Abstract
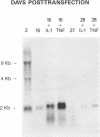
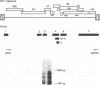
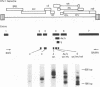
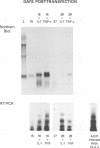
Human immunodeficiency virus type 1 (HIV-1) infection of the developing central nervous system results in a dementing process in children, termed HIV-1-associated encephalopathy. Infection of astroglial elements of the pediatric nervous system has been demonstrated and suggests that direct infection of some astrocytes may contribute to the neurologic deficit. In this model, HIV-1 establishes a persistent state of infection in astrocytes, which can be reactivated by the cytokines tumor necrosis factor alpha (TNF-alpha) and interleukin 1 beta (IL-1 beta). To better understand the natural history of viral persistence in astroglial cells, we characterized infection at the transcriptional level. The most abundant viral transcript during the establishment of persistence was the subgenomic multiply spliced 2-kb message, similar to mononuclear cell models of HIV-1 latency. Following reactivation with TNF-alpha or IL-1 beta the multiply spliced 2-kb message remained the most abundant viral transcript, in contrast to infected mononuclear cells in which reactivation leads to the reemergence of the 9- and 4-kb transcripts. Further characterization of the persistent 2-kb transcript by PCR amplification of in vitro-synthesized viral cDNA showed that, in the absence of cytokine stimulation, the most abundant multiply spliced transcripts were the Nef- and Rev-specific messages. However, following cytokine stimulation, double- and triple-spliced Tat-, Rev-, and Nef-specific messages could be identified. Immunohistochemical staining demonstrated that, during viral persistence, astrocytes expressed Nef protein but few or no viral structural proteins. These results demonstrate that viral persistence in astrocytes at the transcriptional level is fundamentally different from that seen in mononuclear cells and could account for the virtual absence of astroglial expression of viral structural antigens in vivo.
Full text
PDF



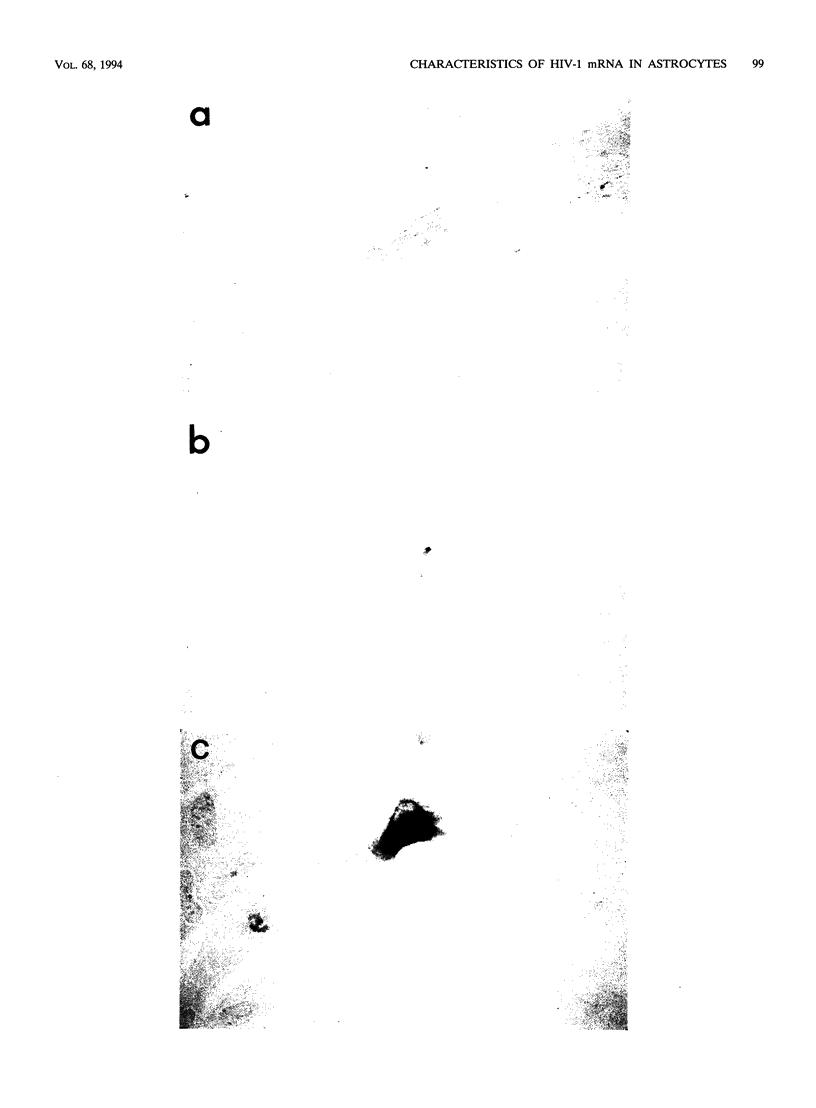



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adachi A., Gendelman H. E., Koenig S., Folks T., Willey R., Rabson A., Martin M. A. Production of acquired immunodeficiency syndrome-associated retrovirus in human and nonhuman cells transfected with an infectious molecular clone. J Virol. 1986 Aug;59(2):284–291. doi: 10.1128/jvi.59.2.284-291.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachelerie F., Alcami J., Hazan U., Israël N., Goud B., Arenzana-Seisdedos F., Virelizier J. L. Constitutive expression of human immunodeficiency virus (HIV) nef protein in human astrocytes does not influence basal or induced HIV long terminal repeat activity. J Virol. 1990 Jun;64(6):3059–3062. doi: 10.1128/jvi.64.6.3059-3062.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagasra O., Hauptman S. P., Lischner H. W., Sachs M., Pomerantz R. J. Detection of human immunodeficiency virus type 1 provirus in mononuclear cells by in situ polymerase chain reaction. N Engl J Med. 1992 May 21;326(21):1385–1391. doi: 10.1056/NEJM199205213262103. [DOI] [PubMed] [Google Scholar]
- Belman A. L., Ultmann M. H., Horoupian D., Novick B., Spiro A. J., Rubinstein A., Kurtzberg D., Cone-Wesson B. Neurological complications in infants and children with acquired immune deficiency syndrome. Ann Neurol. 1985 Nov;18(5):560–566. doi: 10.1002/ana.410180509. [DOI] [PubMed] [Google Scholar]
- Bernton E. W., Bryant H. U., Decoster M. A., Orenstein J. M., Ribas J. L., Meltzer M. S., Gendelman H. E. No direct neuronotoxicity by HIV-1 virions or culture fluids from HIV-1-infected T cells or monocytes. AIDS Res Hum Retroviruses. 1992 Apr;8(4):495–503. doi: 10.1089/aid.1992.8.495. [DOI] [PubMed] [Google Scholar]
- Blumberg B. M., Epstein L. G., Saito Y., Chen D., Sharer L. R., Anand R. Human immunodeficiency virus type 1 nef quasispecies in pathological tissue. J Virol. 1992 Sep;66(9):5256–5264. doi: 10.1128/jvi.66.9.5256-5264.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brack-Werner R., Kleinschmidt A., Ludvigsen A., Mellert W., Neumann M., Herrmann R., Khim M. C., Burny A., Müller-Lantzsch N., Stavrou D. Infection of human brain cells by HIV-1: restricted virus production in chronically infected human glial cell lines. AIDS. 1992 Mar;6(3):273–285. [PubMed] [Google Scholar]
- Brenneman D. E., Westbrook G. L., Fitzgerald S. P., Ennist D. L., Elkins K. L., Ruff M. R., Pert C. B. Neuronal cell killing by the envelope protein of HIV and its prevention by vasoactive intestinal peptide. Nature. 1988 Oct 13;335(6191):639–642. doi: 10.1038/335639a0. [DOI] [PubMed] [Google Scholar]
- Cheng-Mayer C., Rutka J. T., Rosenblum M. L., McHugh T., Stites D. P., Levy J. A. Human immunodeficiency virus can productively infect cultured human glial cells. Proc Natl Acad Sci U S A. 1987 May;84(10):3526–3530. doi: 10.1073/pnas.84.10.3526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiodi F., Fuerstenberg S., Gidlund M., Asjö B., Fenyö E. M. Infection of brain-derived cells with the human immunodeficiency virus. J Virol. 1987 Apr;61(4):1244–1247. doi: 10.1128/jvi.61.4.1244-1247.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christofinis G., Papadaki L., Sattentau Q., Ferns R. B., Tedder R. HIV replicates in cultured human brain cells. AIDS. 1987 Dec;1(4):229–234. [PubMed] [Google Scholar]
- Constantoulakis P., Campbell M., Felber B. K., Nasioulas G., Afonina E., Pavlakis G. N. Inhibition of Rev-mediated HIV-1 expression by an RNA binding protein encoded by the interferon-inducible 9-27 gene. Science. 1993 Feb 26;259(5099):1314–1318. doi: 10.1126/science.7680491. [DOI] [PubMed] [Google Scholar]
- Dewhurst S., Sakai K., Bresser J., Stevenson M., Evinger-Hodges M. J., Volsky D. J. Persistent productive infection of human glial cells by human immunodeficiency virus (HIV) and by infectious molecular clones of HIV. J Virol. 1987 Dec;61(12):3774–3782. doi: 10.1128/jvi.61.12.3774-3782.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreyer E. B., Kaiser P. K., Offermann J. T., Lipton S. A. HIV-1 coat protein neurotoxicity prevented by calcium channel antagonists. Science. 1990 Apr 20;248(4953):364–367. doi: 10.1126/science.2326646. [DOI] [PubMed] [Google Scholar]
- Embretson J., Zupancic M., Beneke J., Till M., Wolinsky S., Ribas J. L., Burke A., Haase A. T. Analysis of human immunodeficiency virus-infected tissues by amplification and in situ hybridization reveals latent and permissive infections at single-cell resolution. Proc Natl Acad Sci U S A. 1993 Jan 1;90(1):357–361. doi: 10.1073/pnas.90.1.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein L. G., Sharer L. R., Cho E. S., Myenhofer M., Navia B., Price R. W. HTLV-III/LAV-like retrovirus particles in the brains of patients with AIDS encephalopathy. AIDS Res. 1984;1(6):447–454. doi: 10.1089/aid.1.1983.1.447. [DOI] [PubMed] [Google Scholar]
- Epstein L. G., Sharer L. R., Joshi V. V., Fojas M. M., Koenigsberger M. R., Oleske J. M. Progressive encephalopathy in children with acquired immune deficiency syndrome. Ann Neurol. 1985 May;17(5):488–496. doi: 10.1002/ana.410170512. [DOI] [PubMed] [Google Scholar]
- Folks T., Benn S., Rabson A., Theodore T., Hoggan M. D., Martin M., Lightfoote M., Sell K. Characterization of a continuous T-cell line susceptible to the cytopathic effects of the acquired immunodeficiency syndrome (AIDS)-associated retrovirus. Proc Natl Acad Sci U S A. 1985 Jul;82(13):4539–4543. doi: 10.1073/pnas.82.13.4539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genis P., Jett M., Bernton E. W., Boyle T., Gelbard H. A., Dzenko K., Keane R. W., Resnick L., Mizrachi Y., Volsky D. J. Cytokines and arachidonic metabolites produced during human immunodeficiency virus (HIV)-infected macrophage-astroglia interactions: implications for the neuropathogenesis of HIV disease. J Exp Med. 1992 Dec 1;176(6):1703–1718. doi: 10.1084/jem.176.6.1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giulian D., Vaca K., Noonan C. A. Secretion of neurotoxins by mononuclear phagocytes infected with HIV-1. Science. 1990 Dec 14;250(4987):1593–1596. doi: 10.1126/science.2148832. [DOI] [PubMed] [Google Scholar]
- Hammes S. R., Dixon E. P., Malim M. H., Cullen B. R., Greene W. C. Nef protein of human immunodeficiency virus type 1: evidence against its role as a transcriptional inhibitor. Proc Natl Acad Sci U S A. 1989 Dec;86(23):9549–9553. doi: 10.1073/pnas.86.23.9549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klotman M. E., Kim S., Buchbinder A., DeRossi A., Baltimore D., Wong-Staal F. Kinetics of expression of multiply spliced RNA in early human immunodeficiency virus type 1 infection of lymphocytes and monocytes. Proc Natl Acad Sci U S A. 1991 Jun 1;88(11):5011–5015. doi: 10.1073/pnas.88.11.5011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohleisen B., Neumann M., Herrmann R., Brack-Werner R., Krohn K. J., Ovod V., Ranki A., Erfle V. Cellular localization of Nef expressed in persistently HIV-1-infected low-producer astrocytes. AIDS. 1992 Dec;6(12):1427–1436. doi: 10.1097/00002030-199212000-00002. [DOI] [PubMed] [Google Scholar]
- Lipton S. A., Sucher N. J., Kaiser P. K., Dreyer E. B. Synergistic effects of HIV coat protein and NMDA receptor-mediated neurotoxicity. Neuron. 1991 Jul;7(1):111–118. doi: 10.1016/0896-6273(91)90079-f. [DOI] [PubMed] [Google Scholar]
- Lyman W. D., Kress Y., Kure K., Rashbaum W. K., Rubinstein A., Soeiro R. Detection of HIV in fetal central nervous system tissue. AIDS. 1990 Sep;4(9):917–920. doi: 10.1097/00002030-199009000-00014. [DOI] [PubMed] [Google Scholar]
- Mano H., Chermann J. C. Fetal human immunodeficiency virus type 1 infection of different organs in the second trimester. AIDS Res Hum Retroviruses. 1991 Jan;7(1):83–88. doi: 10.1089/aid.1991.7.83. [DOI] [PubMed] [Google Scholar]
- Merrill J. E., Koyanagi Y., Zack J., Thomas L., Martin F., Chen I. S. Induction of interleukin-1 and tumor necrosis factor alpha in brain cultures by human immunodeficiency virus type 1. J Virol. 1992 Apr;66(4):2217–2225. doi: 10.1128/jvi.66.4.2217-2225.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michael N. L., Morrow P., Mosca J., Vahey M., Burke D. S., Redfield R. R. Induction of human immunodeficiency virus type 1 expression in chronically infected cells is associated primarily with a shift in RNA splicing patterns. J Virol. 1991 Mar;65(3):1291–1303. doi: 10.1128/jvi.65.3.1291-1303.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muesing M. A., Smith D. H., Cabradilla C. D., Benton C. V., Lasky L. A., Capon D. J. Nucleic acid structure and expression of the human AIDS/lymphadenopathy retrovirus. Nature. 1985 Feb 7;313(6002):450–458. doi: 10.1038/313450a0. [DOI] [PubMed] [Google Scholar]
- Navia B. A., Cho E. S., Petito C. K., Price R. W. The AIDS dementia complex: II. Neuropathology. Ann Neurol. 1986 Jun;19(6):525–535. doi: 10.1002/ana.410190603. [DOI] [PubMed] [Google Scholar]
- Navia B. A., Jordan B. D., Price R. W. The AIDS dementia complex: I. Clinical features. Ann Neurol. 1986 Jun;19(6):517–524. doi: 10.1002/ana.410190602. [DOI] [PubMed] [Google Scholar]
- Niederman T. M., Garcia J. V., Hastings W. R., Luria S., Ratner L. Human immunodeficiency virus type 1 Nef protein inhibits NF-kappa B induction in human T cells. J Virol. 1992 Oct;66(10):6213–6219. doi: 10.1128/jvi.66.10.6213-6219.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novello A. C., Wise P. H., Willoughby A., Pizzo P. A. Final report of the United States Department of Health and Human Services Secretary's Work Group on pediatric human immunodeficiency virus infection and disease: content and implications. Pediatrics. 1989 Sep;84(3):547–555. [PubMed] [Google Scholar]
- Pomerantz R. J., Trono D., Feinberg M. B., Baltimore D. Cells nonproductively infected with HIV-1 exhibit an aberrant pattern of viral RNA expression: a molecular model for latency. Cell. 1990 Jun 29;61(7):1271–1276. doi: 10.1016/0092-8674(90)90691-7. [DOI] [PubMed] [Google Scholar]
- Price R. W., Brew B., Sidtis J., Rosenblum M., Scheck A. C., Cleary P. The brain in AIDS: central nervous system HIV-1 infection and AIDS dementia complex. Science. 1988 Feb 5;239(4840):586–592. doi: 10.1126/science.3277272. [DOI] [PubMed] [Google Scholar]
- Pulliam L., Herndier B. G., Tang N. M., McGrath M. S. Human immunodeficiency virus-infected macrophages produce soluble factors that cause histological and neurochemical alterations in cultured human brains. J Clin Invest. 1991 Feb;87(2):503–512. doi: 10.1172/JCI115024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes R. H., Ward J. M. AIDS meningoencephalomyelitis. Pathogenesis and changing neuropathologic findings. Pathol Annu. 1991;26(Pt 2):247–276. [PubMed] [Google Scholar]
- Robbins D. S., Shirazi Y., Drysdale B. E., Lieberman A., Shin H. S., Shin M. L. Production of cytotoxic factor for oligodendrocytes by stimulated astrocytes. J Immunol. 1987 Oct 15;139(8):2593–2597. [PubMed] [Google Scholar]
- Robert-Guroff M., Popovic M., Gartner S., Markham P., Gallo R. C., Reitz M. S. Structure and expression of tat-, rev-, and nef-specific transcripts of human immunodeficiency virus type 1 in infected lymphocytes and macrophages. J Virol. 1990 Jul;64(7):3391–3398. doi: 10.1128/jvi.64.7.3391-3398.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz O., Rivière Y., Heard J. M., Danos O. Reduced cell surface expression of processed human immunodeficiency virus type 1 envelope glycoprotein in the presence of Nef. J Virol. 1993 Jun;67(6):3274–3280. doi: 10.1128/jvi.67.6.3274-3280.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz S., Felber B. K., Benko D. M., Fenyö E. M., Pavlakis G. N. Cloning and functional analysis of multiply spliced mRNA species of human immunodeficiency virus type 1. J Virol. 1990 Jun;64(6):2519–2529. doi: 10.1128/jvi.64.6.2519-2529.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selmaj K. W., Raine C. S. Tumor necrosis factor mediates myelin and oligodendrocyte damage in vitro. Ann Neurol. 1988 Apr;23(4):339–346. doi: 10.1002/ana.410230405. [DOI] [PubMed] [Google Scholar]
- Sharer L. R., Epstein L. G., Cho E. S., Joshi V. V., Meyenhofer M. F., Rankin L. F., Petito C. K. Pathologic features of AIDS encephalopathy in children: evidence for LAV/HTLV-III infection of brain. Hum Pathol. 1986 Mar;17(3):271–284. doi: 10.1016/s0046-8177(83)80220-2. [DOI] [PubMed] [Google Scholar]
- Sharpless N., Gilbert D., Vandercam B., Zhou J. M., Verdin E., Ronnett G., Friedman E., Dubois-Dalcq M. The restricted nature of HIV-1 tropism for cultured neural cells. Virology. 1992 Dec;191(2):813–825. doi: 10.1016/0042-6822(92)90257-p. [DOI] [PubMed] [Google Scholar]
- Stoler M. H., Eskin T. A., Benn S., Angerer R. C., Angerer L. M. Human T-cell lymphotropic virus type III infection of the central nervous system. A preliminary in situ analysis. JAMA. 1986 Nov 7;256(17):2360–2364. [PubMed] [Google Scholar]
- Swingler S., Easton A., Morris A. Cytokine augmentation of HIV-1 LTR-driven gene expression in neural cells. AIDS Res Hum Retroviruses. 1992 Apr;8(4):487–493. doi: 10.1089/aid.1992.8.487. [DOI] [PubMed] [Google Scholar]
- Tyor W. R., Glass J. D., Griffin J. W., Becker P. S., McArthur J. C., Bezman L., Griffin D. E. Cytokine expression in the brain during the acquired immunodeficiency syndrome. Ann Neurol. 1992 Apr;31(4):349–360. doi: 10.1002/ana.410310402. [DOI] [PubMed] [Google Scholar]
- Vitković L., Wood G. P., Major E. O., Fauci A. S. Human astrocytes stimulate HIV-1 expression in a chronically infected promonocyte clone via interleukin-6. AIDS Res Hum Retroviruses. 1991 Sep;7(9):723–727. doi: 10.1089/aid.1991.7.723. [DOI] [PubMed] [Google Scholar]
- Wahl S. M., Allen J. B., McCartney-Francis N., Morganti-Kossmann M. C., Kossmann T., Ellingsworth L., Mai U. E., Mergenhagen S. E., Orenstein J. M. Macrophage- and astrocyte-derived transforming growth factor beta as a mediator of central nervous system dysfunction in acquired immune deficiency syndrome. J Exp Med. 1991 Apr 1;173(4):981–991. doi: 10.1084/jem.173.4.981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward J. M., O'Leary T. J., Baskin G. B., Benveniste R., Harris C. A., Nara P. L., Rhodes R. H. Immunohistochemical localization of human and simian immunodeficiency viral antigens in fixed tissue sections. Am J Pathol. 1987 May;127(2):199–205. [PMC free article] [PubMed] [Google Scholar]
- Werner T., Ferroni S., Saermark T., Brack-Werner R., Banati R. B., Mager R., Steinaa L., Kreutzberg G. W., Erfle V. HIV-1 Nef protein exhibits structural and functional similarity to scorpion peptides interacting with K+ channels. AIDS. 1991 Nov;5(11):1301–1308. doi: 10.1097/00002030-199111000-00003. [DOI] [PubMed] [Google Scholar]
- Wiley C. A., Schrier R. D., Nelson J. A., Lampert P. W., Oldstone M. B. Cellular localization of human immunodeficiency virus infection within the brains of acquired immune deficiency syndrome patients. Proc Natl Acad Sci U S A. 1986 Sep;83(18):7089–7093. doi: 10.1073/pnas.83.18.7089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie W. Q., Rothblum L. I. Rapid, small-scale RNA isolation from tissue culture cells. Biotechniques. 1991 Sep;11(3):324, 326-7. [PubMed] [Google Scholar]