Abstract
In a recent report, the neurovirulence of herpes simplex virus type 1 (HSV-1) was mapped to the ICP34.5 gene (J. Chou, E. R. Kern, R. J. Whitley, and B. Roizman, Science 250:1262-1266, 1990). In this report, specific mutations within ICP34.5 were constructed in HSV-1 strain 17syn+ to determine the effects of these mutations in a fully neurovirulent isolate. It was found that termination of the ICP34.5 gene after the N-terminal 30 amino acids resulted in a mutant, 17termA, which was 25- to 90-fold reduced in neurovirulence. This reduction of neurovirulence was associated with restricted replication of the mutant virus in mouse brain. The reduced replication phenotype was also evident in the trigeminal and dorsal root ganglia following inoculation at the periphery. 17termA was capable of replicating with wild-type kinetics in mouse footpads, and therefore the restriction seen in neural tissues was not due to a generalized replication defect in mouse cells. Significantly, replication of the mutant was also restricted in the mouse cornea in vivo and in confluent primary mouse embryo cells and mouse 10T1/2 cells in vitro. However, 17termA replicated with much greater efficiency in subconfluent mouse embryo cells, suggesting that the physiological state of the cell may be an important factor for productive replication of this mutant. Restoration of the ICP34.5 gene to the mutant resulted in a virus which displayed wild-type neurovirulence and replication kinetics in all cells and tissues tested.
Full text
PDF
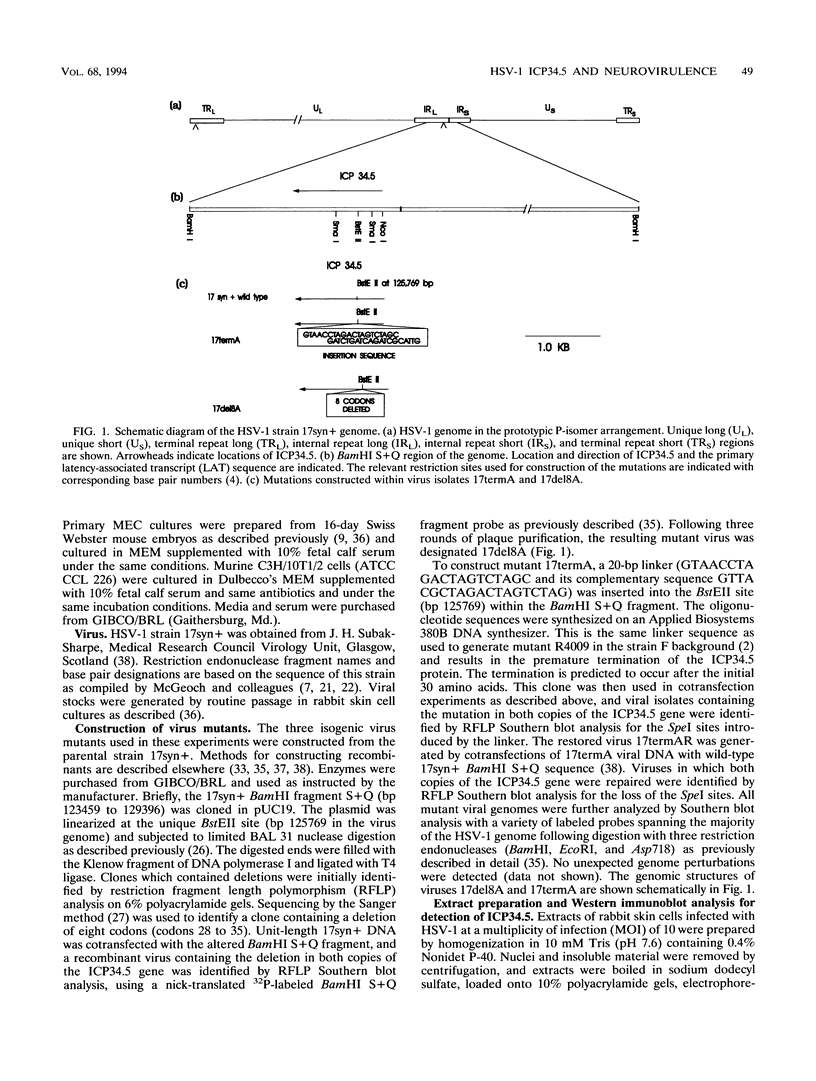

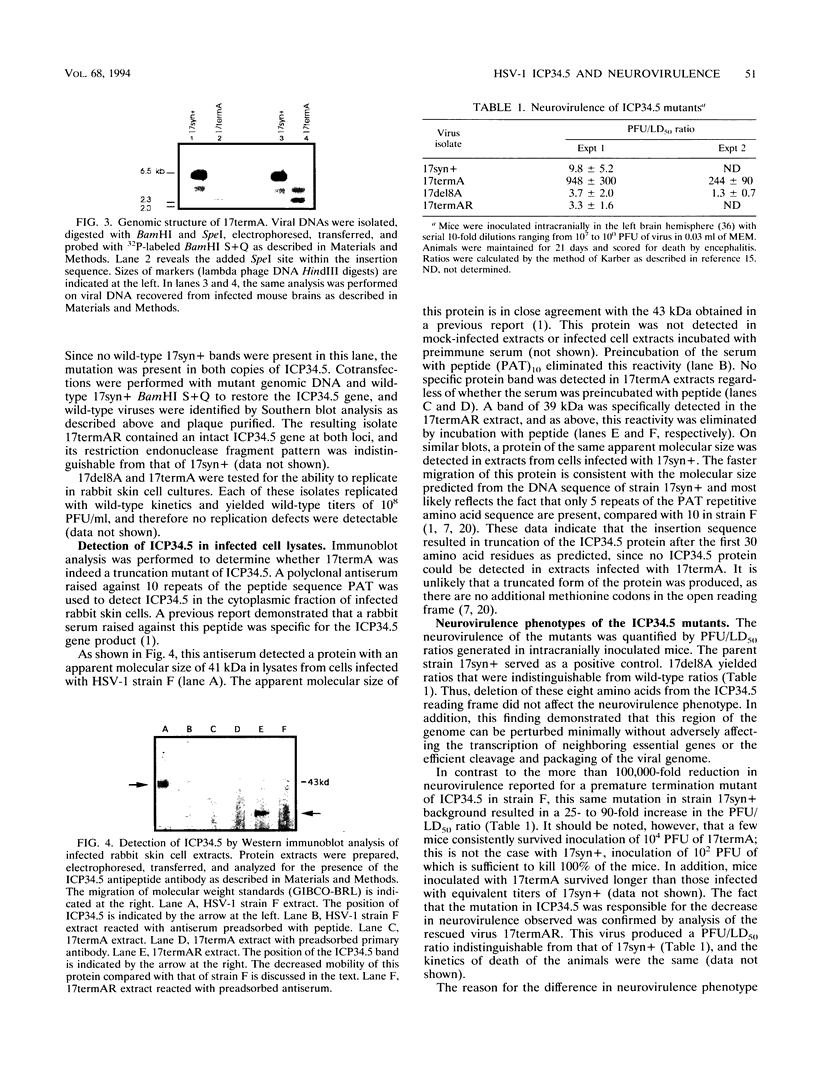
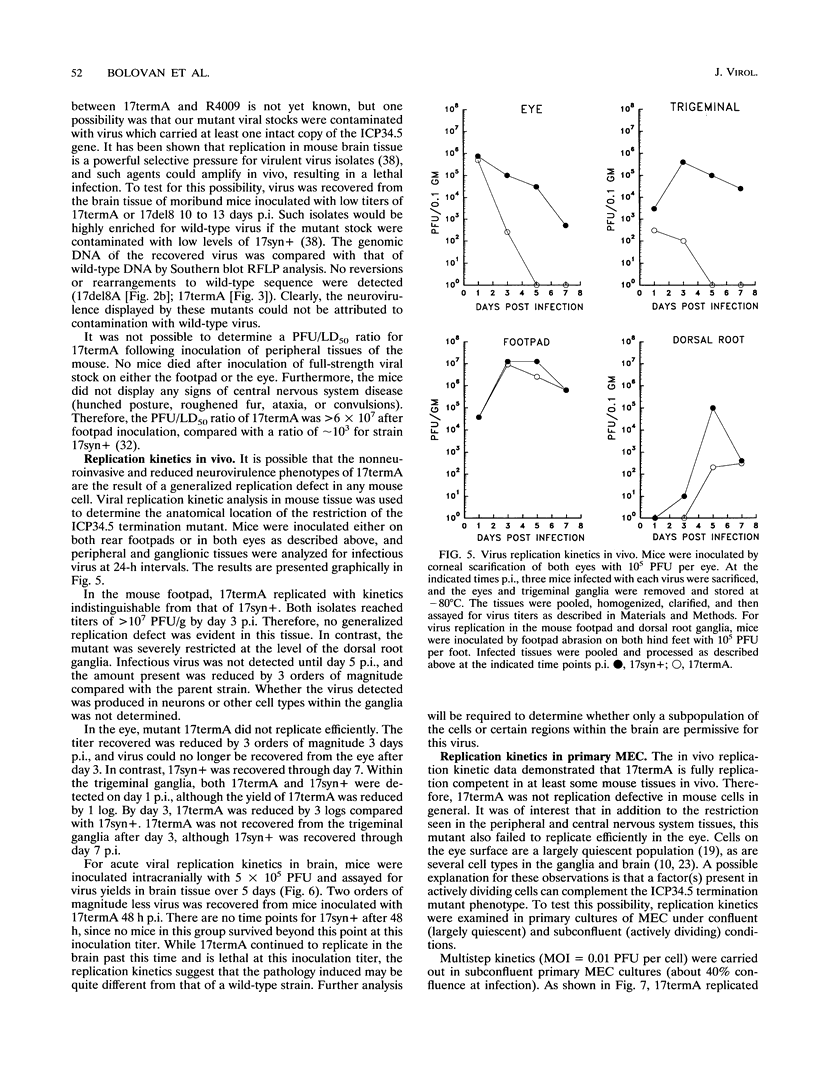


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ackermann M., Chou J., Sarmiento M., Lerner R. A., Roizman B. Identification by antibody to a synthetic peptide of a protein specified by a diploid gene located in the terminal repeats of the L component of herpes simplex virus genome. J Virol. 1986 Jun;58(3):843–850. doi: 10.1128/jvi.58.3.843-850.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou J., Kern E. R., Whitley R. J., Roizman B. Mapping of herpes simplex virus-1 neurovirulence to gamma 134.5, a gene nonessential for growth in culture. Science. 1990 Nov 30;250(4985):1262–1266. doi: 10.1126/science.2173860. [DOI] [PubMed] [Google Scholar]
- Chou J., Roizman B. Isomerization of herpes simplex virus 1 genome: identification of the cis-acting and recombination sites within the domain of the a sequence. Cell. 1985 Jul;41(3):803–811. doi: 10.1016/s0092-8674(85)80061-1. [DOI] [PubMed] [Google Scholar]
- Chou J., Roizman B. The gamma 1(34.5) gene of herpes simplex virus 1 precludes neuroblastoma cells from triggering total shutoff of protein synthesis characteristic of programed cell death in neuronal cells. Proc Natl Acad Sci U S A. 1992 Apr 15;89(8):3266–3270. doi: 10.1073/pnas.89.8.3266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deiss L. P., Chou J., Frenkel N. Functional domains within the a sequence involved in the cleavage-packaging of herpes simplex virus DNA. J Virol. 1986 Sep;59(3):605–618. doi: 10.1128/jvi.59.3.605-618.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dix R. D., McKendall R. R., Baringer J. R. Comparative neurovirulence of herpes simplex virus type 1 strains after peripheral or intracerebral inoculation of BALB/c mice. Infect Immun. 1983 Apr;40(1):103–112. doi: 10.1128/iai.40.1.103-112.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolan A., McKie E., MacLean A. R., McGeoch D. J. Status of the ICP34.5 gene in herpes simplex virus type 1 strain 17. J Gen Virol. 1992 Apr;73(Pt 4):971–973. doi: 10.1099/0022-1317-73-4-971. [DOI] [PubMed] [Google Scholar]
- Leib D. A., Coen D. M., Bogard C. L., Hicks K. A., Yager D. R., Knipe D. M., Tyler K. L., Schaffer P. A. Immediate-early regulatory gene mutants define different stages in the establishment and reactivation of herpes simplex virus latency. J Virol. 1989 Feb;63(2):759–768. doi: 10.1128/jvi.63.2.759-768.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord K. A., Hoffman-Liebermann B., Liebermann D. A. Complexity of the immediate early response of myeloid cells to terminal differentiation and growth arrest includes ICAM-1, Jun-B and histone variants. Oncogene. 1990 Mar;5(3):387–396. [PubMed] [Google Scholar]
- Lord K. A., Hoffman-Liebermann B., Liebermann D. A. Sequence of MyD116 cDNA: a novel myeloid differentiation primary response gene induced by IL6. Nucleic Acids Res. 1990 May 11;18(9):2823–2823. doi: 10.1093/nar/18.9.2823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynn D. E. A BASIC computer program for analyzing endpoint assays. Biotechniques. 1992 Jun;12(6):880–881. [PubMed] [Google Scholar]
- MacLean A. R., ul-Fareed M., Robertson L., Harland J., Brown S. M. Herpes simplex virus type 1 deletion variants 1714 and 1716 pinpoint neurovirulence-related sequences in Glasgow strain 17+ between immediate early gene 1 and the 'a' sequence. J Gen Virol. 1991 Mar;72(Pt 3):631–639. doi: 10.1099/0022-1317-72-3-631. [DOI] [PubMed] [Google Scholar]
- Mackem S., Roizman B. Differentiation between alpha promoter and regulator regions of herpes simplex virus 1: the functional domains and sequence of a movable alpha regulator. Proc Natl Acad Sci U S A. 1982 Aug;79(16):4917–4921. doi: 10.1073/pnas.79.16.4917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackem S., Roizman B. Structural features of the herpes simplex virus alpha gene 4, 0, and 27 promoter-regulatory sequences which confer alpha regulation on chimeric thymidine kinase genes. J Virol. 1982 Dec;44(3):939–949. doi: 10.1128/jvi.44.3.939-949.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGeoch D. J., Barnett B. C. Neurovirulence factor. Nature. 1991 Oct 17;353(6345):609–609. doi: 10.1038/353609b0. [DOI] [PubMed] [Google Scholar]
- McGeoch D. J., Cunningham C., McIntyre G., Dolan A. Comparative sequence analysis of the long repeat regions and adjoining parts of the long unique regions in the genomes of herpes simplex viruses types 1 and 2. J Gen Virol. 1991 Dec;72(Pt 12):3057–3075. doi: 10.1099/0022-1317-72-12-3057. [DOI] [PubMed] [Google Scholar]
- McGeoch D. J., Dalrymple M. A., Davison A. J., Dolan A., Frame M. C., McNab D., Perry L. J., Scott J. E., Taylor P. The complete DNA sequence of the long unique region in the genome of herpes simplex virus type 1. J Gen Virol. 1988 Jul;69(Pt 7):1531–1574. doi: 10.1099/0022-1317-69-7-1531. [DOI] [PubMed] [Google Scholar]
- Pardee A. B. G1 events and regulation of cell proliferation. Science. 1989 Nov 3;246(4930):603–608. doi: 10.1126/science.2683075. [DOI] [PubMed] [Google Scholar]
- Reznikoff C. A., Brankow D. W., Heidelberger C. Establishment and characterization of a cloned line of C3H mouse embryo cells sensitive to postconfluence inhibition of division. Cancer Res. 1973 Dec;33(12):3231–3238. [PubMed] [Google Scholar]
- Sacks W. R., Schaffer P. A. Deletion mutants in the gene encoding the herpes simplex virus type 1 immediate-early protein ICP0 exhibit impaired growth in cell culture. J Virol. 1987 Mar;61(3):829–839. doi: 10.1128/jvi.61.3.829-839.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawtell N. M., Thompson R. L. Rapid in vivo reactivation of herpes simplex virus in latently infected murine ganglionic neurons after transient hyperthermia. J Virol. 1992 Apr;66(4):2150–2156. doi: 10.1128/jvi.66.4.2150-2156.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stow N. D., Stow E. C. Isolation and characterization of a herpes simplex virus type 1 mutant containing a deletion within the gene encoding the immediate early polypeptide Vmw110. J Gen Virol. 1986 Dec;67(Pt 12):2571–2585. doi: 10.1099/0022-1317-67-12-2571. [DOI] [PubMed] [Google Scholar]
- Sussman M. D., Lu Z., Kutish G., Afonso C. L., Roberts P., Rock D. L. Identification of an African swine fever virus gene with similarity to a myeloid differentiation primary response gene and a neurovirulence-associated gene of herpes simplex virus. J Virol. 1992 Sep;66(9):5586–5589. doi: 10.1128/jvi.66.9.5586-5589.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taha M. Y., Brown S. M., Clements G. B., Graham D. I. The JH2604 deletion variant of herpes simplex virus type 2 (HG52) fails to produce necrotizing encephalitis following intracranial inoculation of mice. J Gen Virol. 1990 Jul;71(Pt 7):1597–1601. doi: 10.1099/0022-1317-71-7-1597. [DOI] [PubMed] [Google Scholar]
- Thompson R. L., Cook M. L., Devi-Rao G. B., Wagner E. K., Stevens J. G. Functional and molecular analyses of the avirulent wild-type herpes simplex virus type 1 strain KOS. J Virol. 1986 Apr;58(1):203–211. doi: 10.1128/jvi.58.1.203-211.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson R. L., Devi-Rao G. V., Stevens J. G., Wagner E. K. Rescue of a herpes simplex virus type 1 neurovirulence function with a cloned DNA fragment. J Virol. 1985 Aug;55(2):504–508. doi: 10.1128/jvi.55.2.504-508.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson R. L., Nakashizuka M., Stevens J. G. Vaccine potential of a live avirulent herpes simplex virus. Microb Pathog. 1986 Aug;1(4):409–416. doi: 10.1016/0882-4010(86)90072-0. [DOI] [PubMed] [Google Scholar]
- Thompson R. L., Rogers S. K., Zerhusen M. A. Herpes simplex virus neurovirulence and productive infection of neural cells is associated with a function which maps between 0.82 and 0.832 map units on the HSV genome. Virology. 1989 Oct;172(2):435–450. doi: 10.1016/0042-6822(89)90186-4. [DOI] [PubMed] [Google Scholar]
- Thompson R. L., Stevens J. G. Biological characterization of a herpes simplex virus intertypic recombinant which is completely and specifically non-neurovirulent. Virology. 1983 Nov;131(1):171–179. doi: 10.1016/0042-6822(83)90543-3. [DOI] [PubMed] [Google Scholar]
- Thompson R. L., Wagner E. K. Partial rescue of herpes simplex virus neurovirulence with a 3.2 kb cloned DNA fragment. Virus Genes. 1988 Jun;1(3):261–273. doi: 10.1007/BF00572705. [DOI] [PubMed] [Google Scholar]
- Thompson R. L., Wagner E. K., Stevens J. G. Physical location of a herpes simplex virus type-1 gene function(s) specifically associated with a 10 million-fold increase in HSV neurovirulence. Virology. 1983 Nov;131(1):180–192. doi: 10.1016/0042-6822(83)90544-5. [DOI] [PubMed] [Google Scholar]
- Varmuza S. L., Smiley J. R. Signals for site-specific cleavage of HSV DNA: maturation involves two separate cleavage events at sites distal to the recognition sequences. Cell. 1985 Jul;41(3):793–802. doi: 10.1016/s0092-8674(85)80060-x. [DOI] [PubMed] [Google Scholar]