Abstract
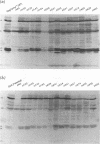
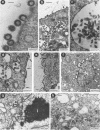
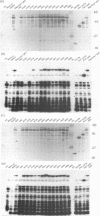
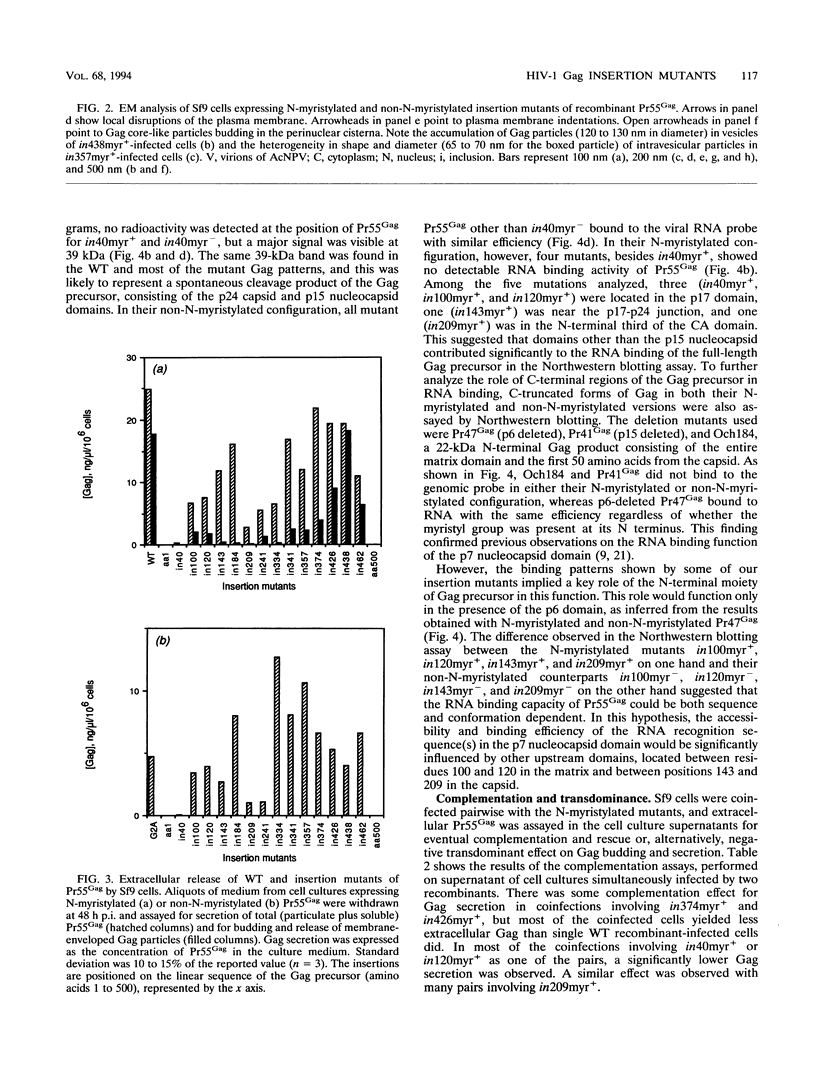
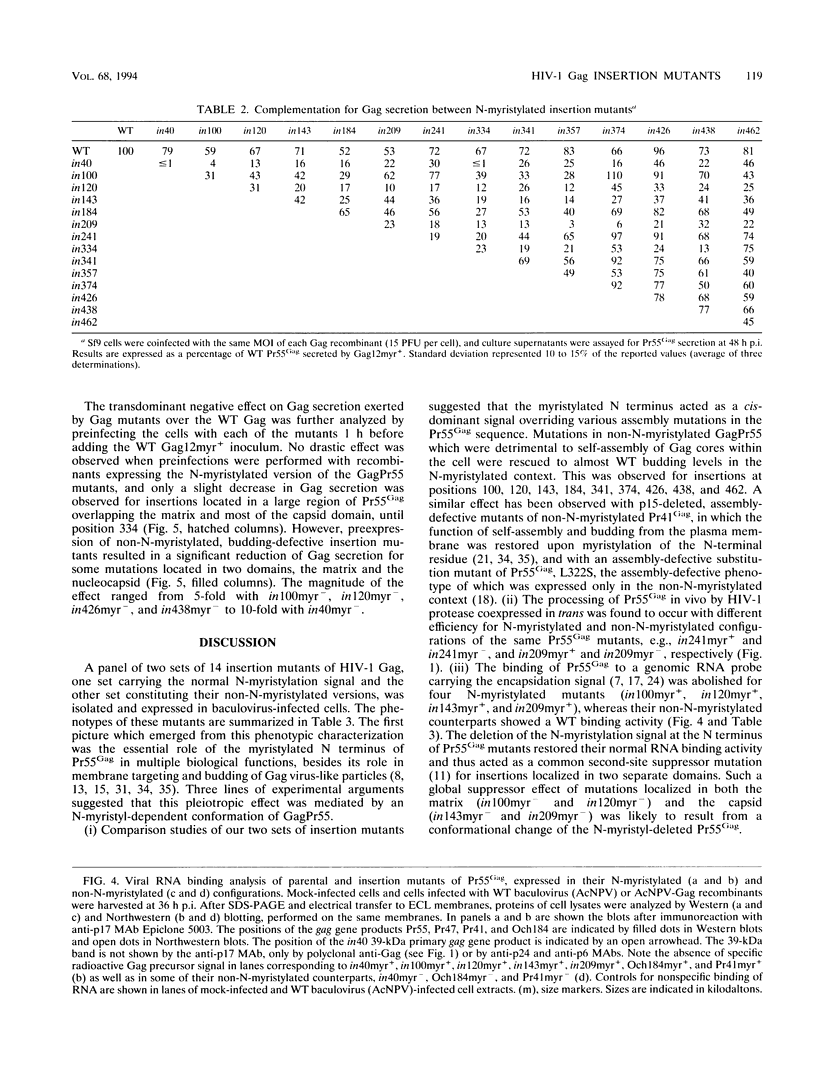
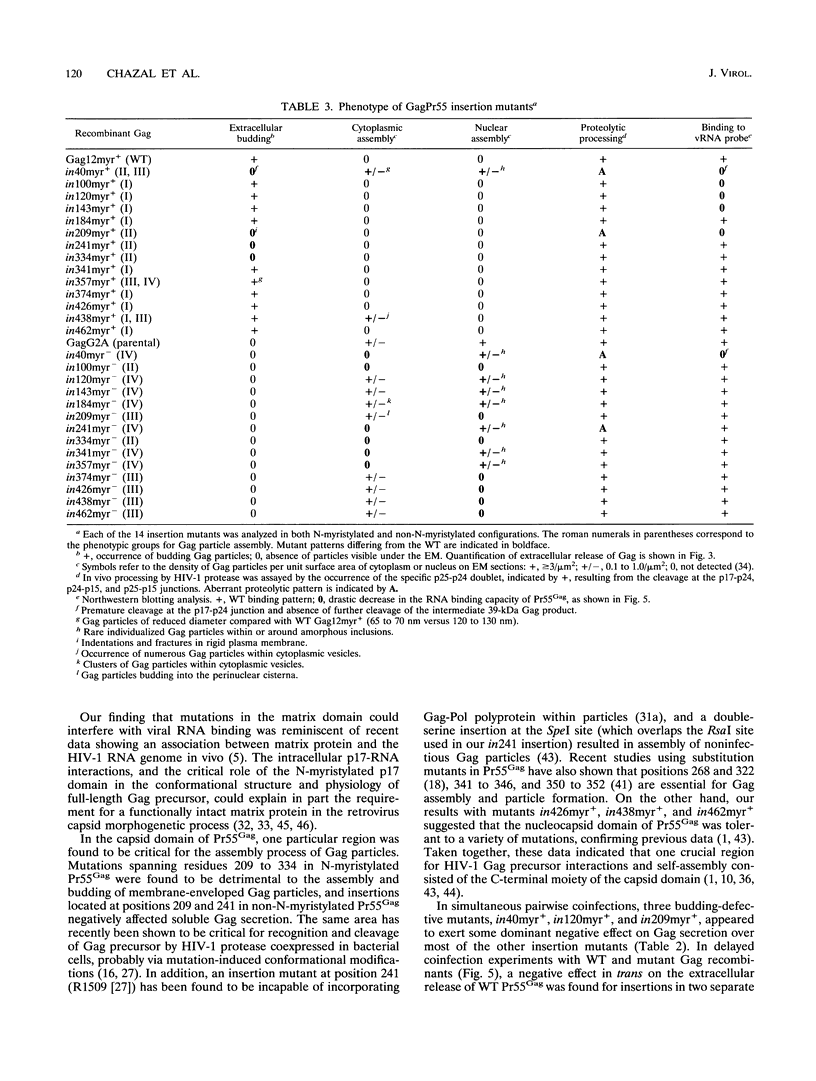
A panel of 28 insertion mutants of the human immunodeficiency virus type 1 (HIV-1) Gag precursor (Pr55Gag) was constructed by linker-insertion mutagenesis and expressed in recombinant baculovirus-infected insect cells. One set of 14 mutants carried the normal N-myristylation signal; the other set constituted their non-N-myristylated counterparts. The mutants were characterized with respect to (i) assembly and extracellular release of membrane-enveloped budding Gag particles, (ii) intracellular assembly and nuclear transport of Gag cores, (iii) specific processing of Pr55Gag by HIV-1 protease in vivo, and (iv) binding of Pr55Gag to an HIV-1 genomic RNA probe in Northwestern blotting. Insertions within the region between amino acid residues 209 and 334 in the CA domain appeared to be the most detrimental to Gag particle assembly and release of Gag into the external medium, whereas a narrower window, between residues 209 and 241, was found to be critical for secretion of soluble Pr55Gag. Differences in Pr55Gag processing in vivo and RNA binding in vitro between N-myristylated and non-N-myristylated Gag mutants suggested a major conformational role for the myristylated N terminus of Gag precursor. In coinfection experiments using wild-type Gag- and mutant Gag-expressing recombinants, a transdominant negative effect on Gag particle assembly and release was observed for insertions located in two separate domains, the matrix and nucleocapsid.
Full text
PDF



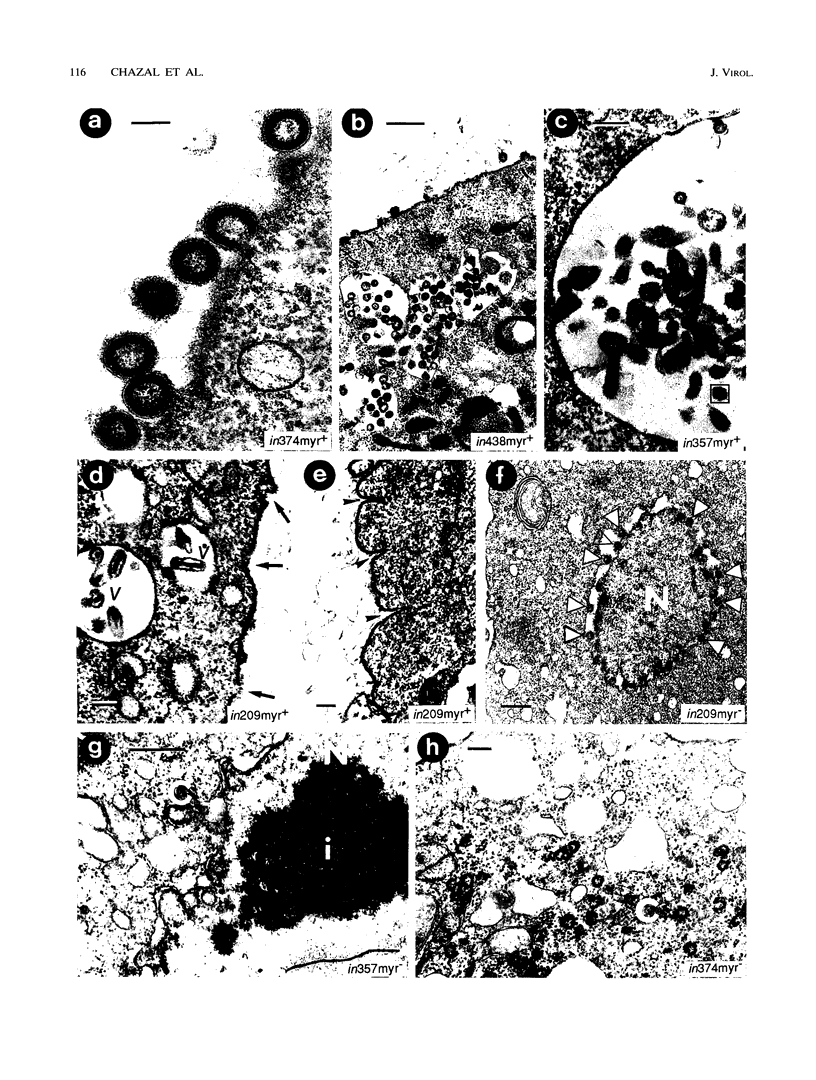



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aldovini A., Young R. A. Mutations of RNA and protein sequences involved in human immunodeficiency virus type 1 packaging result in production of noninfectious virus. J Virol. 1990 May;64(5):1920–1926. doi: 10.1128/jvi.64.5.1920-1926.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreassen H., Bohr H., Bohr J., Brunak S., Bugge T., Cotterill R. M., Jacobsen C., Kusk P., Lautrup B., Petersen S. B. Analysis of the secondary structure of the human immunodeficiency virus (HIV) proteins p17, gp120, and gp41 by computer modeling based on neural network methods. J Acquir Immune Defic Syndr. 1990;3(6):615–622. [PubMed] [Google Scholar]
- Argos P. A possible homology between immunodeficiency virus p24 core protein and picornaviral VP2 coat protein: prediction of HIV p24 antigenic sites. EMBO J. 1989 Mar;8(3):779–785. doi: 10.1002/j.1460-2075.1989.tb03438.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blomberg J., Medstrand P. A sequence in the carboxyl terminus of the HIV-1 matrix protein is highly similar to sequences in membrane-associated proteins of other RNA viruses: possible functional implications. New Biol. 1990 Nov;2(11):1044–1046. [PubMed] [Google Scholar]
- Bukrinskaya A. G., Vorkunova G. K., Tentsov YYu HIV-1 matrix protein p17 resides in cell nuclei in association with genomic RNA. AIDS Res Hum Retroviruses. 1992 Oct;8(10):1795–1801. doi: 10.1089/aid.1992.8.1795. [DOI] [PubMed] [Google Scholar]
- Cann A. J., Karn J. Molecular biology of HIV: new insights into the virus life-cycle. AIDS. 1989;3 (Suppl 1):S19–S34. [PubMed] [Google Scholar]
- Clavel F., Orenstein J. M. A mutant of human immunodeficiency virus with reduced RNA packaging and abnormal particle morphology. J Virol. 1990 Oct;64(10):5230–5234. doi: 10.1128/jvi.64.10.5230-5234.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Rocquigny H., Gabus C., Vincent A., Fournié-Zaluski M. C., Roques B., Darlix J. L. Viral RNA annealing activities of human immunodeficiency virus type 1 nucleocapsid protein require only peptide domains outside the zinc fingers. Proc Natl Acad Sci U S A. 1992 Jul 15;89(14):6472–6476. doi: 10.1073/pnas.89.14.6472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delchambre M., Gheysen D., Thines D., Thiriart C., Jacobs E., Verdin E., Horth M., Burny A., Bex F. The GAG precursor of simian immunodeficiency virus assembles into virus-like particles. EMBO J. 1989 Sep;8(9):2653–2660. doi: 10.1002/j.1460-2075.1989.tb08405.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrlich L. S., Agresta B. E., Carter C. A. Assembly of recombinant human immunodeficiency virus type 1 capsid protein in vitro. J Virol. 1992 Aug;66(8):4874–4883. doi: 10.1128/jvi.66.8.4874-4883.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fane B., Villafane R., Mitraki A., King J. Identification of global suppressors for temperature-sensitive folding mutations of the P22 tailspike protein. J Biol Chem. 1991 Jun 25;266(18):11640–11648. [PubMed] [Google Scholar]
- Gelderblom H. R. Assembly and morphology of HIV: potential effect of structure on viral function. AIDS. 1991 Jun;5(6):617–637. [PubMed] [Google Scholar]
- Gheysen D., Jacobs E., de Foresta F., Thiriart C., Francotte M., Thines D., De Wilde M. Assembly and release of HIV-1 precursor Pr55gag virus-like particles from recombinant baculovirus-infected insect cells. Cell. 1989 Oct 6;59(1):103–112. doi: 10.1016/0092-8674(89)90873-8. [DOI] [PubMed] [Google Scholar]
- Göttlinger H. G., Dorfman T., Sodroski J. G., Haseltine W. A. Effect of mutations affecting the p6 gag protein on human immunodeficiency virus particle release. Proc Natl Acad Sci U S A. 1991 Apr 15;88(8):3195–3199. doi: 10.1073/pnas.88.8.3195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Göttlinger H. G., Sodroski J. G., Haseltine W. A. Role of capsid precursor processing and myristoylation in morphogenesis and infectivity of human immunodeficiency virus type 1. Proc Natl Acad Sci U S A. 1989 Aug;86(15):5781–5785. doi: 10.1073/pnas.86.15.5781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen M., Jelinek L., Whiting S., Barklis E. Transport and assembly of gag proteins into Moloney murine leukemia virus. J Virol. 1990 Nov;64(11):5306–5316. doi: 10.1128/jvi.64.11.5306-5316.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi T., Shioda T., Iwakura Y., Shibuta H. RNA packaging signal of human immunodeficiency virus type 1. Virology. 1992 Jun;188(2):590–599. doi: 10.1016/0042-6822(92)90513-o. [DOI] [PubMed] [Google Scholar]
- Hong S. S., Boulanger P. Assembly-defective point mutants of the human immunodeficiency virus type 1 Gag precursor phenotypically expressed in recombinant baculovirus-infected cells. J Virol. 1993 May;67(5):2787–2798. doi: 10.1128/jvi.67.5.2787-2798.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshikawa N., Kojima A., Yasuda A., Takayashiki E., Masuko S., Chiba J., Sata T., Kurata T. Role of the gag and pol genes of human immunodeficiency virus in the morphogenesis and maturation of retrovirus-like particles expressed by recombinant vaccinia virus: an ultrastructural study. J Gen Virol. 1991 Oct;72(Pt 10):2509–2517. doi: 10.1099/0022-1317-72-10-2509. [DOI] [PubMed] [Google Scholar]
- Hughes B. P., Booth T. F., Belyaev A. S., McIlroy D., Jowett J., Roy P. Morphogenic capabilities of human immunodeficiency virus type 1 gag and gag-pol proteins in insect cells. Virology. 1993 Mar;193(1):242–255. doi: 10.1006/viro.1993.1120. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Langedijk J. P., Schalken J. J., Tersmette M., Huisman J. G., Meloen R. H. Location of epitopes on the major core protein p24 of human immunodeficiency virus. J Gen Virol. 1990 Nov;71(Pt 11):2609–2614. doi: 10.1099/0022-1317-71-11-2609. [DOI] [PubMed] [Google Scholar]
- Lever A., Gottlinger H., Haseltine W., Sodroski J. Identification of a sequence required for efficient packaging of human immunodeficiency virus type 1 RNA into virions. J Virol. 1989 Sep;63(9):4085–4087. doi: 10.1128/jvi.63.9.4085-4087.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobel L. I., Goff S. P. Construction of mutants of Moloney murine leukemia virus by suppressor-linker insertional mutagenesis: positions of viable insertion mutations. Proc Natl Acad Sci U S A. 1984 Jul;81(13):4149–4153. doi: 10.1073/pnas.81.13.4149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luban J., Goff S. P. Binding of human immunodeficiency virus type 1 (HIV-1) RNA to recombinant HIV-1 gag polyprotein. J Virol. 1991 Jun;65(6):3203–3212. doi: 10.1128/jvi.65.6.3203-3212.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luban J., Lee C., Goff S. P. Effect of linker insertion mutations in the human immunodeficiency virus type 1 gag gene on activation of viral protease expressed in bacteria. J Virol. 1993 Jun;67(6):3630–3634. doi: 10.1128/jvi.67.6.3630-3634.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mervis R. J., Ahmad N., Lillehoj E. P., Raum M. G., Salazar F. H., Chan H. W., Venkatesan S. The gag gene products of human immunodeficiency virus type 1: alignment within the gag open reading frame, identification of posttranslational modifications, and evidence for alternative gag precursors. J Virol. 1988 Nov;62(11):3993–4002. doi: 10.1128/jvi.62.11.3993-4002.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overton H. A., Fujii Y., Price I. R., Jones I. M. The protease and gag gene products of the human immunodeficiency virus: authentic cleavage and post-translational modification in an insect cell expression system. Virology. 1989 May;170(1):107–116. doi: 10.1016/0042-6822(89)90357-7. [DOI] [PubMed] [Google Scholar]
- Pal R., Reitz M. S., Jr, Tschachler E., Gallo R. C., Sarngadharan M. G., Veronese F. D. Myristoylation of gag proteins of HIV-1 plays an important role in virus assembly. AIDS Res Hum Retroviruses. 1990 Jun;6(6):721–730. doi: 10.1089/aid.1990.6.721. [DOI] [PubMed] [Google Scholar]
- Rhee S. S., Hunter E. A single amino acid substitution within the matrix protein of a type D retrovirus converts its morphogenesis to that of a type C retrovirus. Cell. 1990 Oct 5;63(1):77–86. doi: 10.1016/0092-8674(90)90289-q. [DOI] [PubMed] [Google Scholar]
- Rhee S. S., Hunter E. Amino acid substitutions within the matrix protein of type D retroviruses affect assembly, transport and membrane association of a capsid. EMBO J. 1991 Mar;10(3):535–546. doi: 10.1002/j.1460-2075.1991.tb07980.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Royer M., Cerutti M., Gay B., Hong S. S., Devauchelle G., Boulanger P. Functional domains of HIV-1 gag-polyprotein expressed in baculovirus-infected cells. Virology. 1991 Sep;184(1):417–422. doi: 10.1016/0042-6822(91)90861-5. [DOI] [PubMed] [Google Scholar]
- Royer M., Hong S. S., Gay B., Cerutti M., Boulanger P. Expression and extracellular release of human immunodeficiency virus type 1 Gag precursors by recombinant baculovirus-infected cells. J Virol. 1992 May;66(5):3230–3235. doi: 10.1128/jvi.66.5.3230-3235.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartzberg P., Colicelli J., Gordon M. L., Goff S. P. Mutations in the gag gene of Moloney murine leukemia virus: effects on production of virions and reverse transcriptase. J Virol. 1984 Mar;49(3):918–924. doi: 10.1128/jvi.49.3.918-924.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommerfelt M. A., Roberts C. R., Hunter E. Expression of simian type D retroviral (Mason-Pfizer monkey virus) capsids in insect cells using recombinant baculovirus. Virology. 1993 Jan;192(1):298–306. doi: 10.1006/viro.1993.1033. [DOI] [PubMed] [Google Scholar]
- Trono D., Feinberg M. B., Baltimore D. HIV-1 Gag mutants can dominantly interfere with the replication of the wild-type virus. Cell. 1989 Oct 6;59(1):113–120. doi: 10.1016/0092-8674(89)90874-x. [DOI] [PubMed] [Google Scholar]
- Valverde V., Lemay P., Masson J. M., Gay B., Boulanger P. Autoprocessing of the human immunodeficiency virus type 1 protease precursor expressed in Escherichia coli from a synthetic gene. J Gen Virol. 1992 Mar;73(Pt 3):639–651. doi: 10.1099/0022-1317-73-3-639. [DOI] [PubMed] [Google Scholar]
- Veronese F. D., Rahman R., Copeland T. D., Oroszlan S., Gallo R. C., Sarngadharan M. G. Immunological and chemical analysis of P6, the carboxyl-terminal fragment of HIV P15. AIDS Res Hum Retroviruses. 1987 Fall;3(3):253–264. doi: 10.1089/aid.1987.3.253. [DOI] [PubMed] [Google Scholar]
- Wain-Hobson S., Sonigo P., Danos O., Cole S., Alizon M. Nucleotide sequence of the AIDS virus, LAV. Cell. 1985 Jan;40(1):9–17. doi: 10.1016/0092-8674(85)90303-4. [DOI] [PubMed] [Google Scholar]
- Wang C. T., Barklis E. Assembly, processing, and infectivity of human immunodeficiency virus type 1 gag mutants. J Virol. 1993 Jul;67(7):4264–4273. doi: 10.1128/jvi.67.7.4264-4273.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wills J. W., Craven R. C. Form, function, and use of retroviral gag proteins. AIDS. 1991 Jun;5(6):639–654. doi: 10.1097/00002030-199106000-00002. [DOI] [PubMed] [Google Scholar]
- Yu X., Yu Q. C., Lee T. H., Essex M. The C terminus of human immunodeficiency virus type 1 matrix protein is involved in early steps of the virus life cycle. J Virol. 1992 Sep;66(9):5667–5670. doi: 10.1128/jvi.66.9.5667-5670.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu X., Yuan X., Matsuda Z., Lee T. H., Essex M. The matrix protein of human immunodeficiency virus type 1 is required for incorporation of viral envelope protein into mature virions. J Virol. 1992 Aug;66(8):4966–4971. doi: 10.1128/jvi.66.8.4966-4971.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Poblotzki A., Wagner R., Niedrig M., Wanner G., Wolf H., Modrow S. Identification of a region in the Pr55gag-polyprotein essential for HIV-1 particle formation. Virology. 1993 Apr;193(2):981–985. doi: 10.1006/viro.1993.1210. [DOI] [PubMed] [Google Scholar]