Abstract
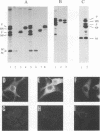
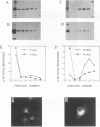
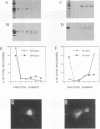
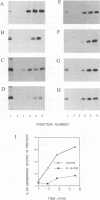
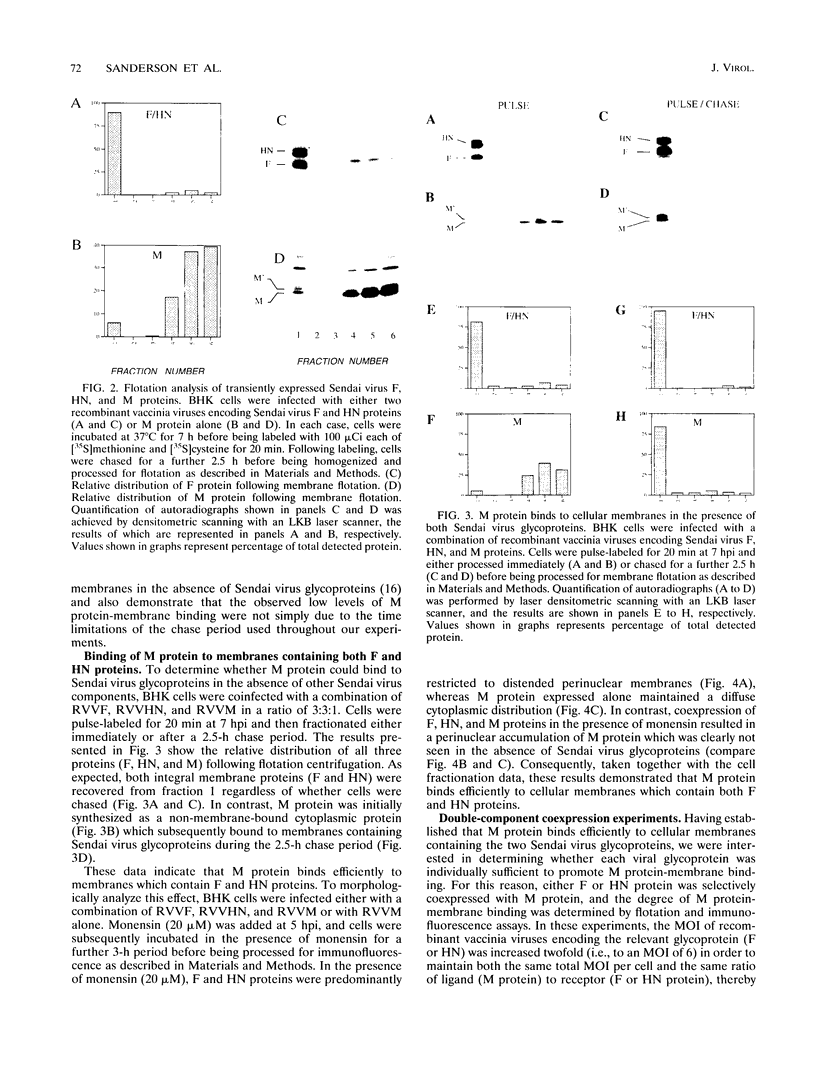
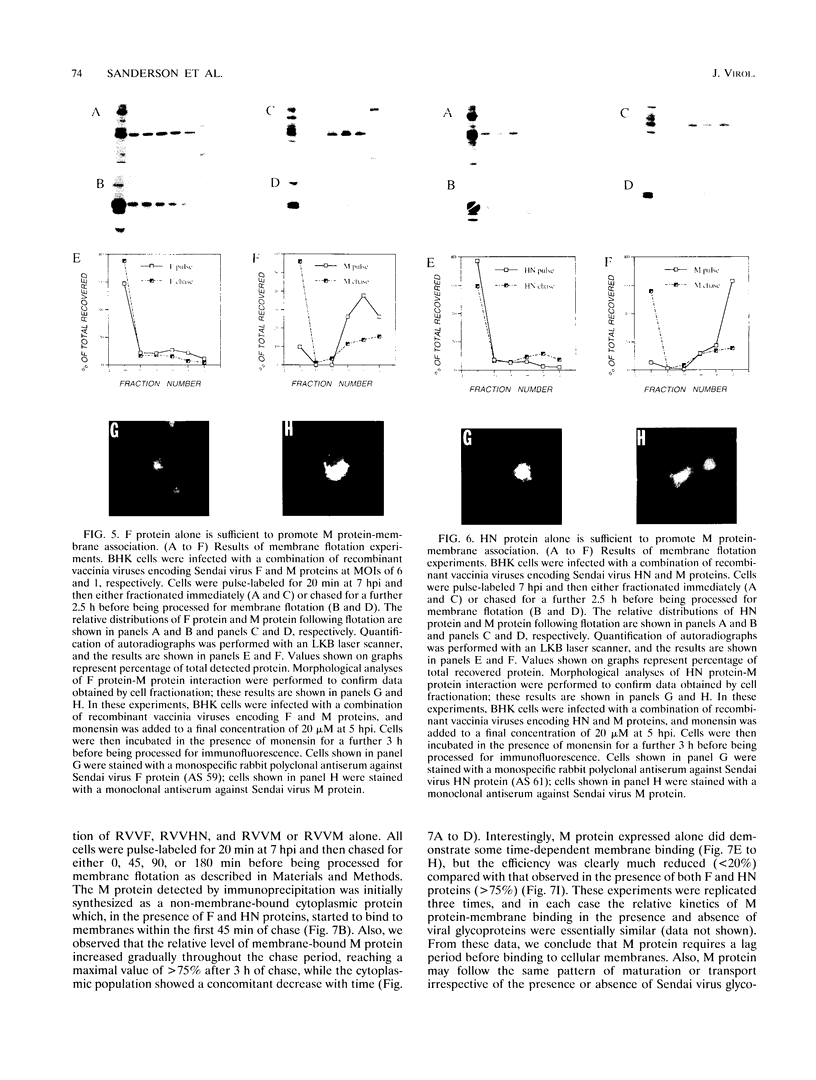
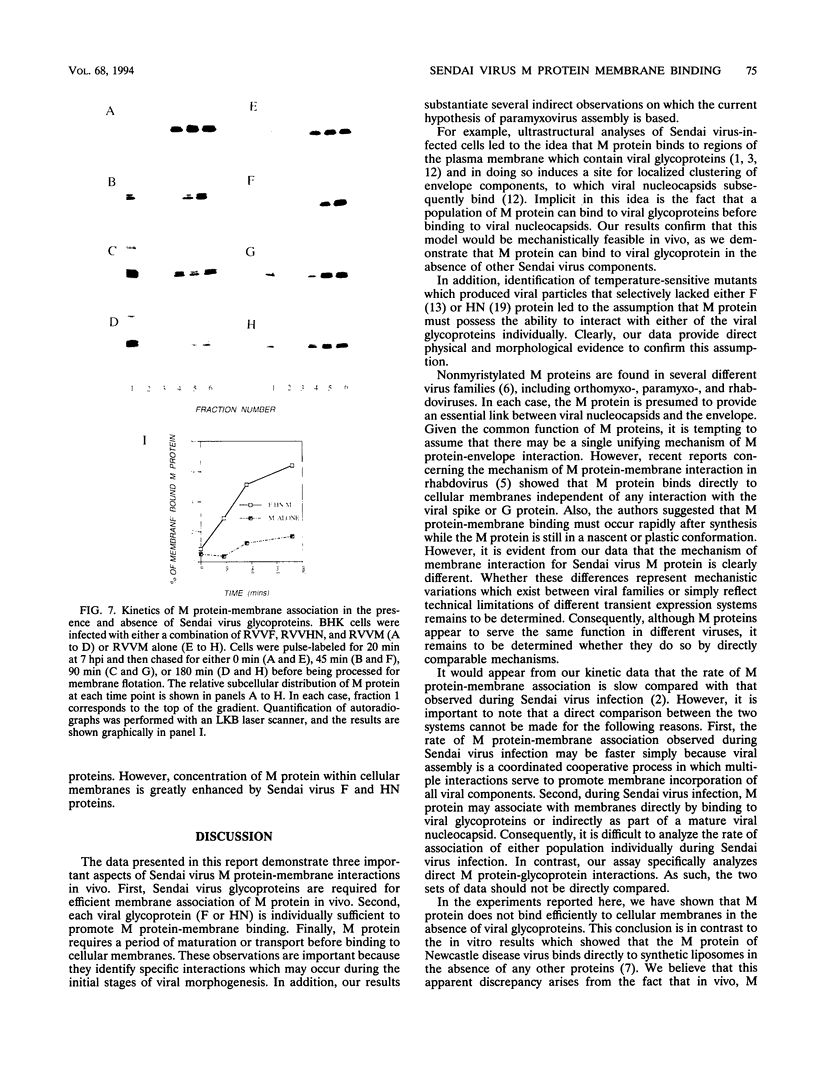
We have analyzed the mechanism by which M protein interacts with components of the viral envelope during Sendai virus assembly. Using recombinant vaccinia viruses to selectively express combinations of Sendai virus F, HN, and M proteins, we have successfully reconstituted M protein-glycoprotein interaction in vivo and determined the molecular interactions which are necessary and sufficient to promote M protein-membrane binding. Our results showed that M protein accumulates on cellular membranes via a direct interaction with both F and HN proteins. Specifically, our data demonstrated that a small fraction (8 to 16%) of M protein becomes membrane associated in the absence of Sendai virus glycoproteins, while > 75% becomes membrane bound in the presence of both F and HN proteins. Selective expression of M protein together with either F or HN protein showed that each viral glycoprotein is individually sufficient to promote efficient (56 to 73%) M protein-membrane binding. Finally, we observed that M protein associates with cellular membranes in a time-dependent manner, implying a need for either maturation or transport before binding to glycoproteins.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bowen H. A., Lyles D. S. Kinetics of incorporation of Sendai virus proteins into host plasma membranes and virions. Virology. 1982 Aug;121(1):1–11. doi: 10.1016/0042-6822(82)90113-1. [DOI] [PubMed] [Google Scholar]
- Bächi T. Intramembrane structural differentiation in Sendai virus maturation. Virology. 1980 Oct 15;106(1):41–49. doi: 10.1016/0042-6822(80)90219-6. [DOI] [PubMed] [Google Scholar]
- Büechi M., Bächi T. Microscopy of internal structures of Sendai virus associated with the cytoplasmic surface of host membranes. Virology. 1982 Jul 30;120(2):349–359. doi: 10.1016/0042-6822(82)90036-8. [DOI] [PubMed] [Google Scholar]
- Chakrabarti S., Brechling K., Moss B. Vaccinia virus expression vector: coexpression of beta-galactosidase provides visual screening of recombinant virus plaques. Mol Cell Biol. 1985 Dec;5(12):3403–3409. doi: 10.1128/mcb.5.12.3403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong L. D., Rose J. K. Membrane association of functional vesicular stomatitis virus matrix protein in vivo. J Virol. 1993 Jan;67(1):407–414. doi: 10.1128/jvi.67.1.407-414.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faaberg K. S., Peeples M. E. Association of soluble matrix protein of Newcastle disease virus with liposomes is independent of ionic conditions. Virology. 1988 Sep;166(1):123–132. doi: 10.1016/0042-6822(88)90153-5. [DOI] [PubMed] [Google Scholar]
- Griffiths G., Quinn P., Warren G. Dissection of the Golgi complex. I. Monensin inhibits the transport of viral membrane proteins from medial to trans Golgi cisternae in baby hamster kidney cells infected with Semliki Forest virus. J Cell Biol. 1983 Mar;96(3):835–850. doi: 10.1083/jcb.96.3.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb R. A., Choppin P. W. The synthesis of Sendai virus polypeptides in infected cells. III. Phosphorylation of polypeptides. Virology. 1977 Sep;81(2):382–397. doi: 10.1016/0042-6822(77)90154-4. [DOI] [PubMed] [Google Scholar]
- Lyles D. S. Glycoproteins of Sendai virus are transmembrane proteins. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5621–5625. doi: 10.1073/pnas.76.11.5621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markwell M. A., Fox C. F. Protein-protein interactions within paramyxoviruses identified by native disulfide bonding or reversible chemical cross-linking. J Virol. 1980 Jan;33(1):152–166. doi: 10.1128/jvi.33.1.152-166.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peeples M. E., Bratt M. A. Mutation in the matrix protein of Newcastle disease virus can result in decreased fusion glycoprotein incorporation into particles and decreased infectivity. J Virol. 1984 Jul;51(1):81–90. doi: 10.1128/jvi.51.1.81-90.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portner A., Scroggs R. A., Marx P. S., Kingsbury D. W. A temperature-sensitive mutant of Sendai virus with an altered hemagglutinin-neuraminidase polypeptide: consequences for virus assembly and cytopathology. Virology. 1975 Sep;67(1):179–187. doi: 10.1016/0042-6822(75)90415-8. [DOI] [PubMed] [Google Scholar]
- Sakaguchi T., Takao S., Kiyotani K., Fujii Y., Nakayama T., Yoshida T. Expression of the HN, F, NP and M proteins of Sendai virus by recombinant vaccinia viruses and their contribution to protective immunity against Sendai virus infections in mice. J Gen Virol. 1993 Mar;74(Pt 3):479–484. doi: 10.1099/0022-1317-74-3-479. [DOI] [PubMed] [Google Scholar]
- Sanderson C. M., McQueen N. L., Nayak D. P. Sendai virus assembly: M protein binds to viral glycoproteins in transit through the secretory pathway. J Virol. 1993 Feb;67(2):651–663. doi: 10.1128/jvi.67.2.651-663.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheshberadaran H., Lamb R. A. Simian virus 5 membrane protein maturation: expression in virus-infected cells and from a eukaryotic vector. Virology. 1991 Aug;183(2):803–809. doi: 10.1016/0042-6822(91)91015-9. [DOI] [PubMed] [Google Scholar]
- Shimizu K., Isida N. The smallest protein of Sendi virus: its candidate function of binding nucleocaspsid to envelope. Virology. 1975 Oct;67(2):427–437. [PubMed] [Google Scholar]
- Stricker R., Roux L. The major glycoprotein of Sendai virus is dispensable for efficient virus particle budding. J Gen Virol. 1991 Jul;72(Pt 7):1703–1707. doi: 10.1099/0022-1317-72-7-1703. [DOI] [PubMed] [Google Scholar]
- Yoshida T., Nagai Y'Yoshii S., Maeno K., Matsumoto T. Membrane (M) protein of HVJ (Sendai virus): its role in virus assembly. Virology. 1976 May;71(1):143–161. doi: 10.1016/0042-6822(76)90101-x. [DOI] [PubMed] [Google Scholar]
- Yoshida T., Nagai Y., Maeno K., Iinuma M., Hamaguchi M., Matsumoto T., Nagayoshi S., Hoshino M. Studies on the role of M protein in virus assembly using a ts mutant of HVJ (Sendai virus). Virology. 1979 Jan 15;92(1):139–154. doi: 10.1016/0042-6822(79)90220-4. [DOI] [PubMed] [Google Scholar]