Abstract
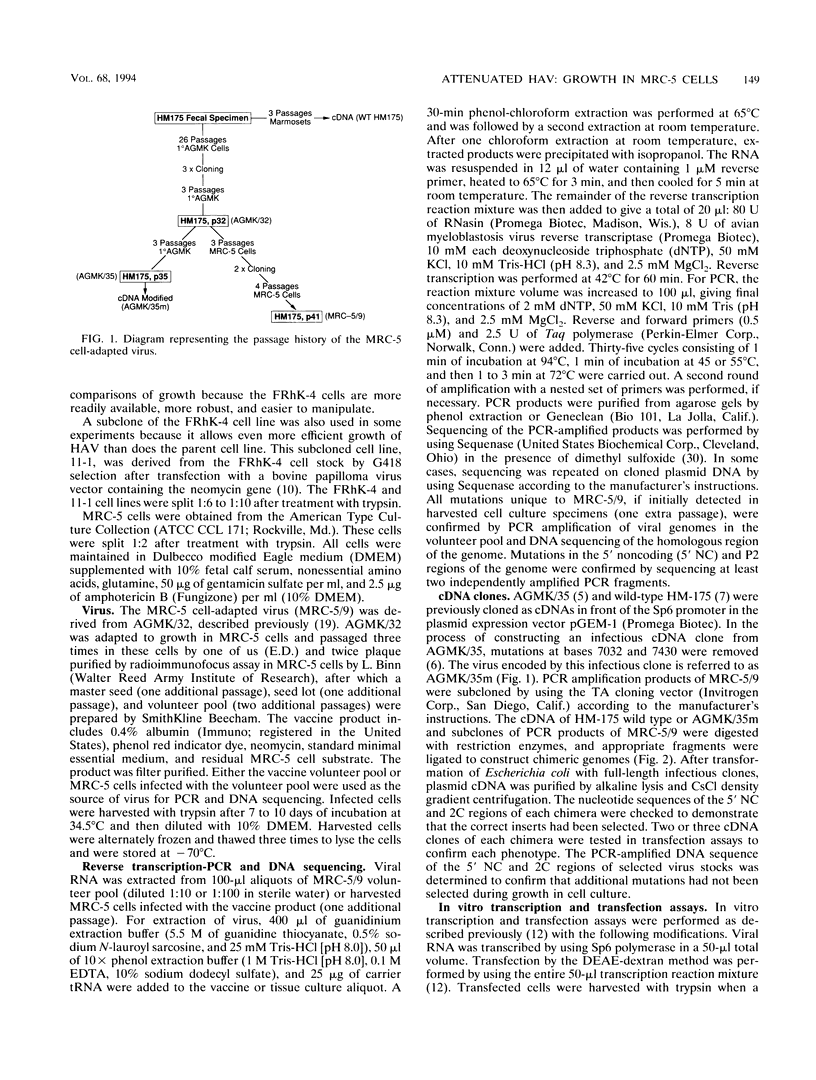
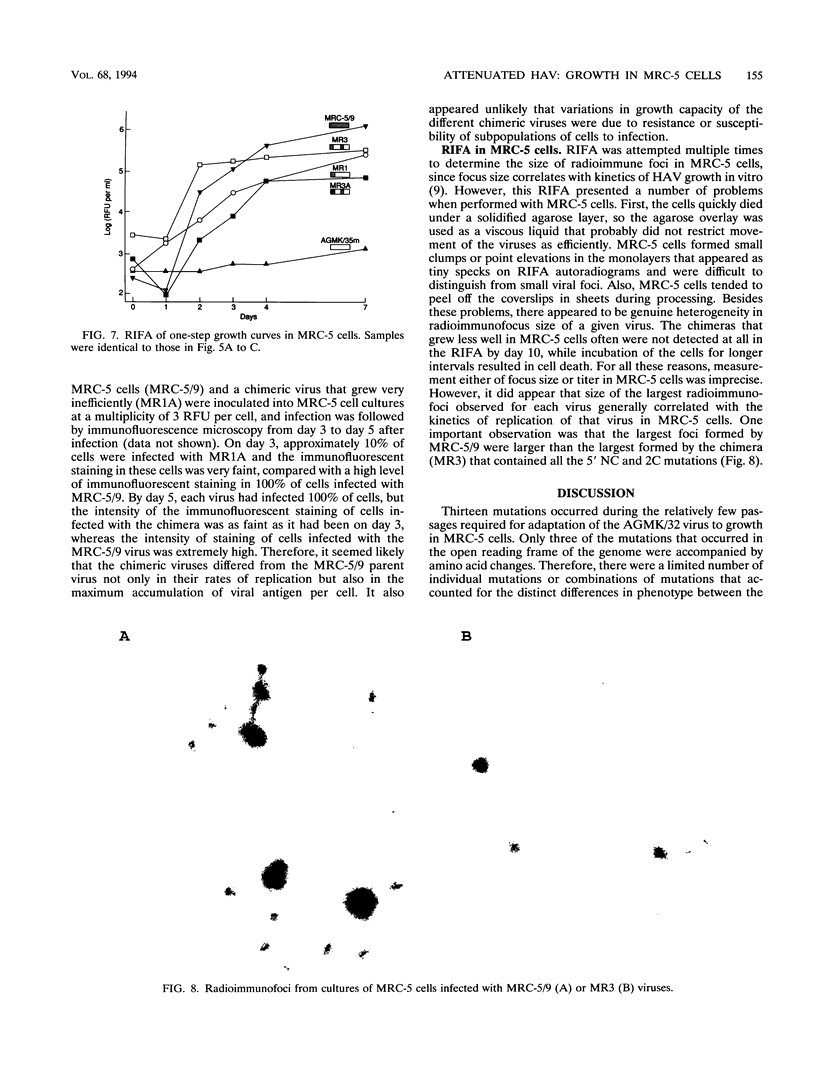
A live candidate hepatitis A virus vaccine, developed from the HM-175 strain and adapted to growth in primary African green monkey kidney (AGMK) cells, was adapted to growth in MRC-5 cells. The nucleotide sequence of the MRC-5 cell-adapted virus was determined and compared with the known sequence of the AGMK cell-adapted virus. Thirteen unique mutations, which occurred during passage in MRC-5 cells, were identified. Four of the unique mutations were located in a cluster in the 5' noncoding region (NC), and three of the remaining nine mutations encoded amino acid changes. Infectious chimeric cDNAs were constructed from infectious cDNA clones of the AGMK cell-adapted and wild-type HM-175 viruses and PCR-amplified cDNA segments of the MRC-5 cell-adapted virus. The viruses encoded by these plasmids were recovered after transfection of cultured cells with in vitro transcripts, and their growth phenotypes in fetal rhesus kidney 4 (FRhK-4) and MRC-5 cells were determined. The important growth-enhancing mutations could be divided into three sets. Two of these were located in the 5' NC region, and the third was located in the 2C nonstructural gene. The mutations in the 5' NC region that developed during passage in MRC-5 cells were indispensable for efficient growth in MRC-5 cells, but a combination of the two groups in the 5' NC region and one in the 2C gene were required to increase growth dramatically in MRC-5 cells.
Full text
PDF

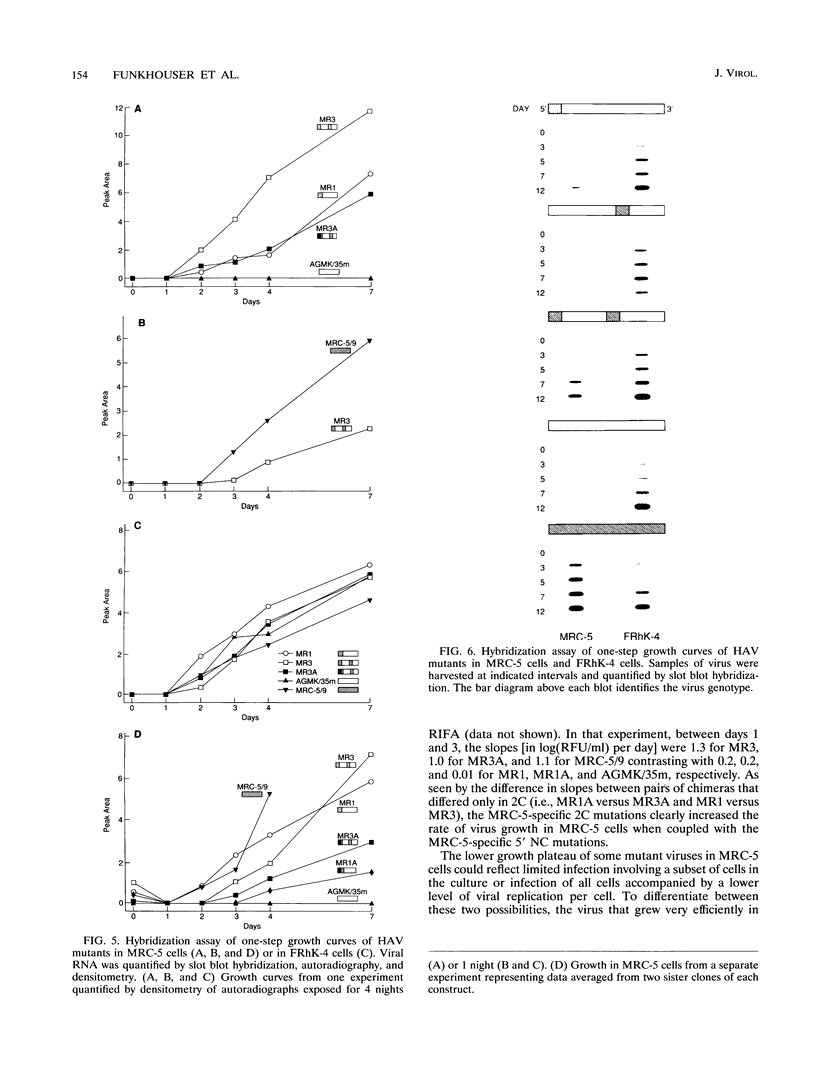


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Borzakian S., Pelletier I., Calvez V., Colbere-Garapin F. Precise missense and silent point mutations are fixed in the genomes of poliovirus mutants from persistently infected cells. J Virol. 1993 May;67(5):2914–2917. doi: 10.1128/jvi.67.5.2914-2917.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J. I., Rosenblum B., Feinstone S. M., Ticehurst J., Purcell R. H. Attenuation and cell culture adaptation of hepatitis A virus (HAV): a genetic analysis with HAV cDNA. J Virol. 1989 Dec;63(12):5364–5370. doi: 10.1128/jvi.63.12.5364-5370.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J. I., Rosenblum B., Ticehurst J. R., Daemer R. J., Feinstone S. M., Purcell R. H. Complete nucleotide sequence of an attenuated hepatitis A virus: comparison with wild-type virus. Proc Natl Acad Sci U S A. 1987 Apr;84(8):2497–2501. doi: 10.1073/pnas.84.8.2497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J. I., Ticehurst J. R., Feinstone S. M., Rosenblum B., Purcell R. H. Hepatitis A virus cDNA and its RNA transcripts are infectious in cell culture. J Virol. 1987 Oct;61(10):3035–3039. doi: 10.1128/jvi.61.10.3035-3039.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J. I., Ticehurst J. R., Purcell R. H., Buckler-White A., Baroudy B. M. Complete nucleotide sequence of wild-type hepatitis A virus: comparison with different strains of hepatitis A virus and other picornaviruses. J Virol. 1987 Jan;61(1):50–59. doi: 10.1128/jvi.61.1.50-59.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daemer R. J., Feinstone S. M., Gust I. D., Purcell R. H. Propagation of human hepatitis A virus in African green monkey kidney cell culture: primary isolation and serial passage. Infect Immun. 1981 Apr;32(1):388–393. doi: 10.1128/iai.32.1.388-393.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day S. P., Murphy P., Brown E. A., Lemon S. M. Mutations within the 5' nontranslated region of hepatitis A virus RNA which enhance replication in BS-C-1 cells. J Virol. 1992 Nov;66(11):6533–6540. doi: 10.1128/jvi.66.11.6533-6540.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emerson S. U., Huang Y. K., Purcell R. H. 2B and 2C mutations are essential but mutations throughout the genome of HAV contribute to adaptation to cell culture. Virology. 1993 Jun;194(2):475–480. doi: 10.1006/viro.1993.1286. [DOI] [PubMed] [Google Scholar]
- Emerson S. U., McRill C., Rosenblum B., Feinstone S., Purcell R. H. Mutations responsible for adaptation of hepatitis A virus to efficient growth in cell culture. J Virol. 1991 Sep;65(9):4882–4886. doi: 10.1128/jvi.65.9.4882-4886.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fineschi N., Cavalieri F., Garelick H., Prugnola A., Pellegrini V., Zuckerman A. J. Characterization of a hepatitis A virus strain suitable for vaccine production. J Hepatol. 1991;13 (Suppl 4):S146–S151. doi: 10.1016/0168-8278(91)90048-g. [DOI] [PubMed] [Google Scholar]
- Flehmig B., Heinricy U., Pfisterer M. Immunogenicity of a killed hepatitis A vaccine in seronegative volunteers. Lancet. 1989 May 13;1(8646):1039–1041. doi: 10.1016/s0140-6736(89)92443-4. [DOI] [PubMed] [Google Scholar]
- Gust I. D., Lehmann N. I., Crowe S., McCrorie M., Locarnini S. A., Lucas C. R. The origin of the HM175 strain of hepatitis A virus. J Infect Dis. 1985 Feb;151(2):365–367. doi: 10.1093/infdis/151.2.365. [DOI] [PubMed] [Google Scholar]
- Horng Y. C., Chang M. H., Lee C. Y., Safary A., Andre F. E., Chen D. S. Safety and immunogenicity of hepatitis A vaccine in healthy children. Pediatr Infect Dis J. 1993 May;12(5):359–362. doi: 10.1097/00006454-199305000-00001. [DOI] [PubMed] [Google Scholar]
- Jansen R. W., Newbold J. E., Lemon S. M. Complete nucleotide sequence of a cell culture-adapted variant of hepatitis A virus: comparison with wild-type virus with restricted capacity for in vitro replication. Virology. 1988 Apr;163(2):299–307. doi: 10.1016/0042-6822(88)90270-x. [DOI] [PubMed] [Google Scholar]
- Karron R. A., Daemer R., Ticehurst J., D'Hondt E., Popper H., Mihalik K., Phillips J., Feinstone S., Purcell R. H. Studies of prototype live hepatitis A virus vaccines in primate models. J Infect Dis. 1988 Feb;157(2):338–345. doi: 10.1093/infdis/157.2.338. [DOI] [PubMed] [Google Scholar]
- Lemon S. M., Binn L. N., Marchwicki R. H. Radioimmunofocus assay for quantitation of hepatitis A virus in cell cultures. J Clin Microbiol. 1983 May;17(5):834–839. doi: 10.1128/jcm.17.5.834-839.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemon S. M., Murphy P. C., Shields P. A., Ping L. H., Feinstone S. M., Cromeans T., Jansen R. W. Antigenic and genetic variation in cytopathic hepatitis A virus variants arising during persistent infection: evidence for genetic recombination. J Virol. 1991 Apr;65(4):2056–2065. doi: 10.1128/jvi.65.4.2056-2065.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathiesen L. R., Feinstone S. M., Purcell R. H., Wagner J. A. Detection of hepatitis A antigen by immunofluorescence. Infect Immun. 1977 Nov;18(2):524–530. doi: 10.1128/iai.18.2.524-530.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul A. V., Tada H., von der Helm K., Wissel T., Kiehn R., Wimmer E., Deinhardt F. The entire nucleotide sequence of the genome of human hepatitis A virus (isolate MBB). Virus Res. 1987 Aug;8(2):153–171. doi: 10.1016/0168-1702(87)90026-8. [DOI] [PubMed] [Google Scholar]
- Ross B. C., Anderson B. N., Edwards P. C., Gust I. D. Nucleotide sequence of high-passage hepatitis A virus strain HM175: comparison with wild-type and cell culture-adapted strains. J Gen Virol. 1989 Oct;70(Pt 10):2805–2810. doi: 10.1099/0022-1317-70-10-2805. [DOI] [PubMed] [Google Scholar]
- Sjogren M. H., Purcell R. H., McKee K., Binn L., Macarthy P., Ticehurst J., Feinstone S., Caudill J., See A., Hoke C. Clinical and laboratory observations following oral or intramuscular administration of a live attenuated hepatitis A vaccine candidate. Vaccine. 1992;10 (Suppl 1):S135–S137. doi: 10.1016/0264-410x(92)90568-5. [DOI] [PubMed] [Google Scholar]
- Tedeschi V., Purcell R. H., Emerson S. U. Partial characterization of hepatitis A viruses from three intermediate passage levels of a series resulting in adaptation to growth in cell culture and attenuation of virulence. J Med Virol. 1993 Jan;39(1):16–22. doi: 10.1002/jmv.1890390105. [DOI] [PubMed] [Google Scholar]
- Werzberger A., Mensch B., Kuter B., Brown L., Lewis J., Sitrin R., Miller W., Shouval D., Wiens B., Calandra G. A controlled trial of a formalin-inactivated hepatitis A vaccine in healthy children. N Engl J Med. 1992 Aug 13;327(7):453–457. doi: 10.1056/NEJM199208133270702. [DOI] [PubMed] [Google Scholar]
- Wiedermann G., Ambrosch F., Kollaritsch H., Hofmann H., Kunz C., D'Hondt E., Delem A., André F. E., Safary A., Stéphenne J. Safety and immunogenicity of an inactivated hepatitis A candidate vaccine in healthy adult volunteers. Vaccine. 1990 Dec;8(6):581–584. doi: 10.1016/0264-410x(90)90013-c. [DOI] [PubMed] [Google Scholar]
- Winship P. R. An improved method for directly sequencing PCR amplified material using dimethyl sulphoxide. Nucleic Acids Res. 1989 Feb 11;17(3):1266–1266. doi: 10.1093/nar/17.3.1266. [DOI] [PMC free article] [PubMed] [Google Scholar]