Abstract
OBJECTIVE
Preeclampsia is a multisystem disease classically defined on the basis of hypertension and proteinuria. As shown in animal studies, complement activation is associated with inflammation in the placenta and adverse pregnancy outcomes. The association between complement activation in humans and adverse pregnancy outcomes is unclear. The purpose of this study was to determine whether elevated levels of the activation fragment Bb in early pregnancy are predictive of preeclampsia.
STUDY DESIGN
This prospective study of 701 women was conducted in Denver, CO. A single plasma sample was obtained from each woman before 20 weeks’ gestation. The cohort was followed up throughout pregnancy for the development of preeclampsia. Analysis included multivariate logistic regression to adjust for established risk factors for preeclampsia.
RESULTS
Preeclampsia developed in 4.6% of the cohort. Women with elevated Bb (90th or greater percentile) were substantially more likely to develop preeclampsia than women who had levels less than the 90th percentile (unadjusted relative risk [RR], 3.3, 95% confidence interval [CI] 1.6 to 7, P = .0009). Other significant risk factors for preeclampsia included nulliparity (RR, 2.1, 95% CI, 1–4), a high body mass index (P = .006 for trend), and maternal medical (preexisting maternal hypertension, type 1 diabetes, systemic lupus erythematosus) disease (RR, 4.4, 95% CI, 2–10). Significant risk factors among multiparous women included a history of hypertension in a previous pregnancy (RR, 5, 95% CI, 1.6 to 16) and a change of paternity (RR, 5.1, 95% CI, 1.6 to 15). Adjustment for risk factors did not attenuate the association between an elevated Bb and preeclampsia (adjusted odds ratio [OR], 3.8, 95% CI, 1.6 to 9, P = .002) in the cohort. After removing women with plasma obtained before 10 weeks, the adjusted OR of Bb in the top decile for preeclampsia was 6.1 (95% CI 2.2, 17, P = .0005).
CONCLUSION
The complement activation product Bb in early pregnancy is a biomarker for elevated risk of preeclampsia. This observation suggests that events linked to activation of complement in early pregnancy are associated with the pathogenesis of preeclampsia.
Keywords: alternative complement pathway, complement activation fragment Bb, preeclampsia
Preeclampsia is a complex multisystem disease that occurs in 3–5% of all pregnancies and contributes significantly to maternal and neonatal mortality and morbidity.1,2 Its cause is unclear. The classical clinical manifestations, de novo hypertension and proteinuria, occur late in pregnancy in the setting of maternal endothelial cell activation.3 However, there is now extensive evidence to suggest that this syndrome starts in early pregnancy, and current data support a pathogenic role for shallow endovascular cytotrophoblastic invasion of the spiral arteries4 and circulating antiangiogenic factors.5,6 In addition, many authors have suggested an important role for the immune system in the etiology of this syndrome.1,7
The complement system is a complex series of more than 30 proteins and, like the clotting cascade, contains a potent amplification mechanism based on the sequential cleavage of inactive zymogen forms of proteins by serine protease mechanisms.8 The complement system combats infection and removes immune complexes as well as ischemic, necrotic, and apoptotic cells. The complement has 3 initiating mechanisms known as the classical, lectin, and alternative pathways (Figure 1). There are many triggers for these pathways including immune complexes, apoptotic bodies, mitochondrial membranes, mitochondrial cardiolipin (classical pathway), carbohydrates on pathogens (lectin pathway), and foreign surfaces (alternative pathway).8
FIGURE 1. Complement activation pathways.
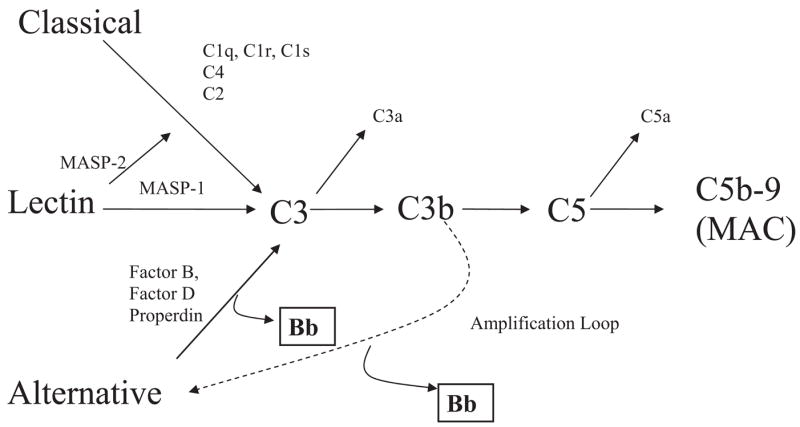
The classical, lectin, and alternative complement pathways converge to generate enzymes called C3 convertases, which cleave C3 into C3b and C3a. C3b is a major effector molecule of the complement system. C3b binds to the surface of foreign cells and opsonizes the cells for phagocytosis. Through a series of reactions, the membrane attack complex (MAC) is formed. This complex can insert into membranes and has the potential to damage cells. Factors B, D, and Properdin initiate activation of C3b directly through the alternative pathway initiation complex or through the amplification loop in which C3b is formed. The activation product Bb (boxed) is derived from factor B. The production of C3b, triggered from engagement of the classical or lectin pathways, is augmented through the alternative pathway amplification loop that is essential to generate all pathogenic proinflammatory mediators in vivo.
Biological functions of the complement system are achieved through the production of activation fragments or complexes (eg, C3a, C5a, C5b-9) that promote chemotaxis, microbial opsonization, cell lysis, phagocyte recruitment, and cellular activation.9,10 The alternative pathway is unique in that it both can serve as an amplification system for the classical and lectin pathways and can also autoactive through a process called tickover.11,12 In addition to C3b, 3 factors are involved in alternative pathway activation, namely factors D and B and properdin.8
The important contribution of complement activation to adverse pregnancy outcomes has emerged from recent studies on the murine model of the antiphospholipid antibody syndrome13–16 as well as other murine models of spontaneous fetal loss,17,18 which demonstrated that complement activation, especially through the alternative pathway, is a proximal cause of vascular injury and fetal demise. The association between complement activation in humans and adverse pregnancy outcomes is, however, unclear. Thus, the purpose of this study was to determine in human pregnancy whether complement activation could contribute to the pathogenesis of preeclampsia. The factor B-derived Bb activation fragment (from the alternative complement pathway) was used as a biomarker of complement activation (Figure 1).12
Materials and Methods
Study design
This prospective study was approved by the Colorado Multiple Institutional Review Board and is part of an ongoing study started in June 2005 to examine the contribution of complement activation to adverse pregnancy outcomes. The explanatory variable of interest was the complement activation fragment Bb, which was examined as a continuous and dichotomous variable (elevated vs not elevated). An elevated Bb was defined as a level of Bb greater than the 90th percentile for the cohort. We chose to dichotomize at the 90th percentile because it was the lowest point at which Bb appeared to give a clinically useful result with a large enough sample of preeclamptic subjects above the cutoff to make modeling meaningful (see Results section).
Other risk factors included in the analysis included race (African American vs others); parity (nulliparity vs multiparity); maternal age (older than 35 years vs younger than 35 years); cigarette smoking at the time of conception (yes vs no); a past history of spontaneous abortion (yes vs no); and the maternal prepregnant body mass index (BMI; kilograms per square meter: 25–30 [overweight], greater than 30 [obese] vs less than 25), calculated from the maternal prepregnant weight and height. A maternal history of medical disease (yes vs no) was a composite of preexisting maternal hypertension, type 1 diabetes, or systemic lupus erythematosus (SLE). Among multiparous women we examined a history of hypertension in a previous pregnancy (yes vs no); a change of paternity since the last delivery (yes vs no), defined as a new father of this baby, compared with the last delivery; and the length of time since the last birth (greater than 5 vs less than 5 years).
The primary outcome of the study was preeclampsia, classically defined as gestational hypertension and proteinuria.19 Gestational hypertension was defined as a systolic blood pressure greater than 140 mm Hg or a diastolic blood pressure greater than 90 mm Hg on at least 2 occasions at least 6 hours apart after 20 weeks’ gestation in women known to be normotensive before pregnancy and before 20 weeks’ gestation. Preeclampsia was defined as: (1) gestational hypertension with proteinuria (300 mg or greater per 24 hour period) or at least 1 or greater on dipstick or (2) in the absence of proteinuria gestational hypertension with cerebral symptoms, epigastric or right upper quadrant pain with nausea or vomiting, or thrombocytopenia and abnormal liver function tests.19 We did not distinguish between mild and severe preeclampsia in this study. Preeclampsia was classified in subjects without knowledge of Bb levels. Intrauterine growth restriction (IUGR) was defined as a birth percentile less than the 10th percentile using the Colorado Intrauterine Growth Charts.20
Study participants were recruited from the prenatal clinics at the University of Colorado Hospital (UCH) (n = 356), the Metro Community Practice Network (MCPN) clinic (a community-based prenatal clinic, n = 98), and the Platte River Prenatal Center, a faculty referral facility (n = 247). The study participants were referred to the complement study team by the prenatal care provider if the woman was in the first half of pregnancy. There were no exclusion criteria for the study, and to date the nonparticipation rate is approximately 3%.
The women were categorized into 1 of 3 strata, depending on the gestation at entry into the study (Table 1). Following informed written consent, an interview was conducted with the study participant, and data were gathered on the maternal medical and obstetrical history. The deidentified data were entered into the complement study data set, which is part of the University of Colorado at Denver and Health Sciences Center perinatal database that is housed in the division of Biostatistics and Bioinformatics at National Jewish Medical and Research Center (NJMRC) Denver.
TABLE 1.
Characteristics of the cohort (n = 701)
| Race/ethnicity non-Hispanic white | 459 | 65% |
| Hispanic white | 149 | 21% |
| African American | 51 | 7% |
| Asian and other | 42 | 6% |
| Age 35 y or older | 293 | 42% |
| Nulliparity | 313 | 45% |
| History of spontaneous abortion | 185 | 26% |
| Prepregnant BMI (kg/m2) | ||
| Less than 25 | 461 | 66% |
| 25–30 | 141 | 20% |
| 30 or greater | 93 | 13% |
| Medical history | ||
| SLE | 6 | 0.9% |
| Diabetes (type 1) | 8 | 1% |
| Chronic hypertension | 22 | 3% |
| Cigarette smoking at conception | 52 | 7% |
| Gestational age at blood draw (wks) | ||
| Less than 10 | 170 | 24% |
| 10–15 | 468 | 67% |
| 15–20 | 63 | 9% |
| Mean gestational age (wks) at delivery (± SD) | 39 ± 2 | |
| Among multiparous women (n = 388) | ||
| History of hypertension in a previous pregnancy | 35 | 9% |
| Length since last delivery longer than 5 y | 93 | 24% |
| Change of paternity | 64 | 16% |
At the same visit, blood (plasma) was drawn for the Bb assay along with the routine prenatal laboratory tests. The women were followed up prospectively throughout pregnancy. At delivery, outcome data were collected by the perinatal database staff and entered into the complement study data set. A secondary review of each completed maternal record was conducted by 1 investigator (A.M.L.) before the participant’s sample was assayed. At this assessment the gestational age at blood draw was verified using results from ultrasound examinations. Women who delivered outside UCH received a detailed questionnaire regarding pregnancy outcome within 2 weeks of the estimated date of delivery.
A diagnosis of preeclampsia was confirmed by the combination of a phone interview and review of the medical record requested from the outside hospital with patient consent. A random selection of the records of women with preeclampsia was also reviewed by a single maternal-fetal medicine specialist (blinded to the results of the assay) to verify classification of the outcome (see Acknowledgments). As of March 2007, pregnancy outcomes were available on 806 women. For this analysis, we excluded women who had a spontaneous abortion (n = 27), multiple gestation (n = 27), or a first presentation for prenatal care after 20 weeks’ gestation (n = 7). Because there is uncertainty whether gestational hypertension is a separate entity from preeclampsia or a mild version of preeclampsia,6,21 we excluded women with gestational hypertension (n = 44) from the cohort to avoid misclassification of the outcome. Following these exclusions, 701 women were available for analysis.
Sample preparation and assay for complement activation fragment Bb
Each sample was centrifuged (within an average time of 11 minutes from phlebotomy), and the supernatant was removed, aliquoted, and placed in a freezer at −80°C. For women recruited outside UCH, the sample was centrifuged immediately and placed on dry ice for transportation back to the repository.
Complement activation fragment Bb was measured using a quantitative sandwich enzyme-linked immunosorbent assay (ELISA) that uses a monoclonal antihuman Bb as the capture antibody and an enzyme-conjugated goat antihuman Bb as the second antibody (Quidel, San Diego, CA). Standards with known concentrations of Bb and high and low plasma controls were run on each ELISA plate. The addition of a chromogenic substrate causes a change in optical density (color) in each well of the ELISA plate. This change in optical density was measured spectrophotometrically and is a function of the amount of antigen (Bb) captured on the plate. The coefficient of variation for the in-house laboratory control was 10.2%. Approximately 100 consecutive samples were analyzed per month on women following completion of pregnancy. The technician performing the assay was blinded to the participant’s pregnancy outcome.
Statistical analysis
The data were analyzed in SAS 9.1 (SAS Institute, Cary, NC). Univariable analysis was used to generate descriptive statistics for the cohort. Differences between dichotomous variables were examined using the relative risk (RR) as a measure of association. Associations were tested using the χ2 or Fisher exact test (P < .05). Differences in means of continuous variables were tested using the Wilcoxon rank-sum and Kruskal-Wallis test. Multivariable logistic regression analysis was used to determine the odds ratio (used as an approximation of the RR) of the main explanatory variable Bb for preeclampsia adjusted for other covariates. These analyses were also done separately for nulliparous and multiparous women and women who had a blood draw for complement Bb between 10 and 20 weeks’ gestation. General test characteristics (sensitivity, specificity, etc) were also calculated for the main explanatory variable.
Results
The characteristics of the 701 women in the cohort are shown in Table 1. The mean level of Bb was 0.7 μg/mL (range, 0.01 to 1.48), and the 90th percentile cutoff was at 0.92 μg/mL. The normal range for nonpregnant controls in our laboratory is 0 to 0.83 μg/mL. The mean (± SD) gestational age (weeks) at delivery for the cohort was 38 (± 2.4), and the mean birthweight was 3.15 kg (± 0.57). There was no statistical difference in the incidence of an elevated Bb between women who had their blood drawn at UCH or at sites outside UCH (P = .5). Fifteen women (2.2%) in the cohort delivered a baby with IUGR. As shown in Table 2, we found significantly higher levels of Bb among African American women and among women with a higher BMI.
TABLE 2.
Association of select maternal risk factors with an elevated complement activation fragment Bb (n = 701)
| Proportion with a high Bba | ||||||
|---|---|---|---|---|---|---|
| Risk factor | n | % | Total | RR | 95% CI | P |
| Maternal age, y | ||||||
| 35 or older | 27 | 9 | 293 | 0.8 | 0.5, 1.3 | .3 |
| Younger than 35b | 47 | 12 | 408 | |||
| African American | 11 | 22 | 51 | 2.2 | 1.2, 4 | .008 |
| Othersb | 63 | 10 | 650 | |||
| Parity | ||||||
| 0 | 32 | 10 | 313 | 0.9 | 0.6, 1.5 | .8 |
| Greater than 0b | 42 | 10 | 388 | |||
| Prepregnant BMIc | ||||||
| Less than 25 | 43 | 9 | 461 | .04 | ||
| 25–30 | 12 | 9 | 141 | |||
| 30 or greater | 17 | 18 | 93 | |||
| Maternal history | ||||||
| Spontaneous abortion | ||||||
| Yes | 15 | 8 | 185 | 0.7 | 0.4, 1.2 | .2 |
| Nob | 59 | 11 | 516 | |||
| Cigarette smoking | ||||||
| Yes | 8 | 15 | 52 | 1.5 | 0.8, 3 | .2 |
| Nob | 66 | 10 | 649 | |||
| Chronic medical disease | ||||||
| Yes | 6 | 17 | 35 | 1.7 | 0.8, 3.6 | .2 |
| Nob | 68 | 10 | 666 | |||
| Among multiparous women | ||||||
| History hypertension in a previous pregnancy | ||||||
| Yes | 8 | 23 | 35 | 2.4 | 1.2, 4.7 | .02 |
| Nob | 34 | 10 | 352 | |||
| Interval since last delivery, yc | ||||||
| 5 or longer | 16 | 17 | 93 | 2.0 | 1.1, 3.4 | .03 |
| Less than 5b | 26 | 9 | 290 | |||
| Change of paternity | ||||||
| Yes | 10 | 16 | 64 | 1.6 | 0.8, 3 | .2 |
| Nob | 32 | 10 | 324 | |||
RR = incidence of preeclampsia in women with a risk factor divided by the incidence of preeclampsia in women without the risk factor; P value by χ2.
Proportion of women with a high Bb (90th percentile or greater) with and without the risk factor.
Referent groups.
Missing heights on 6 women, missing interval since last delivery on 5 women.
Multiparous women with a history of hypertension in a previous pregnancy were more than 2 times more likely to have an elevated Bb in this pregnancy, compared with multiparous women who did not have hypertension in a previous pregnancy (Table 2). Multiparous women who changed their partners had a higher frequency of Bb in the top decile, and levels of Bb were significantly higher among multiparas who changed their partners, compared with multiparas who did not change their partners (P = .0001). The activation fragment was also found to be significantly higher in multiparous women with a long interval since their last delivery. However, it should be noted that there was a strong statistical association between this variable and a change of paternity (P < .0001).
Thirty-two women from the entire cohort (4.6%) developed preeclampsia. Seventy-five percent of the women had an onset of preeclampsia at less than 37 weeks’ gestation and 50% had severe preeclampsia. Fifty percent delivered preterm (less than 37 weeks’ gestation). Only 2 women with preeclampsia delivered a baby with IUGR. The levels of Bb in early pregnancy among women with and without preeclampsia later in pregnancy are shown in Figure 2. The mean (± SD) Bb level was significantly higher in early pregnancy in women who subsequently developed preeclampsia (0.8 ± 0.3), compared with those who did not develop preeclampsia (0.68 ± 0.2, P = .02). The association between elevated Bb (top decile) and select maternal risk factors for preeclampsia is displayed in Table 3.
FIGURE 2. Side-by-side box plots of levels of activation fragment Bb among women with and without preeclampsia.
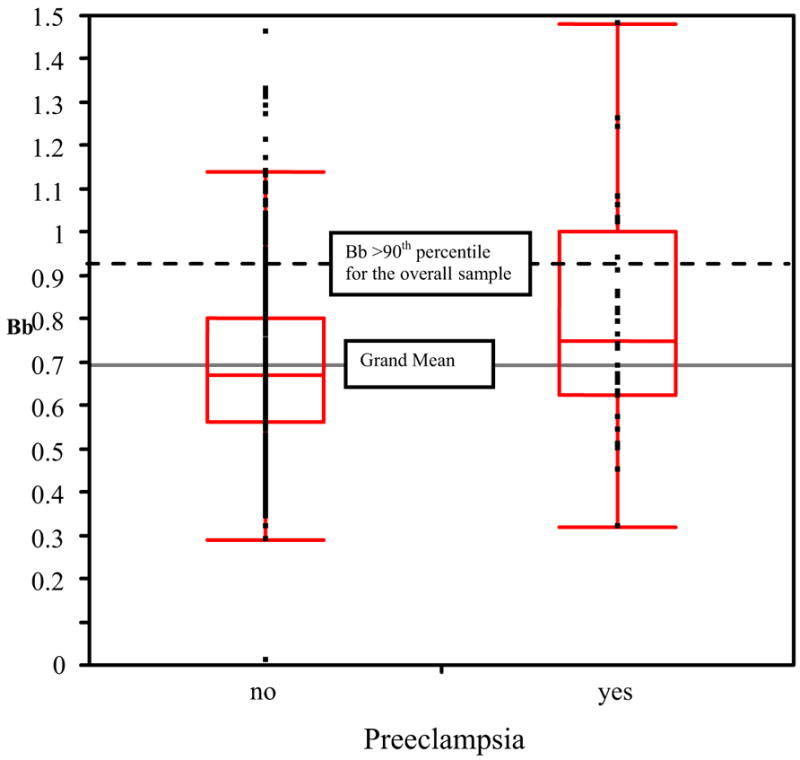
Upper and lower ends of the box are the 75th and 25th percentiles. The difference between the upper and lower lines is the interquartile range. Line across the middle of the box is the median. The upper and lower lines extending from the box are ± 1.5* (interquartile range).
TABLE 3.
Univariate associations of top decile of complement activation fragment Bb and select maternal risk factors with preeclampsia
| Proportion with preeclampsiaa | ||||||
|---|---|---|---|---|---|---|
| Risk factor | n | % | Total | RR | 95% CI | P |
| Bb | ||||||
| 90th percentile or greater | 9 | 12 | 74 | 3.3 | 1.6, 7 | .0009 |
| Less than 90th percentileb | 23 | 3.7 | 627 | |||
| Maternal age, y | ||||||
| 35 or older | 14 | 5 | 293 | 1.1 | 0.5, 2 | .8 |
| Younger than 35b | 18 | 4 | 408 | |||
| African American | 4 | 8 | 51 | 1.8 | 0.7, 5 | .2 |
| Othersb | 28 | 4 | 650 | |||
| Parity | ||||||
| 0 | 20 | 6 | 313 | 2.1 | 1, 4 | .04 |
| Greater than 0b | 12 | 3 | 388 | |||
| Prepregnant BMI, kg/m2‡ | ||||||
| Less than 25 | 15 | 3 | 461 | .006 | ||
| 25–30 | 8 | 6 | 141 | |||
| 30 or greater | 9 | 10 | 93 | |||
| Maternal history | ||||||
| Spontaneous abortion | ||||||
| Yes | 10 | 5 | 185 | 1.3 | 0.6, 2.6 | .5 |
| Nob | 22 | 4 | 515 | |||
| Cigarette smoking | ||||||
| Yes | 3 | 6 | 52 | 1.3 | 0.4, 4 | .7 |
| Nob | 29 | 4 | 649 | |||
| Chronic medical disease | ||||||
| Yes | 6 | 17 | 35 | 4.4 | 2, 10 | .0003 |
| Nob | 26 | 4 | 666 | |||
| Among multiparous women | ||||||
| History hypertension in a previous pregnancy | ||||||
| Yes | 4 | 11 | 35 | 5.0 | 1.6, 16 | .003 |
| Nob | 8 | 2 | 353 | |||
| Interval since last delivery, y | ||||||
| 5 or longer | 5 | 5 | 93 | 2.2 | 0.7, 6.8 | .2 |
| Less than 5b | 7 | 2 | 290 | |||
| Change of paternity | ||||||
| Yes | 6 | 9 | 64 | 5.1 | 1.6, 15 | .002 |
| Nob | 6 | 2 | 324 | |||
Missing heights on 6 women and interval since last delivery on 5 women. RR was unadjusted; P value by χ2.
Proportion of women with preeclampsia with and without the risk factor.
Referent group.
In this univariate analysis, women with levels of Bb over the 90th percentile in a specimen drawn less than 20 weeks’ gestation were 3.3 times more likely to develop preeclampsia, compared with women with lower (less than the 90th percentile) levels of Bb. When the cutoff was placed at the 75th and 95th percentile, the RR of an elevated Bb for preeclampsia was 2.2 (95% confidence interval [CI], 1.2 to 4.4, P = .01) and 4.8 (95% CI, 2.2 to 10, P < .0001 respectively).
Other risk factors significantly associated with preeclampsia were nulliparity, higher BMI, and a history of a maternal medical disease. We saw a similar significant trend in the relationship between Bb and preeclampsia among nulliparous and multiparous women. Analysis of the relationship between risk factors specific to multiparous women showed a significant relationship between a history of hypertension in a previous pregnancy and a change of paternity with preeclampsia.
In the full multivariable logistic regression model (Table 4), women with elevated levels of Bb (top decile) were 3.8 times more likely to develop preeclampsia, compared with women with lower levels of this activation fragment (P = .002). Nulliparity, obesity, and a maternal history of medical disease were other covariates significantly associated with preeclampsia. We also examined Bb as a continuous variable in this same model and found the adjusted odds ratio (OR) to be 15.0 (95% CI, 2.5 to 90, P = .003). In additional exploratory analysis, the adjusted OR of the top decile of Bb for preeclampsia among nulliparous women (incidence of preeclampsia = 6%) was 4.8 (1.4 to 15, P =.01). Among multiparous women, the adjusted OR for preeclampsia (incidence = 3%) was elevated, but the association did not reach statistical significance (OR 2.3, 95% CI, 0.5 to 11, P = .3). Among the 531 study participants who had a blood draw for Bb between 10 and 20 weeks’ gestation, the adjusted OR of a Bb in the top decile for preeclampsia was 6.1 (P = .0005).
TABLE 4.
Full multivariable logistic regression model showing the unadjusted and adjusted OR of complement activation fragment Bb and other select risk factors for preeclampsia
| Unadjusted OR | Adjusted OR | 95% CIa | P value | |
|---|---|---|---|---|
| Complement activation fragment Bb | 3.6 | 3.8 | 1.6, 9 | .002 |
| Maternal age 35 y or older | 0.9 | 1.1 | 0.5, 2.4 | .9 |
| African American | 1.9 | 1.1 | 0.3, 3.8 | .9 |
| Nulliparity | 2.1 | 3.0 | 1.4, 7 | .007 |
| Maternal prepregnant BMI, kg/m2 | ||||
| 25–30 | 1.8 | 2.1 | 0.8, 5 | .1 |
| 30 or greater | 3.2 | 2.7 | 1, 7 | .04 |
| History of spontaneous abortion | 1.3 | 1.4 | 0.6, 3.3 | .4 |
| Cigarette smoking | 1.3 | 0.9 | 0.3, 3.5 | .9 |
| Chronic medical disease | 5.1 | 3.6 | 1.2, 10 | .02 |
For the adjusted OR.
We had inadequate sample power to fully explore this association in the 170 women who had a sample drawn less than 10 weeks’ gestation. Among this group of women, adjusted for other risk factors, the OR of the top decile of Bb for preeclampsia was 0.9 (95% CI, 0.08 to 10, P = .9). The following are the results of the test characteristics of Bb for the outcome preeclampsia with an incidence of 4.6%: sensitivity, 28%; specificity, 90%; positive predictive value, 12%; negative predictive value, 96%; the likelihood ratios for a positive and negative test were 2.8 and 0.8, respectively.
Comment
In our cohort, elevated levels (top decile) of the complement activation fragment Bb in the first 20 weeks of pregnancy were independently associated with preeclampsia later in pregnancy. These results suggest that Bb may be an early biomarker of the pathogenic events in early pregnancy leading to preeclampsia This research question was previously examined by Haeger et al,22 who found that women with severe preeclampsia had increased levels of C5a and the terminal complement complex (drawn at delivery) as compared with uneventful pregnancies. However, ours is the first large prospective study to examine the relationship between Bb levels in the early part of pregnancy with preeclampsia in humans and to find a relationship between elevated levels of Bb (less than 20 weeks’ gestation) and the development of the syndrome later in pregnancy.
A maternal immune-mediated inflammatory response to paternally derived fetal antigens found on the surface of trophoblasts offers a possible explanation for some of the elevated levels of Bb observed in this study and supports the immune maladaptation hypothesis of preeclampsia.1,23 This hypothesis suggests that decidual immune-mediated inflammation contributes to the pathogenesis of preeclampsia.
This hypothesis explains why preeclampsia is seen more frequently in situations in which the woman has limited sperm exposure with the same partner before conception and thus inadequate time to develop immune tolerance to paternally derived antigens. In addition to nulliparity, these situations would include women who are inseminated with a donor sperm, have assisted reproductive technologies with insemination from an intracytoplasmic sperm injection from a surgically obtained sperm, and who use barrier methods of contraception. Moreover, multiparous women lose the protective effect of a previous birth when they change paternity (1,23 for review). Indeed, in our study, multiparous women who changed their partners had higher levels of Bb and were more than 5 times more likely to develop preeclampsia, compared with women who did not have a change in paternity since their last delivery (Tables 2 and 3). Interestingly, in our study a history of hypertension in a previous pregnancy was associated with both an elevation of Bb and preeclampsia (Tables 2 and 3), which may be a marker of a failure of other factors contributing to the maternal immune tolerance necessary to protect the pregnancy from development of preeclampsia.7,23
Another interesting finding of this study was that, when we restricted our analysis to women who had a specimen collected during the time of uteroplacental vascular remodeling (10 to 20 weeks’ gestation), the adjusted OR of an elevated Bb for preeclampsia was consideribly higher than in the full cohort (OR 6.1, P = .0005). This finding suggests that release of Bb into the maternal circulation may be a marker of inflammatory events specific to the placenta.
Normal pregnancy is associated with elevation of some markers of inflammation such as complement.24 However, as shown in murine studies, excessive complement activation at the maternal-fetal interface can present a significant threat to the developing fetus and placenta as a result of local infiltration of inflammatory cells with tissue damage and necrosis.13,25 Furthermore, subcellular debris released from necrotic cells can trigger additional complement activation leading to more tissue destruction.26,27 Indeed, debris from an oxidatively stressed placenta has been shown by other authors to contribute to endothelial cell activation28 and could be a trigger for complement activation at sites outside the placenta.
To protect against the harmful effects of unregulated complement activation on host cells, the placenta is armed with an abundance of complement inhibitory proteins, found on the syncytiotrophoblast.18,29 In this regard, it is also noteworthy that women with a predisposition to inflammation such as those with obesity had significantly higher levels of Bb and were also at a greater risk of developing preeclampsia. This finding suggests that previously inflamed tissues may be a trigger for complement activation or that the inhibitory factors may, in some instances, be insufficient to maintain adequate control of complement activation.
With the addition to this cohort of additional multiparous women as well as women who present in very early pregnancy, we plan to achieve adequate power to fully explore the possible intereaction between Bb with parity and gestational age. Additional numbers will also allow us to explore the relationship between Bb and IUGR (with and without preeclampsia) and pretem onset of preeclampsia. Because the focus of this study was the contribution of the alternative pathway of complement activation to preeclampsia, only the activation fragment Bb was examined. However, future analyses will incorporate measures of other complement activation products alone and in combination and at points in pregnancy beyond 20 weeks’ gestation. By combining measures of complement activation fragmens, we may be able to improve the test characterisitcs for the marker.
We hypothesize that preeclampsia is secondary to the sequence of events in early pregnancy proposed in Figure 3 and that complement activation is an early marker of these events. Our results are in agreement with recent murine studies suggesting that complement activation induces dysregulation of angiogenic factors,17 which are themselves important to the pathogenesis of preeclampsia.5 It will be important to establish the interrelationships in pregnant subjects between complement activation and angiogenic factors. New approaches for inhibiting complement activation that may be able to prevent or modify the inflammatory-related sequelae associated with these events are under development.30,31 Encouraging support for this possibility comes from the fact that complement inhibitors reversed the process of loss and IUGR in the animal model of the antiphospholipid syndrome as well as spontaneous fetal loss associated with complement-dependent increases in angiogenic factors.14,17
FIGURE 3.
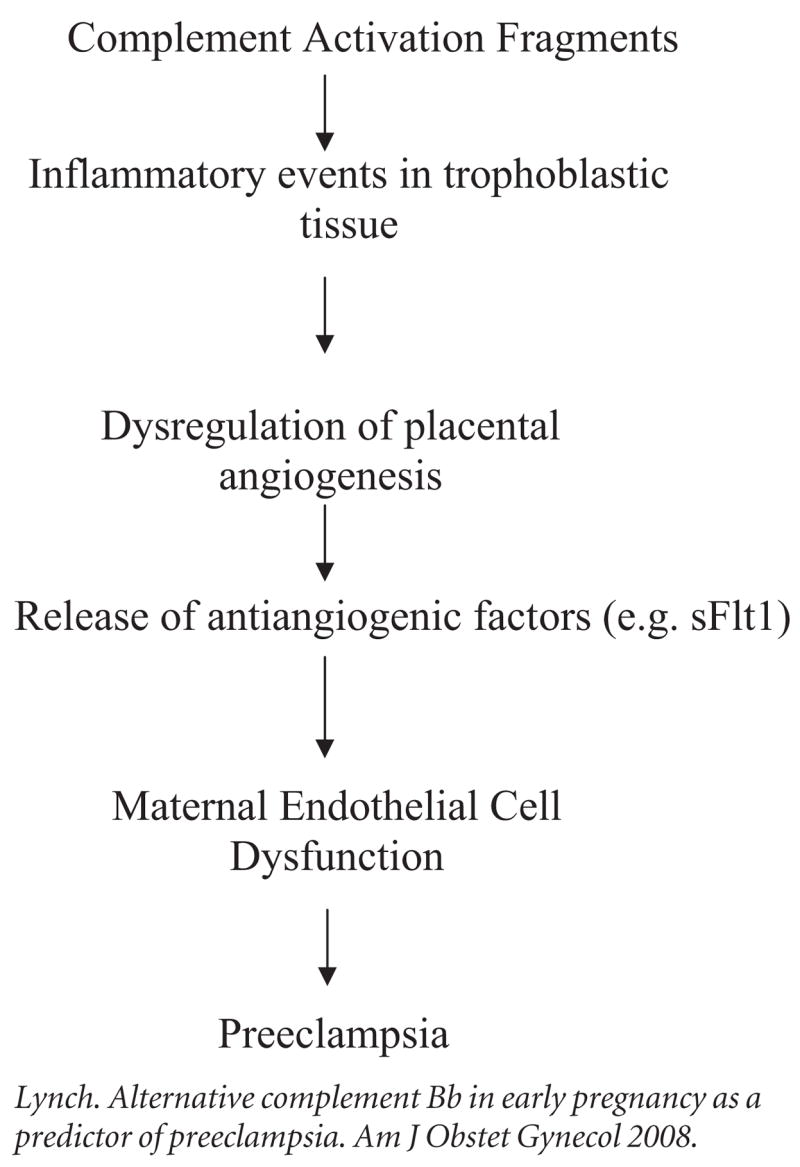
Hypothetical model of the contribution of the complement activation to preeclampsia
Acknowledgments
This study was supported by Grant K23 HD049684 from the National Institute of Child Health and Human Development and Newborn Hope Colorado (to A.M.L.) and National Institutes of Health Grant R01 AI 55007 (to V.M.H.).
We acknowledge Trisha VanHecke, BS, for her careful coordination of this study; Cynthia Marschner, MT(ASCP), for performing the Bb assay; Henry Galan, MD, and William Goddard, MD, for facilitating use of the perinatal database; Joseph Piccoli, BS, and Ted Wade, PhD, for data coordination at NJMRC; Virginia Winn, MD, PhD, for her help in reviewing the medical records; and the administrative, medical, and nursing staff at the UCH, Platte River, and MCPN clinics for their support of the complement study.
Footnotes
Presented at the 20th annual meeting of the Society for Pediatric and Perinatal Epidemiologic Research, Boston, MA, June 18-19, 2007.
References
- 1.Dekker G, Robillard PY. Preeclampsia: is the immune maladaptation hypothesis still standing? An epidemiological update. J Reprod Immunol. 2007;76:8–16. doi: 10.1016/j.jri.2007.03.015. [DOI] [PubMed] [Google Scholar]
- 2.Zhang J, Meikle S, Trumble A. Severe maternal morbidity associated with hypertensive disorders in pregnancy in the United States. Hypertens Pregnancy. 2003;22:203–12. doi: 10.1081/PRG-120021066. [DOI] [PubMed] [Google Scholar]
- 3.Roberts JM. Endothelial dysfunction in preeclampsia. Semin Reprod Endocrinol. 1998;16:5–15. doi: 10.1055/s-2007-1016248. [DOI] [PubMed] [Google Scholar]
- 4.Zhou Y, McMaster M, Woo K, et al. Vascular endothelial growth factor ligands and receptors that regulate human cytotrophoblast survival are dysregulated in severe preeclampsia and hemolysis, elevated liver enzymes, and low platelets syndrome. Am J Pathol. 2002;160:1405–23. doi: 10.1016/S0002-9440(10)62567-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Levine RJ, Maynard SE, Qian C, et al. Circulating angiogenic factors and the risk of preeclampsia. N Engl J Med. 2004;350:672–83. doi: 10.1056/NEJMoa031884. [DOI] [PubMed] [Google Scholar]
- 6.Levine RJ, Qian C, Maynard SE, Yu KF, Epstein FH, Karumanchi SA. Serum sFlt1 concentration during preeclampsia and mid trimester blood pressure in healthy nulliparous women. Am J Obstet Gynecol. 2006;194:1034–41. doi: 10.1016/j.ajog.2005.10.192. [DOI] [PubMed] [Google Scholar]
- 7.Moffett A, Hiby SE. How does the maternal immune system contribute to the development of preeclampsia? Placenta. 2007;28(Suppl A):S51–6. doi: 10.1016/j.placenta.2006.11.008. [DOI] [PubMed] [Google Scholar]
- 8.Holers VM. Complement. In: RR R, editor. Principles and practive of clinical immunology. St Louis, MO: Mosby; 2001. [Google Scholar]
- 9.Bulla R, Bossi F, Fischetti F, De Seta F, Tedesco F. The complement system at the fetomaternal interface. Chem Immunol Allergy. 2005;89:149–57. doi: 10.1159/000087963. [DOI] [PubMed] [Google Scholar]
- 10.Walport MJ. Complement. First of two parts. N Engl J Med. 2001;344:1058–66. doi: 10.1056/NEJM200104053441406. [DOI] [PubMed] [Google Scholar]
- 11.Thurman JM, Holers VM. The central role of the alternative complement pathway in human disease. J Immunol. 2006;176:1305–10. doi: 10.4049/jimmunol.176.3.1305. [DOI] [PubMed] [Google Scholar]
- 12.Janeway C. Immunobiology: the immune system in health and disease. 6. New York: Garland Science; 2005. [Google Scholar]
- 13.Holers VM, Girardi G, Mo L, et al. Complement C3 activation is required for antiphospholipid antibody-induced fetal loss. J Exp Med. 2002;195:211–20. doi: 10.1084/jem.200116116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Salmon JE, Girardi G, Holers VM. Complement activation as a mediator of antiphospholipid antibody induced pregnancy loss and thrombosis. Ann Rheum Dis. 2002;61(ii):46–50. doi: 10.1136/ard.61.suppl_2.ii46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Girardi G, Berman J, Redecha P, et al. Complement C5a receptors and neutrophils mediate fetal injury in the antiphospholipid syndrome. J Clin Invest. 2003;112:1644–54. doi: 10.1172/JCI18817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thurman JM, Kraus DM, Girardi G, et al. A novel inhibitor of the alternative complement pathway prevents antiphospholipid antibody-induced pregnancy loss in mice. Mol Immunol. 2005;42:87–97. doi: 10.1016/j.molimm.2004.07.043. [DOI] [PubMed] [Google Scholar]
- 17.Girardi G, Yarilin D, Thurman JM, Holers VM, Salmon JE. Complement activation induces dysregulation of angiogenic factors and causes fetal rejection and growth restriction. J Exp Med. 2006;203:2165–75. doi: 10.1084/jem.20061022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mao D, Wu X, Deppong C, et al. Negligible role of antibodies and C5 in pregnancy loss associated exclusively with C3-dependent mechanisms through complement alternative pathway. Immunity. 2003;19:813–22. doi: 10.1016/s1074-7613(03)00321-2. [DOI] [PubMed] [Google Scholar]
- 19.Sibai B, Dekker G, Kupferminc M. Preeclampsia. Lancet. 2005;365:785–99. doi: 10.1016/S0140-6736(05)17987-2. [DOI] [PubMed] [Google Scholar]
- 20.Lubchenco LO, Hansman C, Boyd E. Intrauterine growth in length and head circumference as estimated from live births at gestational ages from 26 to 42 weeks. Pediatrics. 1966;37:403–8. [PubMed] [Google Scholar]
- 21.Villar J, Carroli G, Wojdyla D, et al. Preeclampsia, gestational hypertension and intrauterine growth restriction, related or independent conditions? Am J Obstet Gynecol. 2006;194:921–31. doi: 10.1016/j.ajog.2005.10.813. [DOI] [PubMed] [Google Scholar]
- 22.Haeger M, Unander M, Norder-Hansson B, Tylman M, Bengtsson A. Complement, neutrophil, and macrophage activation in women with severe preeclampsia and the syndrome of hemolysis, elevated liver enzymes, and low platelet count. Obstet Gynecol. 1992;79:19–26. [PubMed] [Google Scholar]
- 23.Dekker G. The partner’s role in the etiology of preeclampsia. J Reprod Immunol. 2002;57:203–15. doi: 10.1016/s0165-0378(02)00039-6. [DOI] [PubMed] [Google Scholar]
- 24.Richani K, Soto E, Romero R, et al. Normal pregnancy is characterized by systemic activation of the complement system. J Matern Fetal Neonatal Med. 2005;17:239–45. doi: 10.1080/14767050500072722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xu C, Mao D, Holers VM, Palanca B, Cheng AM, Molina H. A critical role for murine complement regulator crry in fetomaternal tolerance. Science. 2000;287:498–501. doi: 10.1126/science.287.5452.498. [DOI] [PubMed] [Google Scholar]
- 26.Giclas PC, Pinckard RN, Olson MS. In vitro activation of complement by isolated human heart subcellular membranes. J Immunol. 1979;122:146–51. [PubMed] [Google Scholar]
- 27.Walport MJ. Complement. Second of two parts. N Engl J Med. 2001;344:1140–4. doi: 10.1056/NEJM200104123441506. [DOI] [PubMed] [Google Scholar]
- 28.Germain SJ, Sacks GP, Soorana SR, Sargent IL, Redman CW. Systemic inflammatory priming in normal pregnancy and preeclampsia: the role of circulating syncytiotrophoblast microparticles. J Immunol. 2007;178:5949–56. doi: 10.4049/jimmunol.178.9.5949. [DOI] [PubMed] [Google Scholar]
- 29.Holmes CH, Simpson KL. Complement and pregnancy: new insights into the immunobiology of the fetomaternal relationship. Baillieres Clin Obstet Gynaecol. 1992;6:439–60. doi: 10.1016/s0950-3552(05)80005-7. [DOI] [PubMed] [Google Scholar]
- 30.Holers VM. The complement system as a therapeutic target in autoimmunity. Clin Immunol. 2003;107:140–51. doi: 10.1016/s1521-6616(03)00034-2. [DOI] [PubMed] [Google Scholar]
- 31.Girardi G, Redecha P, Salmon JE. Heparin prevents antiphospholipid antibody-induced fetal loss by inhibiting complement activation. Nat Med. 2004;10:1222–6. doi: 10.1038/nm1121. [DOI] [PubMed] [Google Scholar]


