 1986;119:267–276. doi: 10.1016/0076-6879(86)19039-2. [DOI] [PubMed] [Google Scholar]
- 25.Duhe R J, Farrar W L. J Biol Chem. 1995;270:23084–23089. doi: 10.1074/jbc.270.39.23084. [DOI] [PubMed] [Google Scholar]
- 26.Uze G, Lutfalla G, Mogensen K E. J Interferon Cytokine Res. 1995;15:3–26. doi: 10.1089/jir.1995.15.3. [DOI] [PubMed] [Google Scholar]
- 27.Uzé G, DiMarco S, Mouchel-Viehl E, Monneron D, Bandu M T, Horisberger M A. J Mol Biol. 1994;243:245–257. doi: 10.1006/jmbi.1994.1651. [DOI] [PubMed] [Google Scholar]
- 28.Russell-Harde D, Pu H, Betts M, Harkins R N, Perez H D, Croze E. J Biol Chem. 1995;270:26033–26036. doi: 10.1074/jbc.270.44.26033. [DOI] [PubMed] [Google Scholar]
- 29.Uzé G, Lutfalla G, Eid P, Maury C, Bandu M T, Gresser I, Mogensen K E. Eur J Biochem. 1991;21:447–451. doi: 10.1002/eji.1830210229. [DOI] [PubMed] [Google Scholar]
- 30.Benoit P, Maguire D, Plavec I, Kocher H, Tovey M, Meyer F. J Immunol. 1993;150:707–716. [PubMed] [Google Scholar]
- 31.Uzé G, Luftalla G, Bandu M T, Proudhon D, Mogensen K E. Proc Natl Acad Sci USA. 1992;89:4774–4778. doi: 10.1073/pnas.89.10.4774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Muller U, Steihoff U, Reis L F L, Hemmi S, Pavlovic J, Zinkernagel R M, Aguet M. Science. 1994;264:1918–1921. doi: 10.1126/science.8009221. [DOI] [PubMed] [Google Scholar]
- 33.Cutrone E C, Langer J A. FEBS Lett. 1997;404:197–202. doi: 10.1016/s0014-5793(97)00129-4. [DOI] [PubMed] [Google Scholar]
- 34.Darnell J E J, Kerr I M, Stark G R. Science. 1994;264:1415–1421. doi: 10.1126/science.8197455. [DOI] [PubMed] [Google Scholar]
- 35.Krishnan K, Yan H, Lim J T, Krolewski J. Oncogene. 1996;13:125–133. [PubMed] [Google Scholar]
- 36.Klausner R D, Lippincott-Schwartz J, Bonifacino J S. Annu Rev Cell Biol. 1991;6:403–431. doi: 10.1146/annurev.cb.06.110190.002155. [DOI] [PubMed] [Google Scholar]
- 37.Firmbach-Kraft I, Byers M, Shows T, Dalla-Favera R, Krolewski J J. Oncogene. 1990;5:1329–1336. [PubMed] [Google Scholar]
- 38.Finbloom D S, Winestock K D. J Immunol. 1995;155:1079–1090. [PubMed] [Google Scholar]
- 39.Bacon C M, McVicar D W, Ortaldo J R, Rees R C, O’Shea J J, Johnston J A. J Exp Med. 1995;181:399–404. doi: 10.1084/jem.181.1.399. [DOI] [PMC free article] [PubMed] [Google Scholar]