Abstract
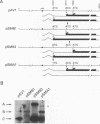
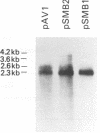
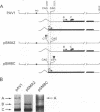
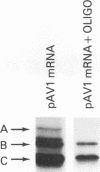
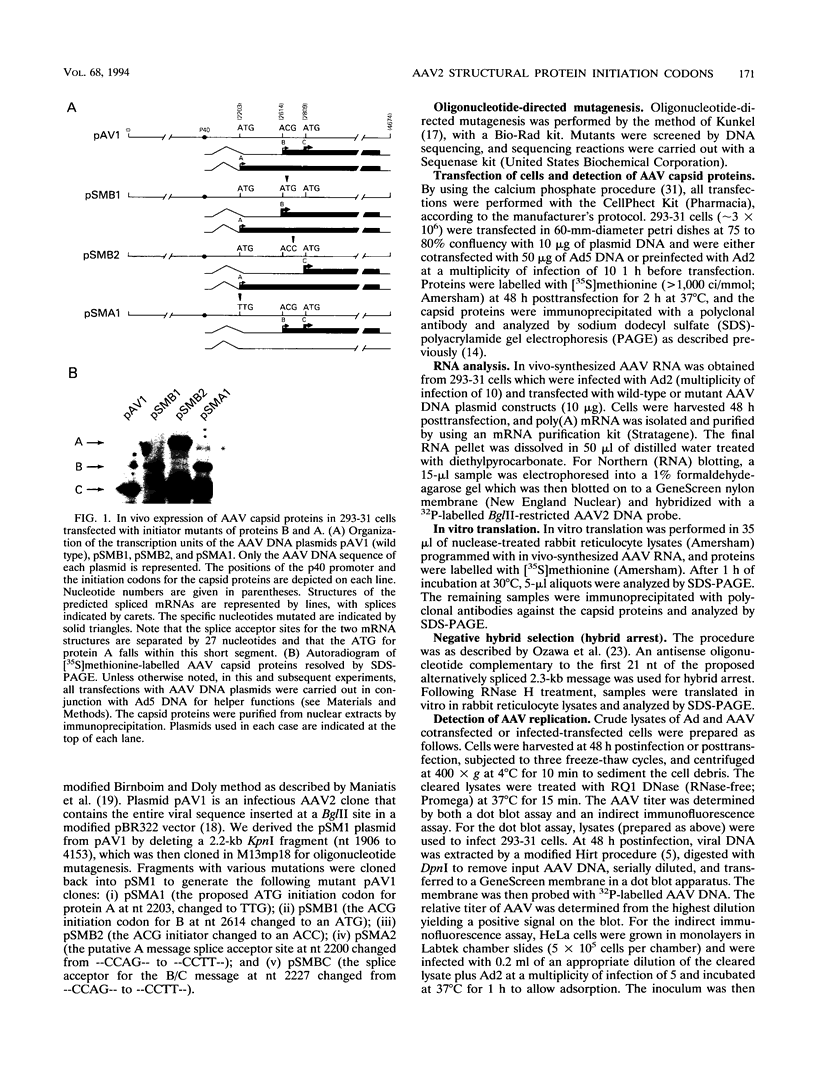
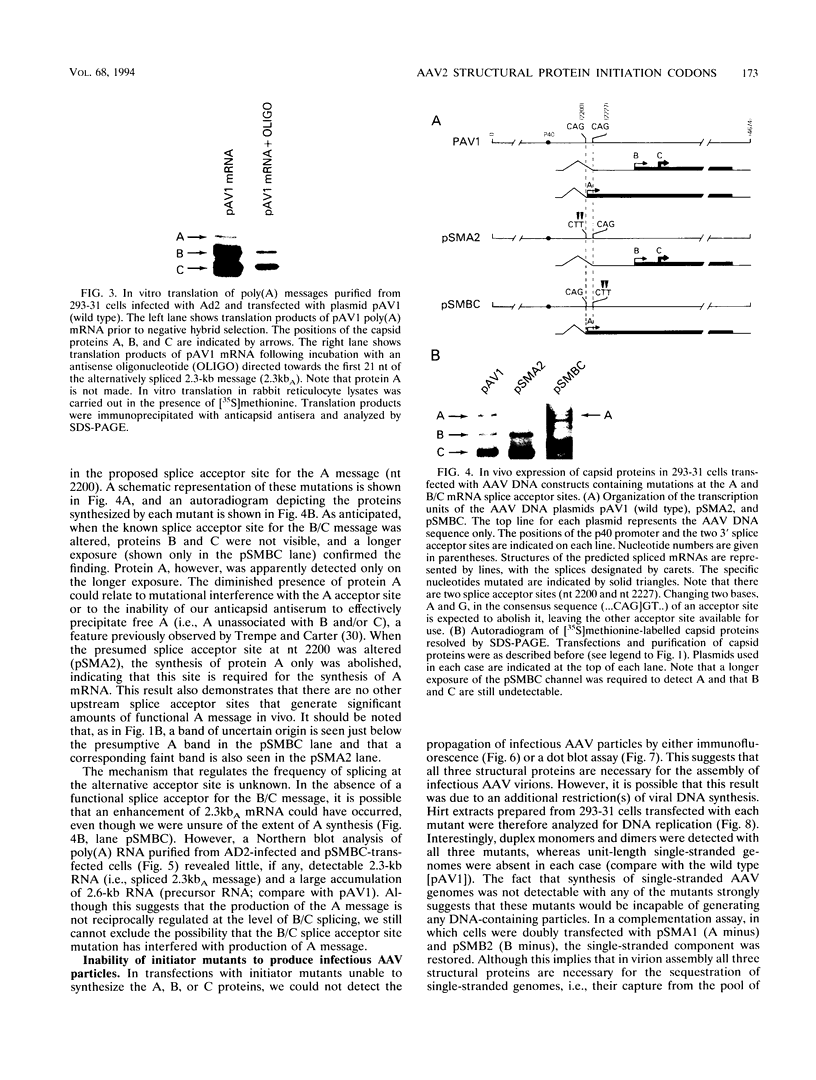
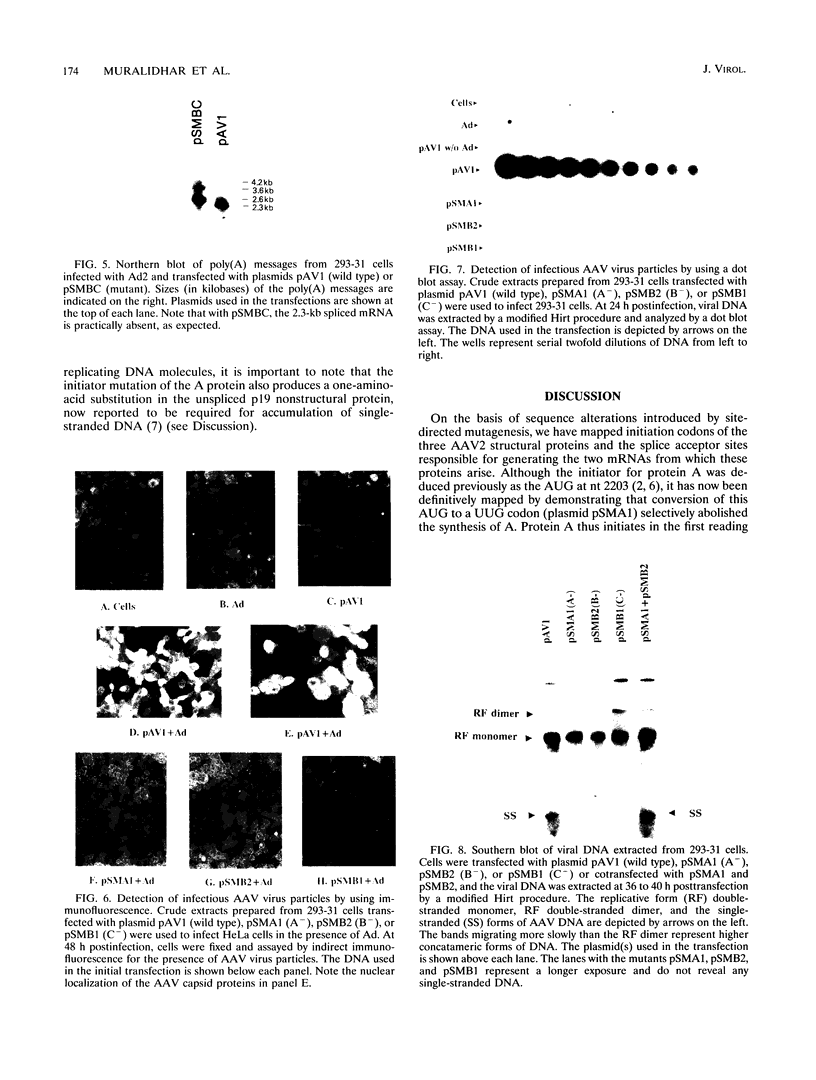
It has been shown that two of the three adeno-associated virus type 2 capsid proteins, B and C, are synthesized from a single spliced transcript. Protein C arises from an AUG codon at nucleotide 2810, whereas protein B is initiated by a unique eucaryotic initiation codon (ACG) that lies 65 triplets upstream from the C origin. The third capsid component, protein A, is synthesized from a second spliced transcript which uses an alternative 3' acceptor site. In this study we used oligonucleotide-directed mutagenesis to confirm the positions of the B initiation codon and the 3' acceptor sites for the alternatively spliced B/C and A protein messages. We also located definitively the protein A initiation codon, an AUG triplet mapping to nucleotide 2203. Mutagenesis of the B initiator permitted a direct test of the effect of increased B initiator strength on the translational efficiencies of the B and C proteins. It was found that conversion of the relatively inefficient protein B initiator (ACG) to an AUG enhanced the level of B synthesis while abolishing the synthesis of C from its downstream AUG initiator. Protein C synthesis thus depends on the strength of the B initiator, i.e., the relatively higher levels of C (approximately 20-fold greater than B) must result from frequent readthrough of the weak B initiator. Finally, we examined the abilities of mutants deficient in the synthesis of A, B, or C to produce infectious virions. We found that at least two of the structural proteins, B and C, are required for the production of infectious virions and that sequestration of single-stranded adeno-associated virus genomes from the pool of replicating DNA molecules does not occur in the absence of either of these proteins.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson C. W., Buzash-Pollert E. Can ACG serve as an initiation codon for protein synthesis in eucaryotic cells? Mol Cell Biol. 1985 Dec;5(12):3621–3624. doi: 10.1128/mcb.5.12.3621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becerra S. P., Koczot F., Fabisch P., Rose J. A. Synthesis of adeno-associated virus structural proteins requires both alternative mRNA splicing and alternative initiations from a single transcript. J Virol. 1988 Aug;62(8):2745–2754. doi: 10.1128/jvi.62.8.2745-2754.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becerra S. P., Rose J. A., Hardy M., Baroudy B. M., Anderson C. W. Direct mapping of adeno-associated virus capsid proteins B and C: a possible ACG initiation codon. Proc Natl Acad Sci U S A. 1985 Dec;82(23):7919–7923. doi: 10.1073/pnas.82.23.7919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buller R. M., Janik J. E., Sebring E. D., Rose J. A. Herpes simplex virus types 1 and 2 completely help adenovirus-associated virus replication. J Virol. 1981 Oct;40(1):241–247. doi: 10.1128/jvi.40.1.241-247.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter B. J., Marcus-Sekura C. J., Laughlin C. A., Ketner G. Properties of an adenovirus type 2 mutant, Ad2dl807, having a deletion near the right-hand genome terminus: failure to help AAV replication. Virology. 1983 Apr 30;126(2):505–516. doi: 10.1016/s0042-6822(83)80008-7. [DOI] [PubMed] [Google Scholar]
- Chejanovsky N., Carter B. J. Mutagenesis of an AUG codon in the adeno-associated virus rep gene: effects on viral DNA replication. Virology. 1989 Nov;173(1):120–128. doi: 10.1016/0042-6822(89)90227-4. [DOI] [PubMed] [Google Scholar]
- Curran J., Kolakofsky D. Ribosomal initiation from an ACG codon in the Sendai virus P/C mRNA. EMBO J. 1988 Jan;7(1):245–251. doi: 10.1002/j.1460-2075.1988.tb02806.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham F. L., Smiley J., Russell W. C., Nairn R. Characteristics of a human cell line transformed by DNA from human adenovirus type 5. J Gen Virol. 1977 Jul;36(1):59–74. doi: 10.1099/0022-1317-36-1-59. [DOI] [PubMed] [Google Scholar]
- Green M. R., Roeder R. G. Definition of a novel promoter for the major adenovirus-associated virus mRNA. Cell. 1980 Nov;22(1 Pt 1):231–242. doi: 10.1016/0092-8674(80)90171-3. [DOI] [PubMed] [Google Scholar]
- Green M. R., Roeder R. G. Transcripts of the adeno-associated virus genome: mapping of the major RNAs. J Virol. 1980 Oct;36(1):79–92. doi: 10.1128/jvi.36.1.79-92.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hann S. R., King M. W., Bentley D. L., Anderson C. W., Eisenman R. N. A non-AUG translational initiation in c-myc exon 1 generates an N-terminally distinct protein whose synthesis is disrupted in Burkitt's lymphomas. Cell. 1988 Jan 29;52(2):185–195. doi: 10.1016/0092-8674(88)90507-7. [DOI] [PubMed] [Google Scholar]
- Hermonat P. L., Labow M. A., Wright R., Berns K. I., Muzyczka N. Genetics of adeno-associated virus: isolation and preliminary characterization of adeno-associated virus type 2 mutants. J Virol. 1984 Aug;51(2):329–339. doi: 10.1128/jvi.51.2.329-339.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janik J. E., Huston M. M., Rose J. A. Adeno-associated virus proteins: origin of the capsid components. J Virol. 1984 Nov;52(2):591–597. doi: 10.1128/jvi.52.2.591-597.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jongeneel C. V., Sahli R., McMaster G. K., Hirt B. A precise map of splice junctions in the mRNAs of minute virus of mice, an autonomous parvovirus. J Virol. 1986 Sep;59(3):564–573. doi: 10.1128/jvi.59.3.564-573.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak M. The scanning model for translation: an update. J Cell Biol. 1989 Feb;108(2):229–241. doi: 10.1083/jcb.108.2.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunkel T. A. Rapid and efficient site-specific mutagenesis without phenotypic selection. Proc Natl Acad Sci U S A. 1985 Jan;82(2):488–492. doi: 10.1073/pnas.82.2.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laughlin C. A., Tratschin J. D., Coon H., Carter B. J. Cloning of infectious adeno-associated virus genomes in bacterial plasmids. Gene. 1983 Jul;23(1):65–73. doi: 10.1016/0378-1119(83)90217-2. [DOI] [PubMed] [Google Scholar]
- Marcus C. J., Laughlin C. A., Carter B. J. Adeno-associated virus RNA transcription in vivo. Eur J Biochem. 1981 Dec;121(1):147–154. doi: 10.1111/j.1432-1033.1981.tb06443.x. [DOI] [PubMed] [Google Scholar]
- McPherson R. A., Rose J. A. Structural proteins of adenovirus-associated virus: subspecies and their relatedness. J Virol. 1983 May;46(2):523–529. doi: 10.1128/jvi.46.2.523-529.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra L., Rose J. A. Adeno-associated virus DNA replication is induced by genes that are essential for HSV-1 DNA synthesis. Virology. 1990 Dec;179(2):632–639. doi: 10.1016/0042-6822(90)90130-j. [DOI] [PubMed] [Google Scholar]
- Ozawa K., Ayub J., Young N. Functional mapping of the genome of the B19 (human) parvovirus by in vitro translation after negative hybrid selection. J Virol. 1988 Jul;62(7):2508–2511. doi: 10.1128/jvi.62.7.2508-2511.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peabody D. S. Translation initiation at an ACG triplet in mammalian cells. J Biol Chem. 1987 Aug 25;262(24):11847–11851. [PubMed] [Google Scholar]
- Rhode S. L., 3rd Nucleotide sequence of the coat protein gene of canine parvovirus. J Virol. 1985 May;54(2):630–633. doi: 10.1128/jvi.54.2.630-633.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose J. A., Maizel J. V., Jr, Inman J. K., Shatkin A. J. Structural proteins of adenovirus-associated viruses. J Virol. 1971 Nov;8(5):766–770. doi: 10.1128/jvi.8.5.766-770.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp P. A. Splicing of messenger RNA precursors. Science. 1987 Feb 13;235(4790):766–771. doi: 10.1126/science.3544217. [DOI] [PubMed] [Google Scholar]
- Smuda J. W., Carter B. J. Adeno-associated viruses having nonsense mutations in the capsid genes: growth in mammalian cells containing an inducible amber suppressor. Virology. 1991 Sep;184(1):310–318. doi: 10.1016/0042-6822(91)90847-5. [DOI] [PubMed] [Google Scholar]
- Tratschin J. D., Miller I. L., Carter B. J. Genetic analysis of adeno-associated virus: properties of deletion mutants constructed in vitro and evidence for an adeno-associated virus replication function. J Virol. 1984 Sep;51(3):611–619. doi: 10.1128/jvi.51.3.611-619.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trempe J. P., Carter B. J. Alternate mRNA splicing is required for synthesis of adeno-associated virus VP1 capsid protein. J Virol. 1988 Sep;62(9):3356–3363. doi: 10.1128/jvi.62.9.3356-3363.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wigler M., Pellicer A., Silverstein S., Axel R., Urlaub G., Chasin L. DNA-mediated transfer of the adenine phosphoribosyltransferase locus into mammalian cells. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1373–1376. doi: 10.1073/pnas.76.3.1373. [DOI] [PMC free article] [PubMed] [Google Scholar]