Abstract
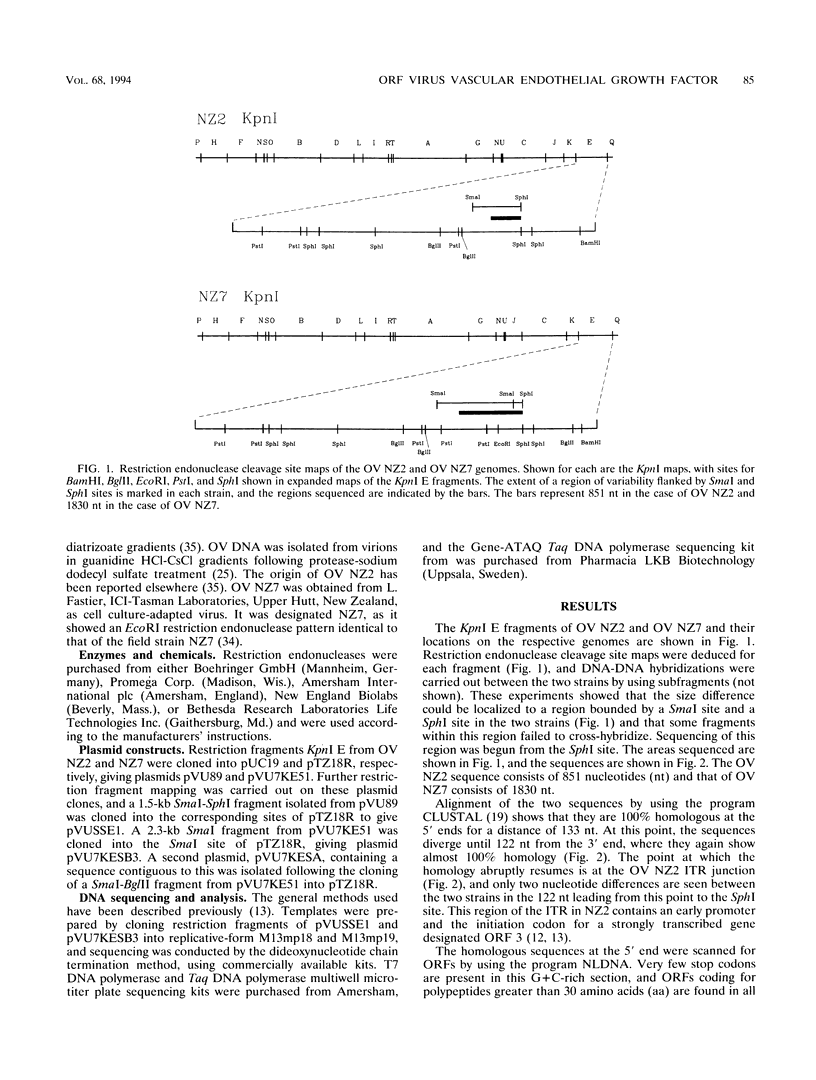
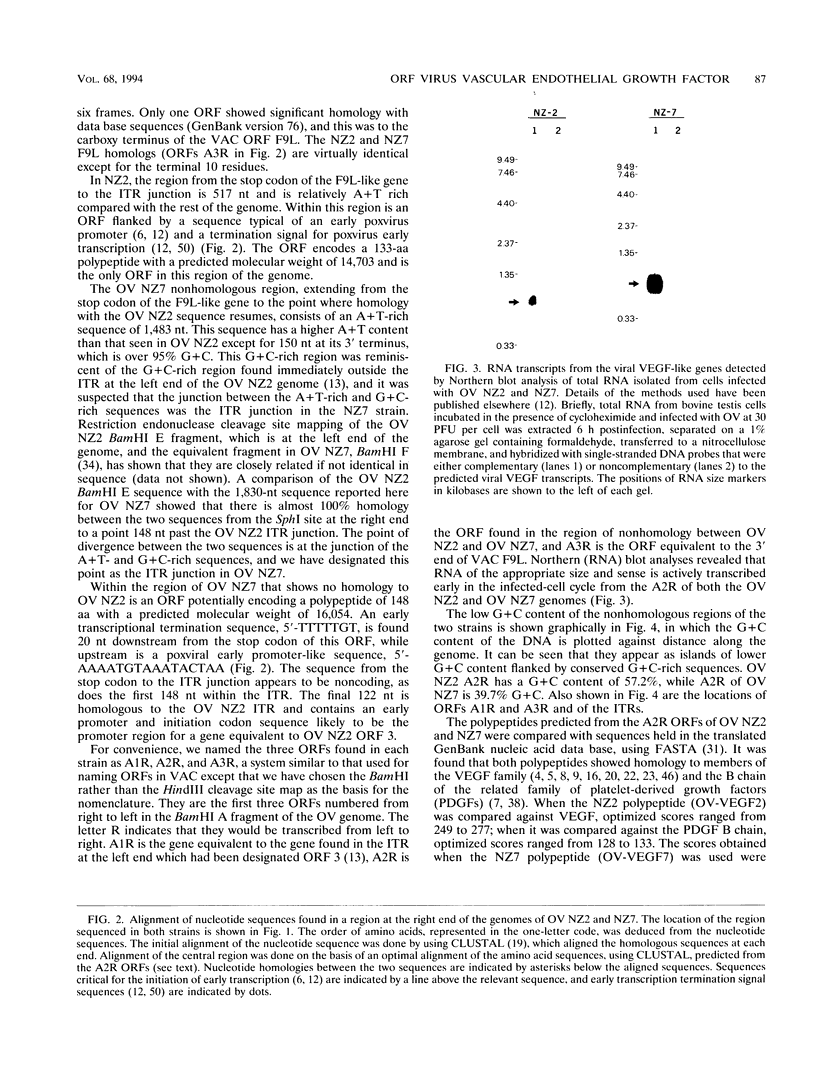
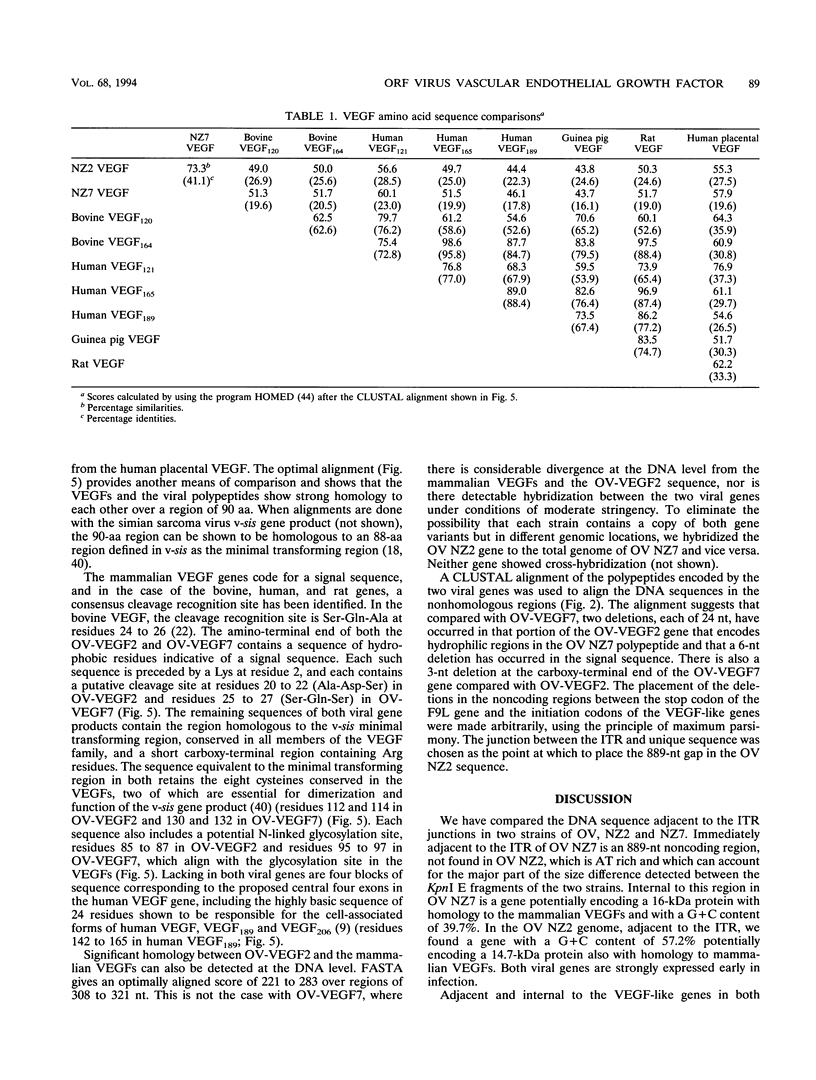
A gene encoding a polypeptide with homology to mammalian vascular endothelial growth factors (VEGFs) has been discovered in the genome of orf virus (OV), a parapoxvirus that affects sheep and goats and, occasionally, humans. The gene is transcribed abundantly early in infection and is found immediately outside the inverted terminal repeat at the right end of the genome. In the NZ2 strain of OV (OV NZ2), the gene encodes a polypeptide with a molecular size of 14.7 kDa, while in another strain, OV NZ7, there is a variant gene that encodes a polypeptide of 16 kDa. The OV NZ2 and OV NZ7 polypeptides show 22 to 27% and 16 to 23% identity, respectively, to the mammalian VEGFs. The viral polypeptides are only 41.1% identical to each other, and there is little homology between the two genes at the nucleotide level. Another unusual feature of these genes is their G+C content, particularly that of OV NZ7. In a genome that is otherwise 63% G+C, the OV NZ2 gene is 57.2% G+C and the OV NZ7 gene is 39.7% G+C. The OV NZ2 gene, but not the OV NZ7 gene, is homologous to the mammalian VEGF genes at the DNA level, suggesting that the gene has been acquired from a mammalian host and is undergoing genetic drift. The lesions induced in sheep and humans after infection with OV show extensive dermal vascular endothelial proliferation and dilatation, and it is likely that this is a direct effect of the expression of the VEGF-like gene.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahn B. Y., Moss B. RNA polymerase-associated transcription specificity factor encoded by vaccinia virus. Proc Natl Acad Sci U S A. 1992 Apr 15;89(8):3536–3540. doi: 10.1073/pnas.89.8.3536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balassu T. C., Robinson A. J. Orf virus replication in bovine testis cells: kinetics of viral DNA, polypeptide, and infectious virus production and analysis of virion polypeptides. Arch Virol. 1987;97(3-4):267–281. doi: 10.1007/BF01314426. [DOI] [PubMed] [Google Scholar]
- Conn G., Bayne M. L., Soderman D. D., Kwok P. W., Sullivan K. A., Palisi T. M., Hope D. A., Thomas K. A. Amino acid and cDNA sequences of a vascular endothelial cell mitogen that is homologous to platelet-derived growth factor. Proc Natl Acad Sci U S A. 1990 Apr;87(7):2628–2632. doi: 10.1073/pnas.87.7.2628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conn G., Soderman D. D., Schaeffer M. T., Wile M., Hatcher V. B., Thomas K. A. Purification of a glycoprotein vascular endothelial cell mitogen from a rat glioma-derived cell line. Proc Natl Acad Sci U S A. 1990 Feb;87(4):1323–1327. doi: 10.1073/pnas.87.4.1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davison A. J., Moss B. Structure of vaccinia virus early promoters. J Mol Biol. 1989 Dec 20;210(4):749–769. doi: 10.1016/0022-2836(89)90107-1. [DOI] [PubMed] [Google Scholar]
- Deuel T. F. Polypeptide growth factors: roles in normal and abnormal cell growth. Annu Rev Cell Biol. 1987;3:443–492. doi: 10.1146/annurev.cb.03.110187.002303. [DOI] [PubMed] [Google Scholar]
- Ferrara N., Henzel W. J. Pituitary follicular cells secrete a novel heparin-binding growth factor specific for vascular endothelial cells. Biochem Biophys Res Commun. 1989 Jun 15;161(2):851–858. doi: 10.1016/0006-291x(89)92678-8. [DOI] [PubMed] [Google Scholar]
- Ferrara N., Houck K. A., Jakeman L. B., Winer J., Leung D. W. The vascular endothelial growth factor family of polypeptides. J Cell Biochem. 1991 Nov;47(3):211–218. doi: 10.1002/jcb.240470305. [DOI] [PubMed] [Google Scholar]
- Fleming S. B., Blok J., Fraser K. M., Mercer A. A., Robinson A. J. Conservation of gene structure and arrangement between vaccinia virus and orf virus. Virology. 1993 Jul;195(1):175–184. doi: 10.1006/viro.1993.1358. [DOI] [PubMed] [Google Scholar]
- Fleming S. B., Fraser K. M., Mercer A. A., Robinson A. J. Vaccinia virus-like early transcriptional control sequences flank an early gene in orf virus. Gene. 1991 Jan 15;97(2):207–212. doi: 10.1016/0378-1119(91)90053-e. [DOI] [PubMed] [Google Scholar]
- Fraser K. M., Hill D. F., Mercer A. A., Robinson A. J. Sequence analysis of the inverted terminal repetition in the genome of the parapoxvirus, orf virus. Virology. 1990 Jun;176(2):379–389. doi: 10.1016/0042-6822(90)90008-f. [DOI] [PubMed] [Google Scholar]
- Goebel S. J., Johnson G. P., Perkus M. E., Davis S. W., Winslow J. P., Paoletti E. The complete DNA sequence of vaccinia virus. Virology. 1990 Nov;179(1):247-66, 517-63. doi: 10.1016/0042-6822(90)90294-2. [DOI] [PubMed] [Google Scholar]
- Gordon J., Mohandas A., Wilton S., Dales S. A prominent antigenic surface polypeptide involved in the biogenesis and function of the vaccinia virus envelope. Virology. 1991 Apr;181(2):671–686. doi: 10.1016/0042-6822(91)90901-M. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gospodarowicz D., Abraham J. A., Schilling J. Isolation and characterization of a vascular endothelial cell mitogen produced by pituitary-derived folliculo stellate cells. Proc Natl Acad Sci U S A. 1989 Oct;86(19):7311–7315. doi: 10.1073/pnas.86.19.7311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groves R. W., Wilson-Jones E., MacDonald D. M. Human orf and milkers' nodule: a clinicopathologic study. J Am Acad Dermatol. 1991 Oct;25(4):706–711. doi: 10.1016/0190-9622(91)70257-3. [DOI] [PubMed] [Google Scholar]
- Hannink M., Sauer M. K., Donoghue D. J. Deletions in the C-terminal coding region of the v-sis gene: dimerization is required for transformation. Mol Cell Biol. 1986 Apr;6(4):1304–1314. doi: 10.1128/mcb.6.4.1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins D. G., Sharp P. M. CLUSTAL: a package for performing multiple sequence alignment on a microcomputer. Gene. 1988 Dec 15;73(1):237–244. doi: 10.1016/0378-1119(88)90330-7. [DOI] [PubMed] [Google Scholar]
- Keck P. J., Hauser S. D., Krivi G., Sanzo K., Warren T., Feder J., Connolly D. T. Vascular permeability factor, an endothelial cell mitogen related to PDGF. Science. 1989 Dec 8;246(4935):1309–1312. doi: 10.1126/science.2479987. [DOI] [PubMed] [Google Scholar]
- LEAVELL U. W., Jr, MACNAMARA M. J., MEULLING R. J. ECTHYMA CONTAGIOSUM (ORF). South Med J. 1965 Feb;58:239–243. doi: 10.1097/00007611-196502000-00021. [DOI] [PubMed] [Google Scholar]
- Leung D. W., Cachianes G., Kuang W. J., Goeddel D. V., Ferrara N. Vascular endothelial growth factor is a secreted angiogenic mitogen. Science. 1989 Dec 8;246(4935):1306–1309. doi: 10.1126/science.2479986. [DOI] [PubMed] [Google Scholar]
- Maglione D., Guerriero V., Viglietto G., Delli-Bovi P., Persico M. G. Isolation of a human placenta cDNA coding for a protein related to the vascular permeability factor. Proc Natl Acad Sci U S A. 1991 Oct 15;88(20):9267–9271. doi: 10.1073/pnas.88.20.9267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGeoch D. J. Protein sequence comparisons show that the 'pseudoproteases' encoded by poxviruses and certain retroviruses belong to the deoxyuridine triphosphatase family. Nucleic Acids Res. 1990 Jul 25;18(14):4105–4110. doi: 10.1093/nar/18.14.4105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercer A. A., Fraser K. M., Stockwell P. A., Robinson A. J. A homologue of retroviral pseudoproteases in the parapoxvirus, orf virus. Virology. 1989 Oct;172(2):665–668. doi: 10.1016/0042-6822(89)90212-2. [DOI] [PubMed] [Google Scholar]
- Mercer A. A., Fraser K., Barns G., Robinson A. J. The structure and cloning of orf virus DNA. Virology. 1987 Mar;157(1):1–12. doi: 10.1016/0042-6822(87)90307-2. [DOI] [PubMed] [Google Scholar]
- Naase M., Nicholson B. H., Fraser K. M., Mercer A. A., Robinson A. J. An orf virus sequence showing homology to the 14K 'fusion' protein of vaccinia virus. J Gen Virol. 1991 May;72(Pt 5):1177–1181. doi: 10.1099/0022-1317-72-5-1177. [DOI] [PubMed] [Google Scholar]
- Pearson W. R., Lipman D. J. Improved tools for biological sequence comparison. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2444–2448. doi: 10.1073/pnas.85.8.2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plouët J., Schilling J., Gospodarowicz D. Isolation and characterization of a newly identified endothelial cell mitogen produced by AtT-20 cells. EMBO J. 1989 Dec 1;8(12):3801–3806. doi: 10.1002/j.1460-2075.1989.tb08557.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson A. J., Barns G., Fraser K., Carpenter E., Mercer A. A. Conservation and variation in orf virus genomes. Virology. 1987 Mar;157(1):13–23. doi: 10.1016/0042-6822(87)90308-4. [DOI] [PubMed] [Google Scholar]
- Robinson A. J., Ellis G., Balassu T. The genome of orf virus: restriction endonuclease analysis of viral DNA isolated from lesions of orf in sheep. Arch Virol. 1982;71(1):43–55. doi: 10.1007/BF01315174. [DOI] [PubMed] [Google Scholar]
- Rodriguez J. F., Esteban M. Mapping and nucleotide sequence of the vaccinia virus gene that encodes a 14-kilodalton fusion protein. J Virol. 1987 Nov;61(11):3550–3554. doi: 10.1128/jvi.61.11.3550-3554.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross R., Raines E. W., Bowen-Pope D. F. The biology of platelet-derived growth factor. Cell. 1986 Jul 18;46(2):155–169. doi: 10.1016/0092-8674(86)90733-6. [DOI] [PubMed] [Google Scholar]
- Sanchez R. L., Hebert A., Lucia H., Swedo J. Orf. A case report with histologic, electron microscopic, and immunoperoxidase studies. Arch Pathol Lab Med. 1985 Feb;109(2):166–170. [PubMed] [Google Scholar]
- Sauer M. K., Hannink M., Donoghue D. J. Deletions in the N-terminal coding region of the v-sis gene: determination of the minimal transforming region. J Virol. 1986 Aug;59(2):292–300. doi: 10.1128/jvi.59.2.292-300.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shuman S., Moss B. Identification of a vaccinia virus gene encoding a type I DNA topoisomerase. Proc Natl Acad Sci U S A. 1987 Nov;84(21):7478–7482. doi: 10.1073/pnas.84.21.7478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slabaugh M. B., Roseman N. A. Retroviral protease-like gene in the vaccinia virus genome. Proc Natl Acad Sci U S A. 1989 Jun;86(11):4152–4155. doi: 10.1073/pnas.86.11.4152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockwell P. A., Petersen G. B. HOMED: a homologous sequence editor. Comput Appl Biosci. 1987 Mar;3(1):37–43. doi: 10.1093/bioinformatics/3.1.37. [DOI] [PubMed] [Google Scholar]
- Tischer E., Gospodarowicz D., Mitchell R., Silva M., Schilling J., Lau K., Crisp T., Fiddes J. C., Abraham J. A. Vascular endothelial growth factor: a new member of the platelet-derived growth factor gene family. Biochem Biophys Res Commun. 1989 Dec 29;165(3):1198–1206. doi: 10.1016/0006-291x(89)92729-0. [DOI] [PubMed] [Google Scholar]
- WHEELER C. E., CAWLEY E. P. The microscopic appearance of ecthyma contagiosum (orf) in sheep, rabbits, and man. Am J Pathol. 1956 May-Jun;32(3):535–545. [PMC free article] [PubMed] [Google Scholar]
- Wittek R., Kuenzle C. C., Wyler R. High C + G content in parapoxvirus DNA. J Gen Virol. 1979 Apr;43(1):231–234. doi: 10.1099/0022-1317-43-1-231. [DOI] [PubMed] [Google Scholar]
- Yeh H. P., Soltani K. Ultrastructural studies in human orf. Arch Dermatol. 1974 Mar;109(3):390–392. [PubMed] [Google Scholar]
- Yuen L., Moss B. Multiple 3' ends of mRNA encoding vaccinia virus growth factor occur within a series of repeated sequences downstream of T clusters. J Virol. 1986 Oct;60(1):320–323. doi: 10.1128/jvi.60.1.320-323.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]




